1Department of Botany, University College, Thiruvananthapuram, Kerala, India
2Biotechnology and Bioinformatics Division, Jawaharlal Nehru Tropical
Botanic Garden and Research Institute, Thiruvananthapuram, Kerala, India
3Phytochemistry Division, Jawaharlal Nehru Tropical Botanic Garden and Research
Institute, Thiruvananthapuram, Kerala, India
4Department of Genomic Science, Central University of Kerala, Kasaragod, Kerala, India
corresponding author email: preethahemanth@yahoo.com
Article Publishing History
Received: 15/09/2021
Accepted After Revision: 18/12/2021
Kaempferia galanga L. or ‘aromatic ginger’ is a stem-less herb in Zingiberaceae having different pharmacological properties like antioxidant, antimicrobial, nemeticidal, vasorelaxant and wound healing activity. The plant is generally a vegetatively propagated annual herb; its conservation using conventional methods takes more time to get sufficient amount of planting materials for commercial cultivation. Micropropagation by in vitro methods helps to overcome the present demand for this high sought medicinal and aromatic species. At present the concern on in vitro propagation is directed to rhizome or storage organ induction for productive acclimatization and to reduce the injury during transportation. Microrhizomes are the small rhizomes developed in in vitro conditions and its induction is an effective biotechnological tool for the production of quality planting materials as they are genetically stable and disease free. The present study is discussing the role of silver nitrate (AgNO3) along with sucrose in in vitro microrhizome induction in K. galanga for the first time.
MS medium fortified with 2.0 mgl-1 AgNO3 along with 6% (w/v) sucrose produced maximum amount of microrhizomes i.e., 4.52±0.11 g after 3 months that increased to 5.70±0.20 g in six months of harvesting. Here we also reports the comparative analysis of chemical constituents in the essential oil of in vivo rhizomes and in vitro microrhizome through GC-MS analysis that further reveals the superior characteristics of the microrhizomes in terms of the bioactive components ethyl p-methoxy cinnamate and ethyl cinnamate, the esters that contribute the nematicidal, antituberculosis, anti-inflammatory, antifungal and larvicidal properties to the oil. This protocol for in vitro microrhizome induction can be used for the commercial production of rhizomes and essential oil in K. galanga and the outcome of this study can be further used for mass production of pathogen-free microrhizomes and conservation for its sustainable utilization of the species.
AgNo3, Essential Oil, Kaempferia galanga, Microrhizome, Sucrose
Vidya V. R, kumar A. S. H, kumar C. B. R, Pillai P, Preetha T. S. In vitro Microrhizome Induction and Essential Oil Production in Aromatic Ginger Kaempferia galanga : An Economically Important Medicinal Herb. Biosc.Biotech.Res.Comm. 2021;14(4).
Vidya V.R, Kumar A.S.H, kumar C.B.R, Pillai P, Preetha T.S. In vitro Microrhizome Induction and Essential Oil Production in Aromatic Ginger Kaempferia galanga : An Economically Important Medicinal Herb. Biosc.Biotech.Res.Comm. 2021;14(4). Available from: <a href=”https://bit.ly/30k7Gpw“>https://bit.ly/30k7Gpw</a>
Copyright © Vidya et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Kaempferia galanga L.is an endangered medicinal plant of Zingiberaceae that is extinct in the wild, but available under cultivation mainly in South East Asia and China for its aromatic rhizome. The plant is economically important as the main ingredient of several ayurvedic preparations such as Dasamularishta, Valiya rasnadi kasaya, Kaccoradi churna, Asanaeladi taila, Valya narayana taila and is used for the healing of rheumatism (Rastogi and Mehrotra 1993; Sivarajan and Balachandran 1994; Kareem 1997). The rhizome extract contains n-pentadecane, ethyl p-methoxy cinnamate, ethyl cinnamate, camphene, borneol, cineol, p-methoxy styrene, kaempferol and kaempferide (Tewtrakul 2005).
The rhizome has stimulatory, expectorant, carminative and diuretic medicinal properties generally. It possesses a camphoraceous odour and the decoction prepared from the rhizome is used for dyspepsia, headache and malaria. K. galanga essential oil (galangal oil) is having high market value (Rs 2000/- for 10 ml, Silky scents L.L.C) (Alsalhi et al. 2020).
Essential oil compounds were analyzed earlier in its rhizome by many workers and the plant contains 2.4 to 3.9% volatile oil (Rao et al. 2009; Raina et al. 2015; Sunitha et al. 2018; Alsalhi et al. 2020). Ethyl p-methoxy cinnamate present was found to exhibit anticancer activity which amount to 30.6% and its vassorelaxation effect was also reported (Srivastava et al. 2019). The species is conventionally propagated by rhizomes and there is no seed setting by natural means. In such circumstances plant tissue culture based micropropagation is the most efficient propagation method for quality planting material production.
However, in the rhizomatous species, the micro clones raised through in vitro cloning are not suitable for direct commercial planting, as they need at least two generations in green house to form sufficient quantity of minirhizome for planting in the field. In vitro microrhizome production can overcome this hurdle and can be exploited for the utilization of commercial product of interest thereby overcoming the delay in the utilization of micropropagated plantlets (Alsalhi et al. 2020; Srivastava et al. 2021).
The chief advantage of these microrhizomes is that they are disease free can be directly transferred to the field without any acclimatization procedure and can be transported internationally as they do not require any special measures as well as quarantine (Regunath and Shameena 2013). Induction of vegetative storage organs under in vitro conditions their application in planting material production, in vitro conservation and germplasm exchange has been reported in many Zingiberaceous species (Raina et al. 2014; Swarnathilalaka et al. 2016; Nguyen et al. 2020).
Microrhizome production can be done in any seasons in sterile conditions and they have advantages of easily storage, transport and can be used in germplasm conservation. Taking into consideration of these merits, the present study was undertaken with an effort to establish an efficient system for microrhizome induction in K. galanga (Mohamed et al. 2014; Zahid et al. 2021).
Here an attempt has been made to develop a convenient and effective method for high frequency in vitro micro rhizome induction in using different concentrations of sucrose and AgNO3 supplementation to the medium followed by comparison of chemical constituents in the essential oil of in vivo rhizomes and in vitro microrhizomes as there exist some lacuna regarding this aspect in the targeted species. The system established will be highly beneficial for mass production of quality planting materials and its economic utilization as well as germplasm exchange thereby providing another means of conservation in this species.
MATERIAL AND METHODS
Rhizomes of K. galanga L. collected from Kundara, Kollam District, Kerala, India (JNTBGRI Herbarium Voucher number TBG 60677) were used as the plant material for the in vitro shoot culture establishment in the present study. Fresh rhizomes with axillary buds collected from the field-grown plants were thoroughly washed under running tap water, outer scales were removed and washed in 5% Teepol (v/v) for 20 minutes, again washed in running tap water and treated with 0.2% bavistin (fungicide) for 10 minutes.
After several rinses in distilled water, they were subjected to sterilization with 0.1% (w/v) HgCl2 for 8-10 minutes followed by 4-5 rinses in sterile distilled water, then inoculated aseptically in MS (Murashige and Skoog) medium (Murashige and Skoog 1962) containing 0.5 mgl-1 BA. The initiated shoots were further subcultured to fresh MS medium augmented with 3.0 mgl-1 BA and 0.5 mgl-1 NAA for stimulating the shoot multiplication (Preetha et al. 2012). For microrhizome induction, in vitro shoots were trimmed to 1-1.5 cm length bearing the rhizomatous base and then inoculated to different media treatments.
To study the effect of sucrose on in vitro microrhizome induction, varying concentrations of sucrose (3, 6, 9 and 12%) along with 3.0 mgl-1 BA and 0.5 mgl-1 NAA was checked. To examine the effect of different concentration of AgNO3 and sucrose in in vitro microrhizome induction, the in vitro shoots established in 0.3 mgl-1BA and 0.5 mgl-1 NAA were subcultured to the fresh medium of the same PGR composition augmented with different concentration of silver nitrate (1.0 mgl-1 and 2.0 mgl-1) and 3, 6 and 9% sucrose (w/v).
Here two different concentrations of AgNO3 and three different concentrations of sucrose were used. The different treatments were 1T1 (1.0 mgl-1AgNO3+3% sucrose), 1T2 (1.0 mgl-1AgNO3+6% sucrose), 1T3 (1.0 mgl-1AgNO3+9% sucrose), 2T1 (2.0 mgl-1AgNO3+3%sucrose), 2T2 (2.0 mgl-1AgNO3+6% sucrose), 2T3 (2.0 mgl-1AgNO3+9% sucrose) respectively. The response of the cultures were periodically noted for up to six months. Bulging of basal portion of the stem was taken as the indication of microrhizome induction.
Experiments were carried out in triplicates with at least ten explants per treatment. The results were taken after 3 and 6 months respectively. Morphogenic response of plants regarding the number of shoots, length of shoots, length of shoots, number of leaves per shoots during these periods and the amount of microrhizomes produced were recorded and statistically analysed by one way analysis of variance (ANOVA) and the means were compared by Duncan’s multiple range test p ≤ 0.05 using the computer software SPSS/ PC + version 4.0 (SPSS Inc., Chicago, USA).
Twenty five grams each of microrhizomes and in vivo rhizomes from the field were subjected to hydro-distillation using a modified Clevenger-type glass apparatus for 4 h. The oil samples were separated by using di-ethyl ether and anhydrous sodium thio sulphate. The GC-MS analysis was done on a Hewlett Packard 6890 gas chromatograph fitted with a cross-linked 5% phenyl methyl siloxane HP-5MS capillary column (30m x 0.32mm, film thickness 0.25 mm) coupled with a 5973 series selective mass detector. 1.0 ml of the essential oil was injected. Helium was used as the carrier gas at 1.4 ml/min.
Constant flow mode, with injector temperature 220 °C and oven temperature 60 °C to 246 °C (3 °C/min). Mass spectra at electron impact (EI+) mode were taken at 70 Ev. The oil constituents were identified by MS library search (WILEY 275), comparison of the relative retention indices were calculated with respect to homologous of n-alkanes (C6-C30, Aldrich Chem.Co.Inc) and by comparison of mass spectrum reported in the literature (Dool and Kratz 1963; Adams 2007).
RESULTS AND DISCUSSION
Effect of sucrose on Morphogenic response of shoot culture: In the present experiment the morphogenic response of K. galanga in different concentration of sucrose (3, 6, 9 and 12%) (w/v) were recorded after 3 months as well as 6 months of inoculation. The mean number of shoot was 4.33±0.31 (w/v) in 3% sucrose (control) which increased to 9.33±0.33 with the increase of concentration of sucrose to 9% (w/v). There after it decreased 6.67±0.30 in 12% (w/v) sucrose. The results agree with the findings in in Z. officinale, where maximum number of shoots were noticed in MS medium supplemented with 8% (w/v) sucrose while in Kaempferia parviflora MS medium fortified with 6 % (w/v) sucrose produced maximum number (8.5) of shoots (Mehaboob et al. 2019; Labrooy et al. 2020).
Here, in K. galanga, maximum shoot length (4.51±0.11 cm) was observed in 6% (w/v) sucrose level. Shoot length reduced considerably as the concentration of sucrose increased further. Very short shoots (1.67±0.07) were noticed in MS medium with 12% (w/v) sucrose (Table 1). Regarding the number of leaves per shoots, maximum number of leaves were recorded in 6% (w/v) sucrose and this parameter remained almost same in the rest of the treatments. Maximum leaf area (15.17±0.17) was observed in the highest sucrose concentration tested, i.e., 12 % (w/v) and the leaves were not much expanded in 6 and 9 % (w/v) sucrose levels. However comparatively large leaves were seen in the control i.e., 3% (w/v) sucrose. Similar trend of morphogenic response was exhibited in six months old cultures also (Fig. 1a).
Table 1. Morphogenic Response of K. galanga in Sucrose Treatments
| Sucrose
(%) |
Number of Shoots | Length of Shoots (cm) | Leaf Number Per Shoot | Leaf Area (cm2) | ||||
| 3 months | 6 months | 3 months | 6 months | 3 months | 6 months | 3 months | 6 months | |
| 3 | 4.30±0.3c | 5.67±0.32d | 2.80±0.09c | 3.59±0.07b | 3.40±0.25b | 4.83±0.21b | 11.75±0.25b | 12.00±0.57b |
| 6 | 6.30±0.32b | 7.33±0.30c | 4.51±0.11a | 4.52±0.08a | 4.00±0.41a | 5.37±0.37a | 8.00 ± 0.20d | 8.50 ± 0.12d |
| 9 | 9.33±0.33a | 11.33±0.33a | 3.53±0.06b | 3.67±0.11b | 3.70±0.21b | 3.92±0.22c | 8.67 ± 0.33c | 9.33 ± 0.66c |
| 12 | 6.67±0.30b | 8.33±0.34b | 1.67±0.07d | 2.54±0.08c | 2.67±0.21c | 2.67±0.16d | 15.17±0.17a | 16.00±0.50a |
*Data represents mean values of ten replicates repeated thrice, recorded after 3 and 6 months of culture. Mean values followed by the same letter in the superscript in a column do not differ significantly based on ANOVA and t-test at p ≤ 0.05
Effect of Sucrose on Microrhizome Induction: The present experiment has analysed the effect of different concentration of sucrose in inducing microrhizomes in K. galanga. In some previous studies on Zingibers, certain specific concentration of sucrose was effective in microrhizome induction such as 6-8% (w/v) sucrose was better for Curcuma longa, while 6% (w/v) sucrose was most effective in Curcuma aromatica and Curcuma zedoaria (Shirgurkar et al. 2001; Nayak 2000; Anisuzzaman et al. 2008; Zahid et al. 2021).
In K. galanga, more amount of rhizomes were observed in 6 and 9% (w/v) sucrose that produced 2.17±0.05 g and 2.93±0.08 g microrhizomes after three months (Table 2). However, the amount of microrhizomes increased predominantly in 6 months old cultures and maximum quantity was observed in 9% (w/v) sucrose which produced 3.94 g microrhizomes, while in 12% (w/v) sucrose 3.01±0.04 g microrhizomes were recorded (Fig. 1b).
Maximum plant weight was noticed in 9% (w/v) sucrose (Table 2). Similarly in Curcuma longa MS medium supplemented with 60-90g sucrose helped in microrhizome induction while in Curcuma amada maximum response was obtained in MS medium supplemented with 80 gl-1 of sucrose where 76.2% explants formed microrhizomes but the control failed to induce microrhizome (Sanghamitra 2002; Pramila et al. 2011). Also, the role of sucrose in in vitro microrhizome induction in another rhizomatous herb Accorus calamus was reported in previous studies (Devi et al. 2012). Agreeing with this, in Zingiber species maximum micorhizome induction was noticed in 8% (w/v) and 10% (w/v) sucrose and medium containing 6% (w/v) and 12 (w/v) sucrose failed to produce microrhizomes (Tombisana and Singh 2015; Zahid et al. 2021).
A healthy and maximum microrhizome production was reported recently in ginger that was obtained in the MS medium with 0.5 mgl-1 BA, 0.5 mgl-1 IAA and 8% (w/v) sucrose under 8-hour photoperiod (Mehaboob et al. 2019). Our result pattern is concomitant with the findings in Zingiber officinale, where microrhizomes were induced when MS medium was supplemented with 4.5 to 9% (w/v) sucrose. All these findings further substantiates that high concentration of sucrose treatments have a profound effect in microrhizome evoking in K. galanga, when compared to control (3% (w/v) sucrose).
High rate of microrhizome induction with increasing concentration of sucrose may be due to the presence of high carbon energy in sucrose because rhizomes mostly store carbohydrate. Very earlier Bhat et al. (1994) suggested that sucrose might act as an energy source and an osmoticum in inducing rhizome formation. Reports of Chirangini et al. (2005) also supports our findings that rhizomes serve as a sink where assimilates are uploaded and in an in vitro culture system assimilates provided as sucrose may have been transported to the stem for rhizome formation (Bhat et al. 1994; Chirangini et al. 2005; Mehaboob et al. 2019; Zahid et al. 2021).
Table 2. Effect of Sucrose on Microrhizome induction in K. galanga
| Sucrose (%) | Weight of Plant (g) | Weight of Plant Without Leaf (g) | Weight of Microrhizome (g) | |||
| 3 months | 6 months | 3 months | 6 months | 3 months | 6 months | |
| 3 | 5.83±0.08d | 11.45±0.09d | 1.01±0.00c | 2.52±0.06c | 0.57±0.01c | 0.07±0.03d |
| 6 | 15.23±0.11b | 13.34±0.07c | 4.31±0.09b | 3.67±0.02b | 2.17±0.05b | 2.58±0.01c |
| 9 | 18.69±0.45a | 20.71±0.13a | 7.26±0.14a | 10.26±0.05b | 2.93±0.08a | 3.94±0.03b |
| 12 | 11.95±0.06c | 19.48±0.43b | 4.43±0.06b | 10.74±0.10a | 1.93±0.03a | 3.01±0.04a |
*Data represents mean values of ten replicates repeated thrice, recorded after 3 and 6 months of culture. Mean values followed by the same letter in the superscript do not differ significantly based on ANOVA and t-test at p ≤ 0.05
Figure 1: Microrhizome induction in K. galanga after six months in sucrose treatments

Effect of Sucrose and AgNO3 on Morphogenic Response of Shoot Cultures: The second set of experiments of the present study has analyzed the morphogenic responses in terms of different concentration of silver nitrate and sucrose supplementation during in vitro culturing of K. galanga. AgNO3 is a salt of silver and it is commonly used as an anti-ethylene compound in plant tissue culture (Sarropoulo et al. 2016). Significant variation was noticed among different parameters analysed in different period of data collection here also. Among the various treatments, 2T1 evoked the production of maximum number of shoots (9.5±0.29) after 3 months of culturing than the control (4.3±0.31) and these shoots were comparatively elongated also.
In MS medium augmented with 1.0 mgl-1 AgNO3 and varying concentration of sucrose (Treatments 1T1, 1T2, 1T3), the mean number of shoots as well as leaf area exhibited a linear increase with the increase in the concentration of sucrose in the nutrient medium both in 3 months and 6 months of observation (Table 3). A gradual increase in the mean shoot length (4.26±0.20 cm to 5.02±0.24 cm) was noticed as the concentration of sucrose was increased to 6% (w/v) which suddenly dropped to lower values (3.23±0.06) when the sucrose concentration was 9% (w/v) upon 3 months of culture. This trend repeated after 6 months of observation also. Effect of AgNO3 on shoot multiplication was already reported in Sphaeranthus indicus and in Moringa oleifera (Harathi et al. 2016; Drisya et al. 2019).
The mean number of leaves per shoots gradually decreased with regard to the increase in the concentration of sucrose in both periods of data recording and the leaf area exhibited linear increase in the values but were having comparatively lesser area than control (Table 3). While the level of AgNO3 was increased to 2.0 mgl-1with varying concentration of sucrose (Treatments 2T1, 2T2, 2T3), a linear reduction in the mean number of shoots and mean length of shoots was noticed. Though 2T1 executed maximum shoot production i.e., 9.52±0.29 after 3 months and 12.22±0.04 after 6 months, it significantly reduced to 7.6±0.24 and 10.4±0.02 after 3 months and 6 months respectively as the sucrose concentration was elevated to 6% (w/v).
Further increase in the concentration of sucrose to 9% (w/v) i.e., treatment 2T3 produced a significant decrease in the mean number of shoots after 3 months (4.63±0.12 shoots) (Fig. 2a) and as the culture period was extended to 6 months, the shoots become stunted. Similar trend was apparent with the mean length of shoots and mean number of leaves per shoot (Table 3). Statistically significant leaf area was observed in 2T2 and control, while in 2T1, the leaves were less expanded and in 2T3 the shoots were stunted and died in six months, hence insufficient to calculate the leaf area (Table 3) (Drisya et al. 2019).
It has been experimentally proved that AgNO3 reduces ethylene production by inhibiting amino cyclopropane-1 carboxylic acid (ACC), present in ethylene biosynthetic pathway (Kumar et al. 2009). Ethylene hormone attaches to its receptors in the presence of copper ions. It has been proved that silver ions could be substituted by copper ions because of similarity in size and thus blocks the receptors and prevent the response from ethylene (Kumar et al. 2016). In addition to the inhibitory effect of silver ions on ethylene and growth stimulation, nitrate in AgNO3 as the main source of nitrogen and its interference in the structure of amino acids and nucleic acids, is one of the growth factors in plants leading to longitudinal growth of roots and shoots and increased leaf area as observed in K. galanga ((Sun et al. 2017).
At the same time AgNO3 increases the production of polyamines, having a common precursor (S-adenosyl methionine) with ethylene. The metabolism of polyamines is related to the production of NO, which is an essential signaling component for plant growth (Pal et al. 2015; Agurla et al. 2017; Mohd et al. 2018). This substantiates the necessity of an in depth study on the role of polyamines in plant growth and development and the effects on plant signaling substances which would further provide a concrete evidence for the stimulatory effect of AgNO3 on plant growth responses as observed in the present study.
Table 3. Morphogenic response of K. galanga in AgNO3 and sucrose supplementation
| Treatments | Number of Shoots | Length of Shoots (cm) | Leaf Number Per Shoot | Leaf Area (cm2) | ||||
| 3 months | 6 months | 3 months | 6 month | 3 month | 6 month | 3 month | 6 month | |
| 1T1 | 7.61±0.24c | 8.41±0.36c | 4.26±0.20b | 5.00±0.23b | 5.41±0.18a | 6.24±0.17a | 6.55±0.31c | 7.5±0.14c |
| 1T2 | 8.40±0.24b | 9.52±0.34b | 5.02±0.24a | 6.32±0.20a | 4.22±0.29b | 5.81±0.25b | 7.76±0.14b | 8.2±0.31b |
| 1T3 | 9.00±0.36a | 10.83±0.24a | 3.23±0.06c | 4.22±0.06c | 3.75±0.18c | 4.23±0.19c | 8.26±0.14a | 9.6±0.42a |
| 2T1 | 9.52±0.28a | 12.22±0.03a | 6.60±0.27a | 6.95±0.039a | 5.00±0.21a | 6.32±0.67a | 8.36±0.20b | 9.0±0.02b |
| 2T2 | 7.61±0.24b | 10.41±0.01b | 4.75±0.27c | 5.01±0.12b | 4.33±0.28b | 5.35±0.04b | 11.80±0.55a | 12.7±0.43a |
| 2T3 | 4.63±0.12c | Stunted growth | 4.16±0.21c | Stunted growth | 3.42±0.34c | Stunted growth | 7.40±0.21c | Stunted growth |
| Control | 4.31±0.31d | 5.60 ±0.32 | 2.84±0.09d | 3.63±0.07d | 3.41±0.25d | 4.86±0.21d | 11.8 ± 0.25a | 12.0±0.05a |
*Data represents mean values of ten replicates repeated thrice, recorded after 3 and 6 months of culture. Mean values followed by the same letter in the superscript do not differ significantly based on ANOVA and t-test at p ≤ 0.05.
Effect of AgNO3 and Sucrose on Microrhizome Induction: To study the effect of AgNO3 on microrhizome induction in K. galanga MS medium supplemented different concentration sucrose with AgNO3 (1.0 and 2.0 mgl-1) or without AgNO3 were tested. Results were taken after three months and six months as in the previous experiment. MS medium supplemented 1.0 mgl-1 AgNO3 along with 9% (w/v) sucrose (1T3) produced 3.24±0.07 g microrhizomes in three months of culture and after six months the weight of rhizomes increased to 4.66±0.01g (Table 4). These cultures showed a significant increase in number of shoots, weight of plant and amount of rhizomes. Weight of the plant recorded was 10.66±0.09 g after three months and it was raised to 14.56±0.07 g after six months.
MS medium containing 2.0 mgl-1 AgNO3 along with 6% (w/v) sucrose produced 4.53±0.11g in vitro rhizomes in three months of culture and the amount increased significantly after 6 months (5.70±0.20g) (Fig. 2b, Table 4). Similarly, in ginger plantlets microrhizomes size was improved in MS medium supplemented with 1.9 mgl-1 AgNO3 and 80 gl-1 sucrose (Nguyen et al. 2020). In the present study, MS medium fortified with 3% (w/v) sucrose, but without AgNO3 (control) produced least number of shoots and microrhizomes. Here it is very clear that AgNO3 has a significant promoting effect on microrhizome induction. The positive effects of AgNO3 on microrhizome induction can be attributed to the binding of Ag2+ cations to ethylene receptors at the cell membrane thereby interfering with the typical inhibitory effect of ethylene on organ size’s elongation and determination as established in ginger (Nguyen et al. 2020).
However, in our experiment, higher concentration of AgNO3 (2.0 mgl-1) along with 9% (w/v) sucrose (2T3 treatment) showed an inhibitory effect on in vitro microrhizome induction in K. galanga. Ethylene inhibitory effect of AgNO3 was reported in many other works. Similar inhibitory effect of AgNO3 in concentrations higher than 11 µM is already reported in two species of ginger. Here we have discussed the combined effect of sucrose and AgNO3 on microrhizome induction in K. galanga. In the light of the findings established here it can be concluded that sucrose and AgNO3 played an important role in in vitro microrhizome induction in K. galanga (Ticona and Oropeza 2013; Singh et al. 2013; Moniuszko 2015; Nguyen et al. 2020).
Table 4. Effect of sucrose and AgNO3 on Microrhizome Induction in K. galanga
| Treatments | Weight of plant (g) | Weight of plant without leaf (g) | Weight of microrhizome (g) | |||
| 3 months | 6 months | 3 months | 6 months | 3 months | 6 months | |
| 1T1 | 7.83±0.12c | 10.25±0.09c | 2.64±0.035c | 3.21±0.09c | 1.57±0.05c | 2.35±0.03c |
| 1T2 | 9.90±0.15b | 12.57±0.09b | 3.18±0.09b | 4.12±0.02b | 2.22±0.01b | 3.54±0.04b |
| 1T3 | 10.66±0.09a | 14.56±0.07a | 4.40±0.08a | 5.57±0.05a | 3.24±0.06a | 4.66±0.01a |
| 2T1 | 16.70±0.11b | 18.78±0.10b | 5.75±0.07b | 7.68±0.05b | 2.65±0.06b | 3.01±0.05b |
| 2T2 | 17.09±0.12a | 19.68±0.16a | 7.47±0.17a | 9.63±0.02a | 4.53±0.11a | 5.70±0.20a |
| 2T3 | 11.10±0.13c | Stunted growth | 4.87±0.05c | Stunted growth | 2.23±0.01b | Stunted growth |
| Control | 3.09±0.09d | 3.42±0.097d | 1.99±0.08d | 2.00±0.0 7d | 0.98±0.02d | 1.08±0.01d |
*Data represents mean values of ten replicates repeated thrice, recorded after 3 and 6 months of culture. Mean values followed by the same letter in the superscript do not differ significantly based on ANOVA and t-test at p ≤ 0.05.
Figure 2: Microrhizome induction in K. galanga shoots in sucrose and AgNO3

GCMS Analysis of Essential oils from In vivo rhizomes and Microrhizomes: Essential oils collected from in vivo rhizomes and in vitro microrhizomes of K. galanga were analysed by GC-MS and the composition of both rhizome oils are shown in Tables 5 and 6 respectively. As per GC-MS analysis, there were 79 components present in in vivo rhizome oil and most of the compounds are present in lower amounts (Fig. 3). Some of the major compounds and their reported activities are represented in Table 5.
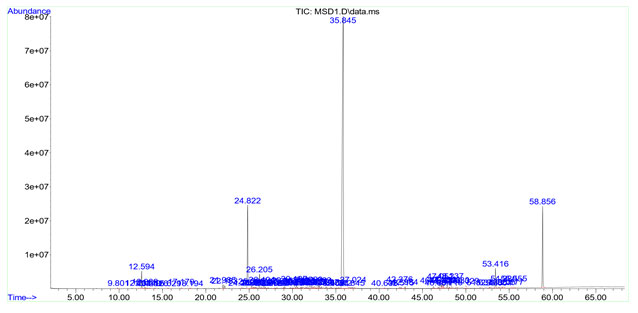
Palmitic acid (12.219%) was detected as the predominant compound in in vivo rhizome oil. In addition to this, capric acid (8.955 %), caprylic acid (4.468 %), lauric acid (3.082 %), myristic acid (3.924 %), stearic acid (3.948%), ethyl-p-methoxy cinnamate (2.132%) and ethyl cinnamate (0.795%) are also detected in in vivo rhizome oil sample. In in vitro microrhizome oil, there were 74 compounds upon by GC-MS analysis. Ethyl-p-methoxy cinnamate (58.088%) was detected as most abundant component. Remaining compounds present in in vitro rhizome oils were ethyl cinnamate (10.155%), octahydro-4a (2H)-naphthalinyl methanol (2.045%), borneol (1.349%), pentadecane (1.482%), α-cadinol (0.729%) and retinol (0.501%) (Table 6).
Great variation was noticed in the case of chemical constituents in in vivo rhizome and in vitro microrhizome as per GC-MS analysis. Based on previous reports ethyl-p-methoxy cinnamate and ethyl cinnamate were the predominant bioactive compounds in the essential oils of K. galanga and their presence was detected in both samples analyzed here. The percentage of ethyl-p- methoxy cinnamate and ethyl cinnamate was comparatively higher in in vitro microrhizome (Fig. 4) than the in vivo rhizome, which was the control sample. Most of the compounds found in the essential extracted here have been reported to exhibit significant biological activities.
The major among them viz. ethyl cinnamate and ethyl-p-methoxycinnamate are esters which contribute the nematicidal, anticancer, antituberculosis, anti-inflammatory, antifungal and larvicidal properties to the oil (Liu et al. 2010; Muhammad et al. 2012). According to Ajay (2014) monoterpenes and sesquiterpenes were found in the essential oil of K. galanga rhizomes which also may contribute the flavour and fragrance properties to the oil. Anti-cancer activity of K. galanga was due to the presence of the compound ethyl p methoxi cinnamate and vassorelaxation effect of ethyl p- methoxy cinnamate in K. galanga was also reported in previous studies (Srivastava et al. 2019; Srivastava et al. 2021). In our experiment a good percentage (58%) of ethylp methoxy cinnamate was detected from microrhizome oil sample.
Table 5. Essential oil components in in vivo rhizome of K. galanga
| Peak number | Retention time | Abundance % | Compound | Common name | Chemical formula |
| 53 | 43.218 | 12.219 | n-Hexadecanoic acid | Palmitic acid | C16H32O2 |
| 8 | 17.00 | 6.447, 2.508 | n-Decanoic acid | Capric acid | CH3(CH2)8COOH |
| 5 | 11.832 | 4.468 | Octanoic Acid | Caprylic Acid | C8H16O2 |
| 29 | 29.144 | 3.082 | Dodecanoic acid | Lauric acid | C12H24O2 |
| 64 | 49.196 | 3.948 | Octadecanoic acid | Stearic acid | C18H36O2 |
| 42 | 36.434 | 3.924 | Tetradecanoic acid | Myristic acid | C14H28O2 |
| 60 | 47.012 | 3.920 | 2(3H)-Furanone,5-dodecyldihydro | Gamma palmitolactone | C16H30O2 |
| 61 | 47.904 | 3.255 | RH-Pyran-2-one,tetrahydro-6-nonyl | δ-tetradecalactone | C14H26O2 |
| 41 | 35.686 | 2.132 | 2-Propenoic acid,3(4-methoxyphenyl)-ethyl ester | Ethyl p-methoxy cinnamate | C12H14O3 |
| 21 | 24.804 | 0.795 | 2-propenoic acid ,3-phenyl,ethyl ester | Ethyl cinnamate | C 11H12O2 |
Figure 3: GC-MS -Chromatogram of in vivo microrhizome oil of K. galanga
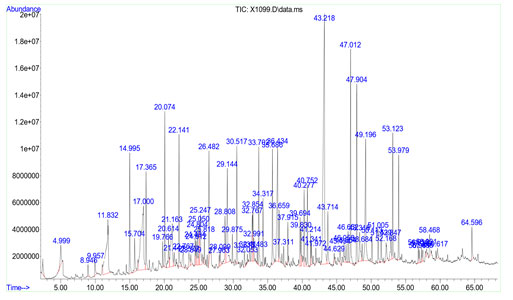
Table 6. Essential oil components in in vitro microrhizome of K. galanga
| Peak number | Retention time | Abundance % | Compound | Common name | Chemical formula |
| 48 | 35.845 | 58.088 | 2-Propenoic acid,3(4-methoxyphenyl)-ethyl ester | Ethyl p-methoxy cinnamate | C12H14O3 |
| 14,15 | 24.822, 25.290 | 9.774.0.381 | 2-propenoic acid ,3-phenyl,ethyl ester | Ethyl cinnamate | C11H12O2 |
| 3 | 12.231 | 1.349 | Borneol | Borneol | C10H18O |
| 19 | 26.205 | 1.482 | Pentadecane | Alkane hydrocarbon | C15H32 |
| 37 | 31.980 | 0.729 | α-Cadinol | Cadinane sesquiterpenoid | C15H26O |
| 30 | 30.187 | 0.633 | 12-Oxybicyclo[9.1.0]dodeca-3,7diene,1,5,5,8-tetramethyl | Humulene epoxide 2 | C15H24O |
| 59 | 47.323 | 0.501 | Retinol | Retinol | C20H30O |
| s40 | 32.517 | 0.413 | Caryophyllene | Bicyclic sesquiterpene | C15H24 |
Figure 4: GC-MS -Chromatogram of in vitro microrhizome oil of K. galanga
CONCLUSION
The findings of the present study reports the technology for microrhizome induction using sucrose and AgNO3 in K. galanga which can be effectively used to produce quality planting material at affordable price for commercial purpose. In GC-MS analysis, there were 79 components present in in vivo rhizome oil and on par with these 74 components were detected in microrhizome oil. The findings established here offers the development of a novel method for the extraction of volatile oil from microrhizomes which can be further scaled up by bioreactor technology. This protocol for microrhizome induction can be used for the commercial production of rhizomes and essential oil in K. galanga and thus ensuring the conservation and sustainable utilization of this species.
ACKNOWLEDGEMENTS
The Study was financially supported by the JNTBGRI, Palode, Thiruvananthapuram. Authors thanks its director for granting facilities regarding GCMS analysis.
Conflict Of Interests: Authors declare no conflict of interests to disclose.
REFERENCES
Adams RS (2007). Identification of essential oil components by Gas chromatography /Mass Spectrometry. Allured Publ, Carol StreamIL, USA, (4).
Agurla S., Gayatri, G. and Raghavendra, A.S. (2017). Polyamines increase nitric oxide and reactive oxygen species in guard cells of Arabidopsis thaliana during stomatal closure. Protoplasma 255, 153–162. doi: 10.1007/s00709-017-1139-3.
Ajay K. (2014). Chemical composition of essential oil isoated from the rhizome of Kaempferia galanga L. International Journal of Pharma and Biosciences, 5(1), 225- 231.
AlSalhi, M.S., Elumalai, K., Devanesan, S., et al. (2020). The aromatic ginger Kaempferia galanga L. (Zingiberaceae) essential oil and its main compounds are effective larvicidal agents against Aedes vittatus and Anopheles maculatus without toxicity on the non-target aquatic fauna. Industrial crops and products, 158. doi: 10.1016/j.indcrop.2020.113012.
Anisuzzaman, M., Sharmin, S.A., Mondal, S.C., et al. (2008). In vitro microrhizome induction in Curcuma zedoaria (Christm.) Roscoe – A conservation prioritized medicinal plant. Journal of Biological Sciences, 8, 1216-1220.
Archana, C., Geetha, S. and Balachandran, I. (2014). In vitro microrhizome and minirhizome production in turmeric (Curcuma longa L.) cultivar Allepy Supreme and its comparative anatomical and histochemical analysis. International Journal of Current Microbiology and Applied Science, 3:535-542.
Arora, J.S., Singh, K., Grewal, H.S., et al. (1996). In vitro cormel production from nodal buds and cormel tips in gladiolus. In: Islam AS (Ed.) Plant Tissue Culture. Oxford & IBH Publishing Co. Pvt. Ltd, New Delhi; 1996. pp. 50-53.
Bhat, S.R., Chandel, K.P.S. and Kackar, A. (1994). In vitro induction of rhizomes in ginger Zingiber officinale Roscoe. Indian Journal of Experimental Biology, 32, 340-344.
Chirangini, P., Sinha, S.K. and Sharma, G.J. (2005). In vitro propagation and microrhizome induction in Kaempferia galanga L. and K. rotunda L. Indian Journal of Biotechnology, 4, 404-408.
Devi, N.S., Kishor, R. and Sharma, G.J. (2012). Microrhizome induction in Acorus calamus Linn. An important medicinal and aromatic plant. Horticulture Environment and Biotechnology, 53, 410-414.
Dool, V.H. and Kratz, P.D. (1963). A generalization of the retension index system including linear temperature programmed gas-lequid partition chromatography. Journal of Chromatography, 11, 463–471.
Drisya, R.S., Siril, E.A. and Bindhu, R. (2019). The effect of Silver nitrate on micropropagation of Moringa oleifera Lam. An important vegetable crop of tropics with substantial nutritional value. Physiology and Molecular Biology of Plants, 25(5), 1311-1322. doi.org/10.1007/s12298-019-00689
Harathi, K., Geetha, G. and Naidu, C.V. (2016). Effect of silver nitrate and different carbon sources on in vitro shoot multiplication of Sphaeranthus indicus (Linn.) – An important antijaundice medicinal plant, International journal of pharmacy and biological science, 6(1), 185-192.
Kareem MA (1997). Plants in ayurveda: A compendium of botanical and Sanskrit names, Foundation for Revitalisation of Local Health Tradition (FRLHT), Bangalore, India, 82, 240.
Kumar, G.P., Sivakumar, S, Siva G, et al. (2016) Silver nitrate promotes high-frequency multiple shoot regeneration in cotton (Gossypium hirsutum L.) by inhibiting ethylene production and phenolic secretion. In Vitro Cellular and developmental Biology, 52(4), 408-418.
Kumar, V., Parvatam, G. and Ravishankar, G.A. (2009). AgNO3: a potential regulator of ethylene activity and plant growth modulator. Electronic Journal of Biotechnology, 12(2), 8-9.
Labrooy, C.D., Abduullah, T.L. and Stanslas, J. (2020). Influence of N6-benzyladenine and sucrose on in vitro direct regeneration and microrhizome induction of Kaempferia parviflora Wall.ex Baker, an important ethnomedicinal herb of Asia.Tropical life science research, 31, 123-139.
Liu, B., Liu, F., Chen, C., et al. (2010). Supercritical carbon dioxide extraction of ethyl p-methoxycinnamate from Kaempferia galanga L. rhizome and its apoptotic induction in human HepG2 cells. Natural Product Research, 24: 1927-1932.
Mehaboob, V.M., Faizal, K., Shamsudheen, K.M., et al. (2019). Direct organogenesis and microrhizome production in ginger (Zingiber officinale Rosc.). Journal of Pharmacognosy and Phytochemistry, 8(3), 2880-2883.
Mohamed, A., Usama, A., Hussein, T., et al. (2014). In vitro production of microrhiomes in ginger (Zingiber officianale Rosco). Journal of Microbiology, Biotechnology and Food Sciences, 4,142-148.
Mohd, A., Tasir, S. P., Susheel V., et al. (2018). Ethylene suplimentation increases PSII efficiency and alleviates chromium inhibited photosynthesis through increased nitrogen and sulphur assimilation in mustard. Journal of Plant Growth Regulation, 37, 1300-1317.
Moniuszko G (2015). Ethylene signaling pathway is not linear, however its lateral part is responsible for sensing and signaling of sulfur status in plants. Plant Signaling and Behavior, 10:11. DOI: 10.1080/15592324.2015.1067742.
Muhammad, I.U., Mohd Zaini A., Amirin S. J., et al. (2012). Bioactivity-Guided Isolation of Ethyl-p-methoxycinnamate, an Anti-inflammatory Constituent, from Kaempferia galanga L. Extracts. Molecules, 17, 8720-8734.
Murashige, T. and Skoog, F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum, 15, 473-497.
Nayak, S. (2000). In vitro multiplication and microrhizome induction in Curcuma aromatica Salisb. Plant Growth Regulation, 32, 41-47.
Nguyen, H.A., Tran, T.M.C., Ho T.H.N., et al. (2020). The effect of sucrose, Silver Nitrate, Plant Growth Regulators, and Ammonium nitrate on microrhizome induction in perennially- Cultivated Ginger (Zingiber officianale) From Hue, Vietnam. Acta Agrobotanica, 73(2), 1-11.
Pal, M., Szalai, G. and Janda, T. (2015). Speculation: polyamines are important in antibiotic stress signalling. Plant Science, 237, 16-23. doi: 10.1016/j.plantsci.2015.05.003
Pramila, S, C., Ramakrishna, V.H., Mokashi, A., et al. (2011). Microrhizome production in turmeric (Curcuma longa L.). Karnataka Journal of Agricultural Sciences, 24(4),s 493-496.
Preetha T.S. (2012). Studies on in vitro conservation in Kaempferia galanga L. PhD thesis, University of Kerala.
Raina, A.P. and Abraham, Z. (2015). Chemical profiling of essential oil of Kaempferia galanga L. germplasm from India. Journal of Essential Oil Research. 28(1), 29:34
Rao, V.K., Rajasekharan, P.E., Roy, T.K., et al. (2009). Comparison of essential oil components in rhizomes and in vitro regenerated whole plants of Kaempferia galanga. Journal of Medicinal and Aromatic Plants, 31, 326-329.
Rastogi, RP and Mehrotra (1993). Compendium of Indian Medicinal Plants, Central Drug Research Institute, Lucknow and publications and Information Directorate, New Delhi, pp.373.
Reghunath, B.R. and Shameena, S. (2013). An Efficient Procedure for in vitro microrhizome induction in Curcuma aromatica Salisb. Journal of Plant Science and Research, 29, 169-176.
Sanghamitra N (2000). In vitro microrhizome production in four cultivars of turmeric (Curcuma longa L.) as regulated by different factors. In: Contributory papers, Centennial conference on spices and aromatic plants: Challenges and opportunities in the new century, Indian Society for Spices, Calicut, Kerala, India, pp. 3-9.
Sarropoulou, V., Dimassi, T. K. and Therios, I. (2016). Effect of the ethylene inhibitors silver nitrate, silver sulfate, and cobalt chloride on micropropagation and biochemical parameters in the cherry rootstocks CAB-6P and Gisela 6.Turkish Journal of Biology, 40,670-683. doi:10.3906/biy-1505-92
Shirgurkar, M.V., John, C.K and Nadgauda, R.S. (2001). Factors affecting in vitro microrhizome production in turmeric. Plant Cell Tissue and Organ Culture, 64(1), 5–11.
Singh, T.D., Chakpram, L. and Devi, H.S. (2013). Induction of in vitro microrhizomes using silver nitrate in Zingiber officianale Rosc.var, Baishey and Nadia. Indian Journal of Biotechnology, 13:256-262.
Sivarajan I and Balachandran (1994). Ayurvedic drugs and their plant sources, Oxford and IBH publishing Co.Pvt.Ltd, New Delhi.
Srivastava, K.C., Rajana, and Amit, C. (2019). Aromatic ginger (Kaempferia galanga) extract with ameliorative and protective potential as a functional food, beyond its flavor and nutritional benefits. Toxicology Reports 6, 521-528. doi.org/10.1016/j.toxrep.2019.05.01
Srivastava, N., Mishra, S., Iqbal, H., et al. (2021). Standardization of Kaempferia galanga L. rhizome and vasorelaxation effect of its key metabolite ethyl p-methoxycinnamate. J Ethnopharmacol. 10, 271. doi: 10.1016/j.jep.2021.113911.
Sun, C.H., Yu, J.Q. and Hu, D.G. (2017). Nitrate: a crucial signal during lateral roots development. Frontiers in Plant Science, 8: 485. https://doi.org/10.3389/fpls.2017.00485.
Sunitha, M., Pompy, S. and Mohan, L. (2018). Chemical composition and biological activity of essential oil of K.galanga : a review , Journal of essential oil research, 30, 303-308.
Tewtrakul, S., Yuenyongsawad, S., Kummee, S., et al. (2005). Chemical components and biological activities of volatile oil of Kaempferia galanga L. Songklanakarin Journal of Science and Technology, 27, 503-507.
Ticona, A.S. and Oropeza, M. (2013). Effect of culture medium consistence and silver nitrate on micropropagation of two potato (Solanum tuberosum) cultivars. Revista Colombiana de Biotechnologia, 15(2), 55-62.
Tombisana, M. N.G. and Noren, S. (2015). Microrhizome induction in Sying Makhir, An important Ginger Cultivar of Meghalaya. Vegetos, 28, 29-34.
Zahid, N.A., Jaafar, H.Z.E. and Hakiman, M. (2021). Alterations in Microrhizome Induction, Shoot Multiplication and Rooting of Ginger (Zingiber officinale Roscoe) var. Bentong with Regards to Sucrose and Plant Growth Regulators Application.11, 320.


