Department of Botany, Bio-informatics, Climate Change Impacts Management,
University School of Science, Gujarat University, Ahmedabad, Gujarat, India.
Corresponding author email: dhruvpandya1309@gmail.com
Article Publishing History
Received: 09/07/2020
Accepted After Revision: 22/09/2020
Pollutants are increasing day by day in the environment. Mitigation of pollutants from the environment is really very difficult task and specially when we are focusing on the soil pollutants, heavy metals are major soil pollutants. Phytoremediation is only the approach by which we can remove the heavy metal from the soil. For that first identification of metal tolerant species is pioneer phase. In this research Heavy Metal stress tolerance capacity of Polyscias fruticosa (L.) Harm. was assessed. Here, two different approaches In-vitro and In-vivo were used for the production of plantlets. In-vitro approach involved tissue culture approach and In-vivo direct through media (soil, cocopeat, mosses). Shoot apexes were used for the production of plantlets. After 30 days of seedlings development all the plantlets which are produced through In-vitro and In-vivo approaches and plants were transplanted in the pots and treated with two metals Lead and Cadmium in the form of Pb (NO3)2 and Cd (NO3)2. Different concentrations were selected for Lead 200mg, 400mg, 600mg, 800mg/Kg and for Cadmium 5mg, 10mg, 15mg, and 20mg/Kg. Each pot was filled with 5Kg of soil. The metals were given directly through root zone of plants in solution form. After incubation time of 75 days mature and treated plants were collected and root length, shoot length, number of branches were measured scientifically. On the basis of the results obtained of physiological parameters of the plants we concluded that for both the metals In-vitro produced plants has more capacity to tolerate the metal stress as compare to In-vivo produced plants.
Micropropagation, Plant Production, Stress Tolerance capacity, Polyscias fruticosa (L.) Harm, Physiological Parameters
Pandya D, Mankad A, Pandya H. In-Vitro Plant Production Approach to Increase Heavy Metal Stress Tolerance Capacity of Polyscias Fruticosa. Biosc.Biotech.Res.Comm. 2020;13(3).
Pandya D, Mankad A, Pandya H. In-Vitro Plant Production Approach to Increase Heavy Metal Stress Tolerance Capacity of Polyscias Fruticosa. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/3325lgD
Copyright © Pandya et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Environmental factors can be of abiotic and biotic nature. Biotic environmental factors, resulting from interactions with other organisms, are, for example, infection or mechanical damage by herbivory or trampling, as well as effects of symbiosis or parasitism. Abiotic environmental factors include temperature, humidity, light intensity, the supply of water and minerals, and heavy metals these are the parameters and resources that determine the growth of a plant. Heavy metals are the major soil pollutants that are emitted from different industries like battery, chemical or steel (Ashwini, Khare and Ganguly 2014). Some plants can survive at high stressful conditions which can be identified and should be grown at high stressful conditions. Some of the stress tolerant plants also has the remediation capacity so the phyto accumulation of the pollutant from the environment can be identified. There are two ways to produce the plants. In-vitro production and In-vivo production. In In-vitro production plants has to be produced under controlled environmental conditions like in Green house or in culture room etc. Stress tolerant capacity of all the plants are different. In-vitro produced plantlets are healthier and stress tolerant after hardening process (Kozminska et al. 2018).
Polyscias fruticosa (L.) Harm is plant which belonging to Araliaceae family, also known as Ming Aralia. It is dicot shrub native to India. This is shade loving and planted for its foliage purposes. It has compound leaves with seven or more than seven leaflets. Generally, the leaves are deeply lobbed and opposite arrangement is observed. The growth of the plant is seen highest from 19-29ºC temperature. Its sensitive plant for any type of stress specially it cannot survive at high temperature. It bears rare flowers and mostly used as an ornamental foliage plant. It is not directly edible by any animals or humans. The leaves have so many important phytochemical constituents and that can be utilized for drug designing. Yang et al. (2009) discovered remediation capacity of Octane through Polyscias fruticosa (L.) Harm. Stanley in 2011 described and reviewed indoor phytoremediant plant species and Polyscias was one of the plants that he reviewed. So, here in this research heavy metal stress was provided to the plant Polyscias fruticosa (L.) Harm (Yang et al. 2009).
Figure 1: Showing In-vitro production of aralia
Figure 2: Showing In-vivo production of Aralia
Figure 3: Showing Hardening of the plantlets
MATERIAL AND METHODS
The shoot apexes (tips) of Polyscias fruticosa (L.) Harm were selected for the propagation of plants through In-vitro and In-vivo approaches. The Experimental work was completed at Plant Biotechnology Laboratory and Botanical Garden of Gujarat University.
Figure 4: Showing ready plants for transplantation
Figure 5: Showing Treatment to the plants in pots
Sources of Explant: Shoot tips were collected with sterilized scalpel from Botanical Garden of Gujarat University. So, shoot tips were used as an explant for the production of plantlets. All the shoot tips were sterilized with the help of 0.1%HgCl2 solution and 70% methanol and rewashed with Grade-1 Distil water (Kanwar, Yu and Zhou 2018).
Figure 6: Showing Plants after metal treatment
Figure 7: Showing lead treated In-vitro plants
Aseptic Conditions for Production: Culture room and the laboratory or transfer room were sterilized through Fumigation technique (Potassium iodide and Formaldehyde were used for it with 2:4 ratio). All the glassware and miscellaneous agents were washed with soap solution and rapped with papers and then sterilized through Autoclave (121ºC for 20 min). Laminar Air flow hood, weighing scale and all the other small equipment like micropipette were sterilized with 0.1% mercuric chloride solution and 70% methanol.
Figure 8: Showing Lead treated In-vivo plants
Figure 9: Showing Cadmium treated In-vitro plants
Figure 10: Showing Cadmium treated In-vivo plants
Preparation of M. S. Media for the production of plantlets: Here for the practical work most widely used media Murashige and Skoog’s media (1962) was used. For the preparation first all the Major, Minor, Iron and Vitamin stalk solutions were prepared as per the Table-1. PGRs were not used because in seeds generally we use to avoid PGRs in In-vitro condition and production of plantlets. Here all the chemicals used for the preparation of stalk solution were Hi Media and SRL company (Ijaz et al. 2016). Different stalk solutions were prepared in the amount of 500ml (Major, Minor and Iorn) and 100ml (Vitamin) and then for the preparation of 1 litre M. S. Media 50ml from Major, 50ml from Minor, 50ml from Iron and 10ml from Vitamin stalk were taken and sequentially dissolved and other chemicals which were separately weighed like Myo Inositol, Agar-Agar, Glycine and Sucrose were added for the preparation of media.
(Here Grade-1 Purified water was used for the preparation of media with the help of Genie Direct Pure (Rephile) Instrument was used for the preparation of Purified water). After the preparation of media, it was sterilized with the help of autoclave at 121ºC temperature for 20 minutes. After Autoclave sterilization the kinetin 0.5mg was added in the media and then under the Laminar Air Flow Hood in all the sterilized culture flasks and Glass jars media was poured about 50ml in each vessel. All the vessels with media were transferred in Culture room where 25±1ºC temperature and sterilized conditions were maintained. After 24 hrs media was ready for the Inoculation process (Yang et al. 2009).
Inoculation of Explant: All the sterilized seeds were inoculated separately in the jars or culture flasks under the sterilized conditions of Laminar Air flow hood. Different small equipment was used like forceps and scalpels for the inoculation process. After the inoculation of the seeds in the media all the jars and flasks were again transferred carefully at Culture room where 25±1ºC temperature and 16hrs light and 8hrs darkness was maintained. Incubation time was of 40 days.
Table 1. Showing the Composition and Components of M. S. Media (1962) preparation
In-vivo production of Plantlets: By same way sterilized seeds were directly sawed in the media (soil, cocopeat and mosses) in separate pots and regular irrigation process was maintained and up to 40 days the plantlets were produced. The production was carried out at Botanical Garden, Gujarat University.
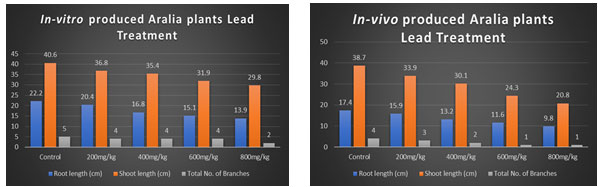
Table 1. Showing Effect of Lead on the In-vitro produced Polyscias fruticosa (L.) Harm
| Treatment Series | Root length (cm) | Shoot length (cm) | Total No. of Branches |
| Control | 22.2 | 40.6 | 5 |
| 200mg/kg | 20.4 | 36.8 | 4 |
| 400mg/kg | 16.8 | 35.4 | 4 |
| 600mg/kg | 15.1 | 31.9 | 4 |
| 800mg/kg | 13.9 | 29.8 | 2 |
Table 2. Showing Effect of Lead on In-vivo produced Polyscias fruticosa (L.) Harm
| Treatment Series | Root length (cm) | Shoot length (cm) | Total No. of Branches |
| Control | 17.4 | 38.7 | 4 |
| 200mg/kg | 15.9 | 33.9 | 3 |
| 400mg/kg | 13.2 | 30.1 | 2 |
| 600mg/kg | 11.6 | 24.3 | 1 |
| 800mg/kg | 9.8 | 20.8 | 1 |
Now same conditions were provided to all the In-vitro and In-vivo produced plantlets. 40 day’s all the plantlets were transferred for the hardening process in the net house of Botanical Garden, Gujarat University where 60% moisture was maintained. Here same media soil, cocopeat were applied for all the In-vitro and In-vivo produced platelets. After 40 days in the Net house all the mature plants with 8-12 compound leaves, they were transplanted in different pots separately with 5kg of soil in each pot. In-vitro and In-vivo produced plants were segregated and potted individually in triplicate sets.
Table 3. Showing Effect of Cadmium on In-vitro produced Polyscias fruticosa (L.) Harm
| Treatment Series | Root length (cm) | Shoot length (cm) | Total No. of Branches |
| Control | 18.9 | 42.6 | 6 |
| 5mg/kg | 15.3 | 40.2 | 3 |
| 10mg/kg | 14.9 | 33.5 | 3 |
| 15mg/kg | 13 | 32 | 2 |
| 20mg/kg | 12.3 | 29 | 2 |
Treatment of Heavy Metal to the plants: Lead and Cadmium metals were used for the treatment in the form of Lead nitrate and Cadmium nitrate. For the treatment lead the concentrations were selected 200mg/kg, 400mg/kg, 600mg/kg, 800mg/kg of soil. And for cadmium the concentrations were selected 5mg/kg, 10mg/kg, 15mg/kg, 20mg/kg of soil. One set was kept as control both the series and both the approaches. Lead nitrate and Cadmium nitrate solution series were prepared and the treatment was provided to individual directly through rootzone via digging the soil near by the roots.
Table 4. Showing Effect of Cadmium on In-vivo produced Polyscias fruticosa (L.) Harm
| Treatment Series | Root length (cm) | Shoot length (cm) | Total No. of Branches |
| Control | 18.8 | 36.9 | 4 |
| 5mg/kg | 15.9 | 32.9 | 3 |
| 10mg/kg | 13.7 | 30.8 | 3 |
| 15mg/kg | 11.8 | 29 | 2 |
| 20mg/kg | 9.1 | 28.4 | 2 |
Incubation time of the plants: After the treatment to all the In-vitro and In-vivo plantlets all the plants are placed at Botanical Garden for 75 days incubation period. Regular irrigation was done to all the plantlets.
RESULTS AND DISCUSSION
After 75 days the plants were taken out. Different parameters like root length, shoot length and total no. of branches were measured and counted. As the table data and graphical representation shows that as the metal concentration increases the root length, shoot length and no. of branches were decreased. For In-vitro plants lead 200mg concentration in the soil plants growth parameters showed 20.4cm root length, 36.8cm shoot length and 4 number of branches but as the concentration increases 800mg concentration in the soil showed that decreased plant growth included 13.9cm root length, 29.8cm shoot length and 2 number of branches. For In-vivo plants lead 200mg concentration in the soil showed 15.9cm root length, 33.9cm shoot length, 3 number of branches and 800mg concentration of lead showed decreased physiological parameters of the plant included 9.8 root length, 20.8 shoot length and 1 branch.
For Cadmium In-vitro produced plants at 5mg concentration in the soil showed 15.3cm root length, 40.2cm shoot length, 3 number of branches and highest concentration 20mg in the soil showed that decreased physiological data included 12.3cm root length, 32.0 cm shoot length and 2 number of branches. For In-vivo plants cadmium 5mg concentration in the soil showed at 15.9cm root length, 32.9cm shoot length, 3 number of branches and highest concentration 20mg in the soil showed 9.1cm root length, 28.4cm shoot length and 2 number of branches. Even the cadmium effect on the plant growth was more than the lead because at lower concentration also it showed the effect on the growth parameters of the Polyscias fruticosa (L.) Harm (Kays 2011; Waoo, Khare and Ganguly 2014).
Graph-1 Showing Effect of Lead on the In-vitro produced Polyscias fruticosa (L.) Harm
Graph-2 Showing Effect of Lead on the In-vivo produced Polyscias fruticosa (L.) Harm
Graph-3 Effect of Cadmium on the In-vitro produced Polyscias fruticosa (L.) Harm
Graph-4 Effect of Cadmium on the In-vivo produced Polyscias fruticosa (L.) Harm
In the comparison of In-vitro and In-vivo produced plants In-vitro produced plants has more capacity to tolerate metal stress because the growth of these plants was observed higher than the In-vivo produced plants. As the table shows that as compare to control the metal stressed plant growth rate of the plants (root length, shoot length, no. of branches) were lower for both the metals lead and cadmium (Mojiri et al. 2013). Thach et al. (2016) worked on the propagation of the plant as a medical plant because of the volatile compounds found in the leaves of Polyscias fruticosa (L) Harm. Boye et al (2018) discovered the effect of the extract of the plant in male rat.
Koffur et al. (2014) discovered the anti-inflammatory effect of the plant. Salva S. Sakar et al. (2014) worked on In-vitro production of Polyscias fruticosa (L) Harm. In this research work heavy metal stress was provided to the different method (In-vitro and In-vivo) produced plants. So many researchers worked on the phytochemicals and different activities of Polyscias fruticosa (L.) Harm there was no any record or the review of articles which showed heavy metal or stress tolerance activity or its effects on the growth parameters of the plant. In future the proteins or the phytochemicals can be identified which are responsible to increase the metal stress activity of the plants. For this study in future phytoremediation study can be assessed of the plants and with the help of In-silico analysis the binding capacity of metal and plant proteins can be analysed and protein molecules can be identified where the metal binds strongly (Hussain et al. 2018).
CONCLUSION
Polyscias fruticosa (L.) Harm is highly metal stress tolerant plant. The plant can survive at high lead and cadmium stress. In-vitro produced plants has more capacity to tolerate metal stress compare to In-vivo produced plants. Cadmium effect on the plants was higher as compare to lead metal stress. The research is also coming up with new application of plant tissue culture too increase metal stress tolerance capacity. After the treatment the plant material can be used for the production of Biochar which can be used in different industries like tier industries, varnish industries. After the identification of proteins which provided stress tolerant activity to the plants, the genes which are responsible for the production of that proteins can be identified and with the help of genetically modified technology it can be inserted in another plants and stress tolerant plants can be produced. So, it can be applicable to molecular genetics level and to improve the crop stress tolerant capacity.
REFERENCES
Ashwini A., Khare Swati, and Ganguly Sujata (2014). Comparative tissue culture on Lantana camera and Datura innoxa at heavy metal contaminated site and phytoremediation approach at industrially contaminated sites. International Journal of Advances in Biology, 1 (1); 55-62.
Bui Dinh Thach, Le Nguyen Tu Linh, Diep Trung Cang, Trinh Thi Ben, Tran Thi Linh Giang, Nguyen Pham Ai Uyen and Ngo Ke Suong (2016). Protoplast establishment for the multiplication and regeneration of Polyscias fruticosa (L) Harm. As medicinal plant. European Journal of Biotechnology and Genetic Engineering, 3(1); 31-37.
Hussain, Z., Arslan, M., Malik, M. H., Mohsin, M., Iqbal, S., and Afzal, M. (2018). Integrated perspectives on the use of bacterial endophytes in horizontal flow constructed wetlands for the treatment of liquid textile effluent: phytoremediation advances in the field. Journal of environmental management, 224, 387-395.
Ijaz, A., Imran, A., ul Haq, M. A., Khan, Q. M., and Afzal, M. (2016). Phytoremediation: recent advances in plant-endophytic synergistic interactions. Plant and Soil, 405(1-2), 179-195.
Kanwar, M. K., Yu, J., and Zhou, J. (2018). Phytomelatonin: recent advances and future prospects. Journal of pineal research, 65(4), e12526.
Kays, S. J. (2011). Phytoremediation of indoor air—Current state of the art. The value creation of plants for future urban agriculture. Nat. Inst. Hort. Herbal Science, RDA, Suwon, Korea, 3-21.
Koźmińska, A., Wiszniewska, A., Hanus-Fajerska, E., and Muszyńska, E. (2018). Recent strategies of increasing metal tolerance and phytoremediation potential using genetic transformation of plants. Plant biotechnology reports, 12(1), 1-14.
Mojiri Amin, Aziz Abdul Hamidi, Aziz Quarani Shuokr, Razip M., and Selmat B. (2013). Phytoremediation of Heavy metals from Urban Waste Leachate by Typha domengenis.
Waoo, A. A., Khare, S., and Ganguly, S. (2014). Comparative In-vitro studies on native plant species at heavy metal polluted soil having phytoremediation potential. International Journal of Scientific Research in Environmental Sciences, 2(2), 49.
Yang, D. S., Pennisi, S. V., Son, K. C., and Kays, S. J. (2009). Screening indoor plants for volatile organic pollutant removal efficiency. HortScience, 44(5), 1377-1381.