1School of Biological Engineering and Life Sciences, Shobhit Institute of
Engineering and Technology, Meerut, Uttar Pradesh, India
2National Bureau of Plant Genetic Resources, Pusa Campus, New Delhi, India
3Department of Microbiology, Mehsana Urban Institute of Sciences,
Ganpat University, Mehsana, Gujarat, India
Corresponding author Email: r.rishi56@gmail.com
Article Publishing History
Received: 13/03/2022
Accepted After Revision: 25/05/2022
In the tissue culture investigations for tree species, the growth medium with a perfect concentration of growth regulators and other components has a crucial role in the in vitro callus initiation from explants material. The present study has highlighted the effects of various concentrations of plant growth regulators on callus initiation from immature fruits of Elaeocarpus ganitrus in Murashige & Skoog (MS) medium. The immature fruits were collected as explants sources during fruiting season and cultured on 1/2, 3/4 and full-strength MS medium. In this study, 1.8mgL-1 of 2, 4-D, and full-strength MS medium was observed best for callus initiation and further supported for the multiplication from immature fruits of E. ganitrus. The callus initiation from immature fruits of E. ganitrus was the crucial step towards the conservation study of this tree species. This study can be helpful for tissue culture investigations concerning callus development from immature fruit explants material of several other species of Elaeocarpus and even for the other endangered tree species.
Callus, E. Ganitrus, Immature Fruits, Ms.
Rishi, Vishwakarma H, Arya R, Garg A. P. In Vitro Callus Induction from Immature Fruits of Elaeocarpus Ganitrus: A Tissue Culture Approach. Biosc.Biotech.Res.Comm. 2022;15(2).
Rishi, Vishwakarma H, Arya R, Garg A. P. In Vitro Callus Induction from Immature Fruits of Elaeocarpus Ganitrus: A Tissue Culture Approach. Biosc.Biotech.Res.Comm. 2022;15(2). Available from: <a href=”https://bit.ly/3LXnr86“>https://bit.ly/3LXnr86</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
The genus Elaeocarpus consists of a large number of species. Among these many, Rudraksha are popularly known. Rudraksha has tremendous importance in rituals and prayers. Several investigations were carried out by researchers in the past on different species of Elaeocarpus. Elaeocarpus sphaericus is well known for its antioxidant properties and through several literature reviews, it was surmised that it owns notable biomedical capability. Through GC-MS E. sphaericus phytochemicals have been analyzed in the previously published studies (Mahajanakatti et al. 2022). E. sphaericus is rich in antimicrobial and anti-inflammatory characteristics. Along with phytochemicals, E. sphaericus is a very important and useful source of antibiotics and antioxidants. Findings on Elaeocarpus ganitrus fruit set and dispersal may have high implications for species regeneration (Khan et al. 2005; Koirala et al. 2022).
The blue colour is most prevalent in Elaeocarpus spp. in its entire distribution. Regeneration failure from seeds of E. venustus leads to an approach toward other techniques. Further vegetative propagation was first reported successfully (Lee 1998; Saravanan et al. 2011). The callus initiation using leaf stalk as explants material of E. grandiflorus was reported on MS medium (Habibah et al. 2019). Using small and immature soft leaves of E. ganitrus, callus was developed (Rishi et al. 2021). A procedure for in-vitro propagation was developed for E. serratus fruit tree. For the investigation, mature nodal explant material was collected. In the study ½, ¾ strength MS medium was used during the study (Koirala et al. 2022).
Other media like White’s, B5 Gamborg and WPM (Woody Plant Medium) were also taken during the investigation. For several other trees of a different genus, tissue culture has shown its importance and necessity (Raji and Siril 2021). For Baramasi mango, a variety of micropropagation protocols were developed by nucellar embryogenesis. B5 Gamborg MS minor and major salts along with several other additives were used for basal media. 2,4-D is very essential for callus induction and pro-embryonic calli generation in nucellar embryogenesis (Al-Busaidi 2016).
Young soft leaves of Litchi chinensis Sonn. were taken as explants for the development of callus to get in vitro plantlets (Puchooa 2004). Somatic embryogenesis of ‘Feizixiao litchi’ was first reported. The lactalbumin hydrolysate helped in getting a high frequency of callus initiation when taken in combination with plant growth regulators in MS medium (Wang et al. 2016; Koirala et al. 2022).
The somatic embryogenesis was achieved in callus cultures of C. wightii, a medicinally important woody tree species (Kumar et al. 2003). The MS medium was found suitable for callus initiation from leaf explants of Ailanthus excelsa Roxb., and plant growth regulator kinetin was found better than TDZ (Dhaval and Nataraj 2018). For the tropical woody plant, Parasponia andersonii Planch the callus initiation was obtained with 0.1-0.2mg L-1 TDZ with 0.05 mgL-1NAA (Knyazev 2018). For direct and indirect shoot organogenesis in Ficus religiosa using hypocotyls as explants, two valuable protocols were developed (Hesami and Daneshvar 2018).
In zygotic embryos of Cedrus deodara formation of adventitious bud was examined. Four different tissue culture growth media variously fortified with and without plant growth regulators were used (Tamta and Palni 2004; Koirala et al. 2022). In the present study growth regulators and antioxidants were used in MS (Murashige and Skoog 1962) medium for in vitro callus initiation from immature fruits of E. ganitrus. It is difficult to do tissue culture work with fruits of E. ganitrus. Therefore, immature fruits of E. ganitrus (Roxb.) were taken into consideration as explants source.
MATERIAL AND METHODS
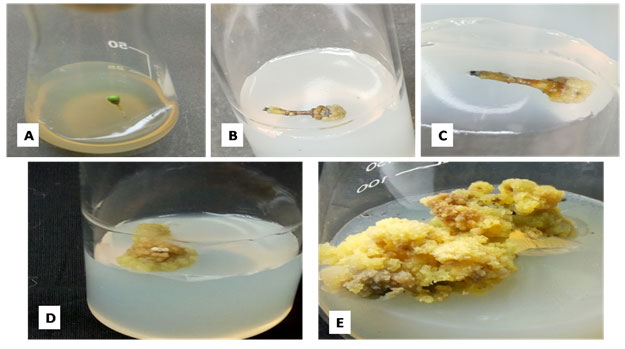
The samples (Fig. 2A) were obtained from 6-7 years old tree located inside SIET, Meerut, Uttar Pradesh, India (Fig.1A) which were brought into the laboratory and further washed gently using distilled water. The samples were dipped in distilled water to treat under bavistin (1%) (w/v) for 35mins (Rishi et al. 2021). Under the laminar air hood the surface sterilization of explants was performed using 70% ethanol for time period of 30 seconds. The explants samples were treated with HgCl2 0.1% (w/v) for 4 mins (Rishi et al. 2021). The explants were rinsed for 6 times each for 4 min. with sterile double distilled water within the laminar hood. The immature fruits after surface sterilization were cultured on the respected medium of various strength. The temperature of25±2ºC was set for maintaining the in vitro cultures with 60-70% humidity. The light and dark conditions of 16h/8h were provided to the respective cultures.
Figure 1: [A] Tree of E. ganitrus [B] Inflourescence on E. ganitrus [C] Growing E. ganitrus fruit.

For the investigation MS medium with 1/2, 3/4 and full strength was used and fortified with 2, 4-D (2, 4-Dichlorophenoxyacetic acid) and TDZ which were taken in the concentration range of 0.2 mgL-1 to 2.0 mgL-1. The antioxidants like ascorbic acid (165 mgL-1), citric acid (5 mgL-1) polyvinyl pyrrolidone (PVP,165 mgL-1) were used. The pH of 5.8±0.5 was maintained for the medium and 0.8% agar was added. The medium was autoclaved for 15-20 min. For statistics, analysis of variance, one way ANOVA was carried out for significant difference p≤ 0.05 (n=10).
RESULTS AND DISCUSSION
The callus induction was started after 5 weeks (Fig.2B) on full strength MS medium. It was observed that the entire explants material converted into callus after 9-10 weeks (Fig.2D). The initial callus appeared to be whitish to light green in colour, but after some time, when it started getting older, the callus started to appear light yellow and green in colour (Fig.2E)
Figure 2: Different stages of callus development from immature fruit explants on full strength MS medium. [A] Immature fruit; [B]&[C] Callus initiation; [D] Entire immature fruit of E. ganitrus converted into callus; [E] Callus multiplication.
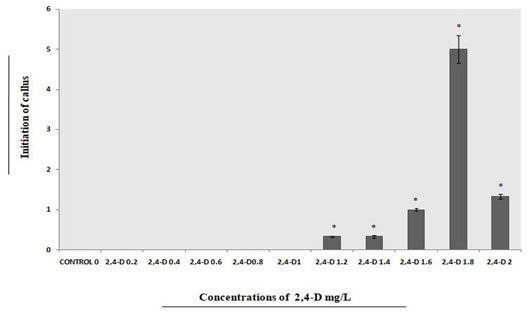
From immature fruits of E. ganitrus initiation of callus was observed maximum at 1.8mgL-1 of 2,4-D as compared to 1.2mgL-1, 1.4mgL-1, 1.6mgL-1 and 2.0mgL-1 of 2,4-D when considered alone (Fig.3).
Figure 3: Different concentrations of 2, 4-D used for callus initiations from explants on full strength MS medium.
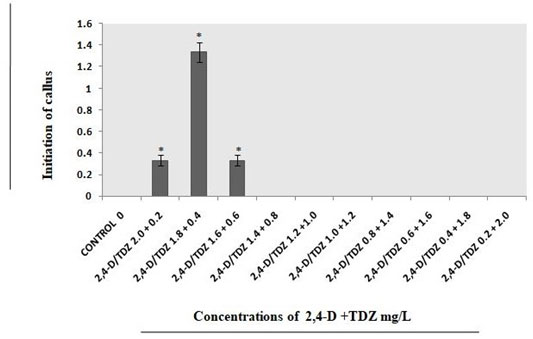
On the other hand, with the concentrations of TDZ 0.6mgL-1 analyzed better in comparison with 0.4mgL-1 and 0.8mgL-1 (Fig.4). With combination of both the growth regulators i.e., 2,4-D 1.8mgL-1 and TDZ 0.4mgL-1 concentration callus developed successfully (Fig.5).
Figure 4: Different concentrations of TDZ used for callus initiations from explants on full strength MS medium
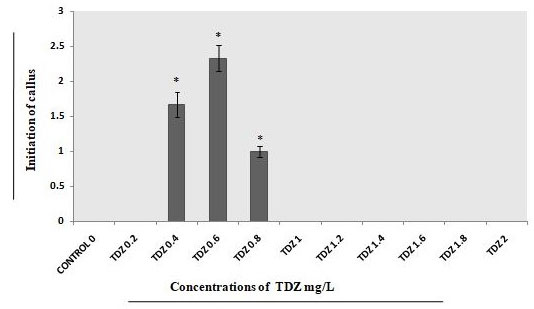
Figure 5: Different concentrations of 2, 4-D+TDZ mg/L used for callus initiations from explants on full strength MS medium
In this study, it was observed that low concentrations of TDZ responded much better in contrast to higher concentrations. The organogenic callus of E. robustus was observed using the MS medium consisting of 2,4-D (Rahman 2004). Callus initiation was reported in E. tuberculatus when MS medium was fortified with 2, 4-D (Arshad and Kumar 2006). Callus initiations for E. sphaericus were best observed in MS medium fortified with 1 mgL-1 BAP in combination with 0.5mgL-1 2, 4-D (Dubey and Das 2011). The compact callus induction and basal callus formation were observed in the in vitro cultures of E. blascoi in WPM using TDZ (Siva et al. 2015). Callus formation was successfully reported in E. grandiflorus when 2,4-D was used (Habibah et al. 2019). The sterilization component i.e., 0.1% HgCl2 was found best for surface sterilization of immature fruit explants. HgCl2 with a similar concentration was analysed as best for surface sterilization of leaf explants of E. ganitrus (Rishi et al. 2021).
The previously published studies on tissue culture for different tree species including E. ganitrus, were observed quite promising based on plant conservation aspects. The results obtained from such reported studies can be utilized to develop deep insights into the tissue culture of Elaeopcarpus due to its high importance in several research disciplines. Therefore, the investigation of immature fruits of E. ganitrus (Rudraksha) was taken for research purposes which further may open the various ways of holding other studies. The study can become very important in the coming future for the conservation of endangered tree species of not only Elaeocarpus but for other species belonging to different genera through plant tissue culture.
CONCLUSION
The findings of the present study have shown that the initiation and multiplication of callus best in full strength MS medium with 2, 4-D at the concentration of 1.8 mgL-1. The study indicated that 2,4-D plays a crucial role during callus induction, growth and development to influence the entire explant material. Hence, the procedure may be useful for callus studies of other Elaeocarpus species, especially the ones from which we got different types Rudraksha beads.
ACKNOWLEDGEMENTS
The research facilities for the study were provided by the Shobhit Insitute of Engineering & Technology, Modipuram, Meerut, Uttar Pradesh, India.
Conflict of interests: Authors declare no conflict of interests to disclose.
Data Availability Statement: The database generated and /or analysed during the current study are not publicly available due to privacy.
REFERENCES
Al-Busaidi KT, Shukla M, Al-Burashdi AH et al. (2016). In vitro regeneration of Mango (Magnifera indica L.) cv. Baramasi through nucellar embryogenesis. Journal of Horticulture and Forestry. 8(5): 37-43. doi:10.5897/JHF2016.0443
Arshad SM and Kumar A (2006). Tissue culture investigation of Elaeocarpus tuberculatus– a highly valued rudraksha. Vegetos-An Int. J. Plant Res. 19: 111-114.
Dhaval P and Nataraj M (2018). Callus induction in Ailanthus excelsa Roxb.- A Multipurpose tree. International Journals of Scientific Reviews. 7(1): 116-129.
Dubey P and Das AK (2011). Micropropagation and rehabilitation of Elaeocarpus sphaericus gaertn k schum a highly valued plant from Arunachal Pradesh. Shodhganga@INFLIBNET / Rajiv Gandhi University / Department of Botany, http://hdl.handle.net/10603/278904.
Habibah NA, Widiatningrum T, Anggraito YU et al. (2019). Growth of Elaeocarpus grandiflorus callus cultures in MS medium with various concentrations of growth regulators, Journal of Physics: Conference Series 1321doi:10.1088/1742-6596/1321/3/032037.
Hesami M and Daneshvar MH (2018). In vitro adventitious shoot regeneration through direct and indirect organogenesis from seedling-derived hypocotyls segments of Ficus religiosa L.: An important medicinal plant, Hort Science, 53(1): 55-61. https://doi.org/10.21273/HORTSCI12637-17
Khan ML, Bhuyan P and Tripathi RS (2005). Effects of forest disturbance on fruit set, seed dispersal and predation of Rudraksh (Elaeocarpus ganitrus Roxb.) in northeast India, Curr. Sci., 88 (1): 133-142.
Knyazev A, Kuluev B, Vershinina Z et al. (2018). Callus induction and plant regeneration from leaf segments of unique tropical woody plant Parasponia andersonii Planch. Plant Tissue Cult. and Biotech. 28(1): 45-55. https://doi.org/10.3329/ptcb.v28i1.37197
Koirala B, Pakuwal E, Rai HJ et al. (2022). Evaluation of antioxidants and antimicrobial properties of Indigenous plants Elaeocarpus sphaericus and Ficus religiosa. International Journal of Environment 10(2): 48-63. doi: https://doi.org/10.3126/ije.v10i2.42821
Kumar S, Suri SS, Sonie KC et al. (2003). Establishment of embryonic cultures and somatic embryogenesis in callus culture of guggul- Commiphora wightii (Arnott) Bhandari. Indian Journal Experimental Biology. 41.69-77.
Lee DW (1998). The biology of Rudraksha. Curr. Sci. 75(1):26-30.
Mahajanakatti AB, Deepak TS, Achar RR, et al. (2022). Nanoconjugate synthesis of Elaeocarpus ganitrus and the assessment of its antimicrobial and antiproliferative properties. 27, 2442. 1-17. https://doi.org/10.3390/molecules27082442
Murashige T and Skoog F (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol. 15: 473-97.
Puchooa D (2004). In vitro regeneration of lychee (Litchi chinensis Sonn.). African Journal of Biotechnology. 3(11): 576-584.
Rahman MM, Amin MN and Ahmed R (2004). In-vitro rapid regeneration from Cotyledon Explant of Native-olive (Elaeocarpus robustus Roxb.). Asian J. Plant Sci. 3(1): 31-35. doi: 10.3923/ajps.2004.31.35
Raji R and Siril EA (2021). Alteration of media enables efficient in vitro cloning of mature Elaeocarpus serratus L. (Ceylon olive): a commercially important fruit tree. Physiol Mol Biol Plants. 27(2): 429-443. https://doi.org/10.1007/s12298-021-00955-x
Kumar SR and Vishwakarma H (2021). Effects of growth regulators on callus initiation of Elaeocarpus ganitrus (Roxb.). Adv. Biores. 12(2):123-127. doi: 10.15515/abr.0976-4585.12.2.123127
Saravanan S, Indra M, Kamalraj P et al. (2011). In-situ vegetative propagation of Elaeocarpus venustus Bedd. a threatened endemic tree of Agasthiamalai Biosphere Reserve, Western Ghats, India. J. Biosci. Res. 2(2): 46-49.
Siva SM, Priya TA, Balasubramanian P et al. (2015). In–vitro regeneration of an endangered tree – Elaeocarpus blascoi Weibel. (Rudraksha) from Southern Western Ghats, Tamil Nadu, India. Eur. J. Biotech. Biosci.3(11): 62-66.
Tamta S and Palni LMS (2004). Studies on in vitro propagation of Himalayan cedar (Cedrus deodara) using zygotic embryos and stem segments. Indian Journal of Biotechnology. 3: 209-215.
Wang G, Li H, Wang S et al. (2016). In vitro regeneration of ‘Feizixiao litchi’ (Litchi chinensis Sonn.). African Journal of Biotechnology. 15(22): 1026-1034. doi: 10.5897/AJB2015.15175


