1Department of Biology, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia.
2King Abdulaziz Hospital, Ministry of Health, Makkah, Saudi Arabia.
3Ministry of Health, Jeddah, Saudi Arabia.
4Alwaha Medical Center (Private clinic), Jeddah, Saudi Arabia.
5Botany and Microbiology Department, Faculty of Science, Kafrelsheikh University, Egypt
Corresponding author email: magdammali@hotmail.com
Article Publishing History
Received: 27/11/2020
Accepted After Revision: 25/02/2021
One of the environmental pollutants in the word is Keratin waste which is produced mostly from the poultry farms, slaughterhouses, and leather industries. Beneficial organisms are found in nature and considered as a well-known microflora (fungi and bacteria), which have the capability to degrade the keratin waste. This review deals with the different application of microbial keratinase. The keratinolytic microflora has been qualified to produce keratinase enzyme for biodegradation (enzymatic degradation). Keratinases are proteolytic enzymes accomplished of degrading insoluble keratin protein present in skin, hair, nail, or feather. Keratinases are active over wide range of conditions and are useful in bio recycling of keratin wastes into feed and fertilizers. They also have potential applications in leather, cosmetic, textile, biomedical and detergent industries. The applications of keratinases extend to energy generation and green synthesis of nanoparticles. Due to their ubiquitous biotechnological applications, techniques such as immobilization, optimization strategies, protein engineering and DNA recombinant technology have been used to improve their activities and stabilities thereby widening the scope for commercialization. This review records recent multi-functional applications of keratinases.
Keratin, Wastes, Keratinase, Microorganism, Application, Environment, Pollutants
Khalel A. F, Aly M. M, Alghamdi A. G, Khalil A. F, Aloryani4 F. A. Insight into the Keratinase Enzymes from Microbial Origins and Their Applications. Biosc.Biotech.Res.Comm. 2021;14(1).
Khalel A. F, Aly M. M, Alghamdi A. G, Khalil A. F, Aloryani F. A. Insight into the Keratinase Enzymes from Microbial Origins and Their Applications. Biosc.Biotech.Res.Comm. 2021;14(1). Available from: <a href=”https://bit.ly/3reMeKo“>https://bit.ly/3reMeKo</a>
Copyright © Khalel et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
All over the world, production of livestock is increasing quickly because of population growth, increasing incomes, changes in lifestyles and nutritional habits. The leftover from animal meat production consists of keratinous materials such as chicken feathers, pig bristles, wool and horns and millions of tons of these co-products are produced each year (Jingwen et al., 2020). Chicken feathers from poultry processing plants is classified as low-risk materials for animals, the public, and the environment because of high producing of keratin (Verma et al., 2017).
Consequently, they can be considered as an abundant protein or amino acid source for new cycling processes targeting potential use in feeding, fertilizer in cosmetics and other applications (Callegaro et al., 2019). Searching for better and “green” ways to support the world health by motivation and utilization of natural byproducts are available. Unique keratinous materials come from the rich of certain amino acids, including in particular the sulfur-containing amino acid, cysteine, other amino acids like glycine, proline, arginine, and the essential amino acids valine, leucine, and threonine.
The highly stability of keratin is due to disulfide bridges formed among cysteine residues within and between keratin polypeptides (Callegaro et al., 2019). Keratins considered as insoluble materials and partial or complete degradation without denaturizing the amino acids are needed (Gindaba et al, 2019). The degradation processes provide useful bio- refinery methods for both protein and amino acids (Koentjoro and Prasetyo, 2021).
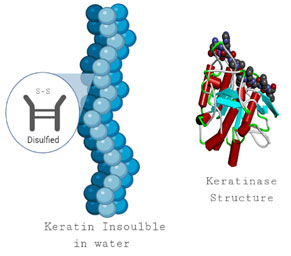
Keratin and its derivatives: Keratins are a major class of structural proteins that are highly resistant to biological degradation. Common enzymes, which break down protein, such as trypsin do not affect Keratins. They are water insoluble proteins. Like other proteins, they are made of a long string of various amino acids, which fold into a final three-dimensional form. There are two types of keratins; α-keratins and β-keratins, consisting of tightly packed protein chains in α-helices and β-sheets, respectively (Parry and North, 1998; Esawy, 2007). Additionally, keratins filament structures are stabilized by their high degree of cross-linking of disulfide bonds, hydrophobic interactions and hydrogen bonds.
Due to their extremely rigid structures, Keratins are insoluble and hard to degrade (Esawy, 2007). Chicken feathers are composed of over 90% of Keratin protein, small amounts of lipids and water. Feathers Keratin consists of high quantities of small and essential amino acid residues (Pencho, 1990). It is also the main protein components of hair, wool, nails, horn and hoofs. Animal hair, hoofs, horns and wool contain α-keratin and bird’s feather contains β-KRT. The polypeptides in α-KRT are closely associated pairs of α-helices, whereas β-KRT has high proportion of β pleated sheets (Figure 1) (Asquith, 1977; Morris et al., 1992; Savitha et al., 2007).
Keratins are macromolecule comprises a tight packing of supercoiled long polypeptide chains with a molecular weight of approximately 10 kDa. High degree of cross linked cystine disulfide bonds between contiguous chains in keratinous material imparts high stability and resistance to degradation (Schmidt and Barone, 2004; Coward-Kelly et al., 2006; Tamilmaniet al., 2008; Weidele, 2009). In summary, a keratinous material is a tough, fibrous matrix being mechanically firm, chemically unreactive, water insoluble and protease-resistant (Savitha et al., 2007;Callegaro et al., 2019; Gindaba et al, 2019; Koentjoro and Prasetyo, 2021).
Figure 1: Keratin structure shows the disulfide bond which makes the keratin insoluble in water and keratinase structure.
Production of keratinases:
Keratinase production in liquid medium (submerged fermentation): Onifade et al., (1998) reported that when bacteria or fungi were grown in liquid medium containing keratinous substrates or feathers as carbon sources, extracellular intracellular keratinases were predominantly obtained (submerged fermentation). It is clear that keratin or feather act as an inducer for keratinases production however soy meal (non-keratin material) may also induce the enzyme release.
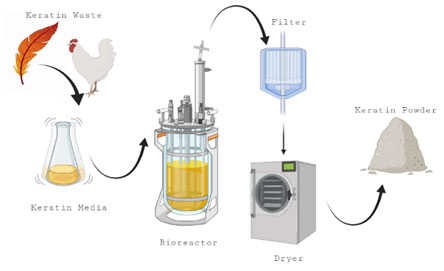
Keratin was degraded in two steps, sulfitolysis, meaning the decrease the number of the disulfide bonds and protein hydrolysis (Gupta and Ramnani, 2006). Under submerged conditions using either shaking or static methods, production of Keratinase is mainly obtained (Lateef et al., 2010; Cai et al., 2011, Aly and Tork, 2018, Aly et al., 2019). Keratinolys is activity of each bacterium differed according to production conditions, the used microorganisms and cultivation techniques. Additionally, addition of simple carbohydrates like glucose suppresses the keratinase production (Daroit et al., 2011, Aly et al., 2019). On contrast, polysaccharide as starch improves the production of keratinases (Syed et al., 2009). Figure 2, showed the steps used for production keratinase in submerged fermentation.
Figure 2: Production keratinase in submerged fermentation.
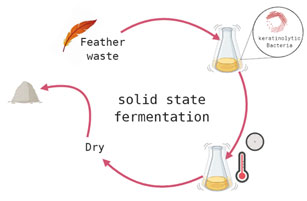
Keratinase production in solid state fermentation: Paul et al., (2013) reported that feathers, hair, horn and sugarcane bagasse can be used as rich carbon source for induction of keratinases from bacteria (solid state fermentation).Likewise , it was reported that under solid state fermentation, presence of 0.1% soybean powder in the growth medium in addition to feather enlarged keratinase production by Bacillus sp. PPKS-2 (Prakash et al., 2010, Tork et al., 2016) while El-Gendy (2010) found that under solid state fermentation and using different agricultural and poultry wastes, the endophytic keratinolytic fungal,Penicilliumsp., extensively produced keratinase. Figure 3 showed the steps for Keratinase production during solid state fermentation. Fermentation method is used to biodegrade whole chicken feather sby keratinolytic microorganisms.
For example, Fervidobacterium islandicum AW-1 is keratinolytic microorganism has been considered as an important microbe for biodegradation of feathers and keratinous wastes. Potential fermentation process enables this bacterium to degrade feather with excellent activities (Yeo et al., 2018). The used technique is advantageous by reason of its ability to apply substrates quite rapidly and is best suited for the bacteria that need high moisture contents (Koentjoro and Prasetyo, 2021).
In this method, the screening and isolation of bacteria are the most power step to start the process. The ability of the isolates to degrade the feather polymers directly and highly fermented was selected. The ability of the microbe to degrade feather is affected by optimization of feather concentration, incubation time, pH and temperature (Osman et al., 2017).
Figure 3: Keratinase production in solid state fermentation.
Keratinase application:
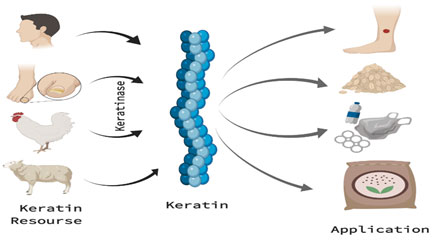
Wound dressings: Keratins is a rich source of proteins and have a clear functions in wound healing because Keratin material found mainly as fundamental components of the skin (Kelly, 2016). Proteins of Keratin may be obtained from wool using processes that do not hydrolyze peptide bonds, which allows the keratin proteins to retain a form and function similar to native keratins. Topical creams can then be prepared from purified keratinase which can be incorporated into dressings and Keraplast. These treatments are often used for the treatment of persistent wounds and have been found to be therapeutic (Kelly, 2016) as shown in Figure 4.
Figure 4: Keratin sources used for keratinase production and its applications.
Keratinase for Keratin waste management: The huge increase in the population is linked to increases in wastes production. Biodegradation of different wastes to useful materials or products is important for meeting the demand of the crowded population. Accordingly, there is an enlarged amounts of agro- industrial wastes, including keratin and feather wastes from slaughterhouses, poultry farms and leather industry (Tesfaye et al., 2017; Srivastava et al., 2020).
It is clear that, feather wastes increased every minute and feather treatments are urgent to decrease bad environmental impacts and maintain healthy environment. Thus, keratinases from bacteria or fungi can degrade different waste components in addition to prions by using cocktail enzymes from active microbes. Microbial keratinase had very remarkable feather degrading ability and thus could be usefully used in management possess (Lateef et al., 2015).
Keratin wastes may be keratins, feathers, collagens, elastins, wool and prion proteins all these wastes are generated during meat industries and considered dangerous wastes must be efficiently degraded by the keratinolytic enzymes (Zhao et al., 2012). Also, wool waste can be degraded by keratinase obtained from Stenotrophomona smaltophilia as reported before (Fang et al., 2013). Therefore, using a mixture of bacterial keratinases can be used as potential application for bio-treatment of slaughterhouse or abattoirwaste stream/effluents to simple important materials.
Thus, professional management of keratin and feather wastes through recycling into value-added and proper products is important. The costs of chemical management process may be high, thus efficient means of biological treatments can be applied to prevent the harmful effects of these wastes on the environment (Nnolim et al., 2020).
Animal feed production: For decades, it is well know that feather meal can be used as supplements or a feed but the nutritive value of the meal is different due to the used protein material in the feed. Proteins structure in feathers or other keratinous materials are difficult to be broken or digested by ruminants or livestock due to structural orientation and presence of different chemical groups (Nnolim et al., 2020). Chemical degradation of feather wastes can be used to prepare feather meal but this method is costly high and need high energy input in addition it denatures important heat sensitive proteins (Dong et al., 2017). Thus, keratinases from keratinolytic microorganisms can be used for the recycling techniques used for waste managements (Gegeckas et al., 2018).
keratinolytic wastes are rich in many amino acids and the hydro-thermal treatments of keratinolytic wastes reduces nutritional values of the products as they destroyed definite essential amino acids like methionine, lysine, histidine and tryptophan and inability to release some amino acids from the keratins. Consequently, keratinases from microorganism origins are a good alternative for bio-treatment and may be used for keratinolytic wastes degradation.
as reported before, the keratinolytic isolate B. subtilis may be used for degradation of keratins and production of proteinous hydrolysate with as potential values from wool and feather wastes (Fakhfakh et al., 2013; Volik et al, 2020). Keratinase degradation of feather is more beneficial than microbial degradation, as it avoids the possibility of exposure of the environment to the microbe which may be a pathogen. Nutritional uses of keratinase daily in growing and nursery pigs as supplements improve immune response, weight gain, nutrient digestibility, intestinal morphology and ecology (Wang et al., 2011).
Bacillus licheniformis hydrolyzed feather to free amino acids which increased the growth chickens (Williams et al., 1991) while feather hydrolyzate obtained by B. licheniformis LMUB05 showed no significant effect on birds performance (Adetunji and Adejumo, 2018). For these reasons, biodegradation of keratins which serves as an actual source of nutrient-rich feeds, and feed supplements with lots of potential applications in animal husbandry needs more detail studied to enhance to biodegradation process using the best bacterium or keratinases (Nnolim et al., 2020).
Production of bio-fertilizer: Keratin composed of different amino acids and many microorganisms are able to degrade it by production of keratinase. An example for a keratinase-producing strain is B. subtilis which is demonstrated plant growth-promoting and broad-spectrum antimicrobial activities, as it produced indole acetic acid (IAA) and antifungal activities in the course of keratinase production (Jeong et al., 2010).
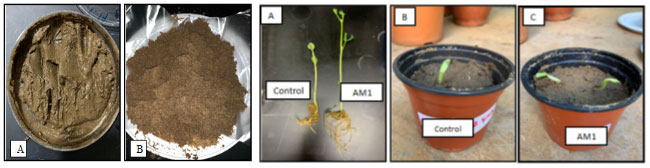
Feather meal produced from the recycling of keratinous wastes is still applicable as a slow-release biofertilizer. Khalel et al. (2020) obtained feather hydrolysate through hydrolysis from Streptomyces enissocaesilis AM1which enhanced plant growth. It also enhanced inorganic elements and microbial activities in the soil, while the N, P, K and the C/N ratio was increased, thereby improving soil fertility (Figure 5).
Figure 5: Feather degradation production biofertilizer to enhance plant growth (Khalel et al., 2020).
CONCLUSION
Microbial keratinases have many industrial and biotechnological applications including management of wastewater, treatments and recycling leathers, feathers and agricultural wastes. They can be used in textile technology, food and feed preparations, medical and pharmaceutical applications, agriculture industries (bio-fertilizers and composting, plant-growth promotion), and bio catalysis preparations. Search for new sources of keratinases from bacteria using new techniques to increase enzyme production, decreased the production costs, enhanced the enzyme characters in addition to enzyme stability at high temperature and different pH are urgently needed. The multi-functionality of keratinases may encourage scientists for more studies which guide us for production of keratinase with excellent characters for bioconversion of feather or different keratinous wastes to free amino acids or bio-fertilizers.
REFERENCES
Adetunji C O and Adejumo I O (2018). Efficacy of crude and immobilized enzymes from Bacillus licheniformis for production of biodegraded feather meal and their assessment on chickens. Environ. Technol. Innov., 11, 116–124.
Aly M M and Tork S (2018). High keratinase production and keratin degradation by a mutant strain KR II, derived from Streptomyces radiopugnans KR 12. Journal of Applied Biological Sciences, 12 (1): 18-25.
Aly M M, Khalel A and Hassan S (2019). Isolation, Identification, and Characterization of a Keratolytic Bacterium From Poultry Wastes. IOSR Journal of Pharmacy and Biological Sciences, 14.5: 46-50.
Asquith RS (1977). Chemistry of natural protein fibers. Plenum Press, New York, USA.
Cai SB, Huang ZH, Zhang XQ, Cao ZJ, Zhou MH et al. (2011). Identification of a keratinase-producing bacterial strain and enzymatic study for its improvement on shrink resistance and tensile strength of wool- and polyester-blend blended fabric. Applied Biochemistry and Biotechnology, 163 (1):112-126.
Callegaro A, Ndour C, Aris E, Legrand C (2019). A note on tests for relevant differences with extremely large sample sizes. Biom J. Jan;61(1):162-165.
Coward-Kelly G, Vincent SC, Frank KA and Mark TH (2006). Lime treatment of keratinous materials for the generation of highly digestible animal feed: Chicken feathers, Biores Technol 97:1337-1343.
Daroit D J, Correa APF and Brandelli A (2011). Production of keratinolytic proteases through bioconversion of feather meal by the Amazonian bacterium Bacillus sp. P45. International Biodeterioration and Biodegradation, 65(1):45-51.
El-Gendy M MA (2010). Keratinase production by endophytic Penicillium spp. Morsy1 under solid-state fermentation using rice straw. Applied Biochemistry and Biotechnology, 162(3):780-794.
Esawy MA (2007). Isolation and partial characterization of extracellular keratinase from a Novel Mesophilic Streptomyces albus. Res J Agricul Biol Sci 3:808- 817.
Fakhfakh N, Ktari N, Siala R and Nasri M (2013). Wool-waste valorization: production of protein hydrolysate with high antioxidative potential by fermentation with a new keratinolytic bacterium, Bacillus pumilus A 1. Journal of Applied Microbiology, 115(2):424-433.
Fang Z, Zhang J, Liu B, Du G and Chen J (2013). Biodegradation of wool waste and keratinase production in scale-up fermenter with different strategies by Stenotrophomonas maltophilia BBE11-1. Bioresource Technology, 140:286-291.
Gegeckas A, Šimkutë A, Gudiukaitë R, and Èitavièius D J (2018). Characterisation and application of keratinolytic paptidases from Bacillus spp. Int. J. Biol. Macromol., 113, 1206–1213.
Gindaba GT, Filate SG, Etana BB (2019). Extraction and characterization of natural protein (keratin) from waste chicken feather. Int J Mod Sci Technol., 4 (7):174-179.
Gupta R and Ramnani P (2006). Microbial keratinases and their prospective applications: an overview. Applied Microbiology and Biotechnology, 70:21-33.
Jeong EJ, Rhee MS, Kim GP, Lim KH, Yi D H et al. (2010). Purification and characterization of a keratinase from a feather-degrading bacterium, Bacillus sp. SH-517. Journal of the Korean Society for Applied Biological Chemistry, 53(1):43-49.
Jingwen Q, Wilkens C, Barrett K, Meyer A S (2020). Microbial enzymes catalyzing keratin degradation: Classification, structure, function, Biotechnology Advances, Volume 44, 107607.
Kelly R (2016). Keratins in wound healing, Wound healing biomaterials, Woodhead Publishing, Pages 353-365.
Khalel A, Alshehri W and Aly M (2020). Enhancing plant growth by chicken feather compost obtained from feather degradation by Streptomyces enissocaesilis. Biosc. Biotec. Res.; 13(4)1847-1853.
Koentjoro M P, Prasetyo EN (2021). Advances in Use of Keratinase from Feather Wastes for Feedstock Modification.Appl Food Biotechnol., 8(1):19-30.
Lateef A, Adelere IA and Gueguim-Kana EB (2015). Bacillus safensis LAU 13: a new novel source of keratinase and its multi-functional biocatalytic applications. Biotechnology and Biotechnological Equipment, 29(1):54-63.
Lateef A, Oloke JK, Gueguim-Kana E B, Sobowale BO, Ajao SO et al. (2010). Keratinolytic activities of a new feather degrading isolate of Bacillus cereus LAU 08 isolated from Nigerian soil. International Biodeterioration and Biodegradation, 64:162-165.
Morris AL, MacArthur MW, Hutchinson EG and Thornton JM (1992). Stereochemical quality of protein structures. Proteins 12:345-364.
Nnolim NE, Udenigwe CC, Okoh AI and Nwodo UU (2020). Microbial Keratinase: Next Generation Green Catalyst and Prospective Applications. Front. Microbiol., 11:580164.
Onifade, A.A., Al-Sane, N.A., Al-Musallam, A.A. and Al-Zarban, S. (1998). A review: Potentials for biotechnological applications of keratin-degrading microorganisms and their enzymes for nutritional improvement of feathers and other keratins as livestock feed resources. Bioresource Technology, 66:1-11.
Osman Y, Elsayed A, Mowafy AM, Abdelrazak A, Fawzy M (2017) Bioprocess enhancement of feather degradation using alkaliphilic microbial mixture. Bri Poultry Sci., 58 (3): 319-328.
Parry DAT and North ACT (1998). Hard α-keratin intermediate filament chains: substructure of the N and C-terminal domains and the predicted structure and function of the C-terminal domains of type I and type II chains. J StructBiol 122:67-75.
Paul T, Das A, Mandal A, Jana A, Maity C et al. (2013). Effective dehairing properties of keratinase from Paenibacillus woosongensis TKB2 obtained under solid state fermentation. Waste Biomass Valorization, 5:97-107.
Pencho D (1990) An enzyme-alkaline hydrolysis of feather keratin for obtaining a protein concentrate for fodder. Biotechnol Lett., 12:71-72.
Prakash P, Jayalakshmi SK and Sreeramulu K (2010). Production of keratinase by free and immobilized cells of Bacillus halodurans strain PPKS-2: Partial characterization and its application in feather degradation and dehairing of the goat skin. Applied Biochemistry and Biotechnology, 160 (7): 1909-1920.
Savitha GJ, Tejashwini MM, Revati N, Sridevi R and Roma D (2007). Isolation, identification and characterization of a feather degrading bacterium. Int J Poul Sci., 6:689-693.
Schmidt WF and Barone JR (2004). New uses for chicken feathers keratin fiber. Poultry Waste Management Symposium Proceedings pp:99-101.
Srivastava B, Khatri M, Singh G, and Arya S K (2020). Microbial keratinases: An overview of biochemical characterization and its eco-friendly approach for industrial applications. J. Clean. Prod. 252:119847.
Syed DG, Lee JC, Li WJ, Kim CJ and Agasar D (2009). Production, characterization and application of keratinase from Streptomyces gulbargensis. Bioresource Technology, 100(5):1868-1871.
Tamilmani P, Umamaheswari A, Vinayagam A and Prakash B (2008). Production of an extra cellular feather degrading enzyme by Bacillus licheniformis isolated from poultry farm soil in Namakkal District (Tamilnadu), Int J Poul Sci., 7:184-188.
Tesfaye T, Sithole B, Ramjugernath D, and Chunilall V (2017). Valorisation of chicken feathers: Characterisation of physical properties and morphological structure. J. Clean Prod., 149: 349–365.
Tork S E, Shahein YE., El-Hakim A E, Abdel-Aty AM, Aly M M (2016). Purification and partial characterization of serine-metallo keratinase from a newly isolated Bacillus pumilus NRC21. International Journal of Biological Macromolecules, Volume 86:189-196.
Verma AK, Kakani RK, Solanki RK and Meena RD (2017). Improvement in yield attributing traits of cumin (Cumin umcyminum) through acute exposure of gamma ray” Int. J. Pure & Appl. Biosci., 22; 1223-1250.
Volik V, Ismailova D, Lukashenko V, Saleeva I, Morozov V. (2020). Biologically active feed additive development based on keratin and collagen containing raw materials from poultry waste. Int Trans J Eng Manag Appl Sci Technol.; 11 (5): 1-10.
Wang D, Piao XS, Zeng Z K, Lu T, Zhang Q et al. (2011). Effects of keratinase on performance, nutrient utilization, intestinal morphology, intestinal ecology and inflammatory response of weaned piglets fed diets with different levels of crude protein. Asian-Australian Journal of Animal Sciences, 24(12):1718-1728.
Weidele T (2009). Method for using biomass in biogas process, US patent online, Pub. No. US 2009/0035834 A1.
Williams C M, Lee C G, Garlich J D, and Shih J C (1991). Evaluation of a bacterial feather fermentation product, feather-lysate, as a feed protein. Poult. Sci., 70, 85–94.
Yeo I, Lee YJ, Song K, Jin HS, Lee JE, Kim D, Lee DW, Kang NJ (2018). Low-molecular weight keratins with anti-skin aging activity produced by anaerobic digestion of poultry feathers with Fervidobacterium islandicum AW-1. J. Biotechnol.; 10 (217): 17-25.
Zhao H, Mitsuiki S, Takasugi M, Sakai M, Goto M et. al (2012). Decomposition of insoluble and hard-to-degrade animal proteins by enzyme E77 and its potential application. Applied Biochemistry and Biotechnology, 166 (7):1758-1768.


