1Faculty of Sciences,University of Central Punjab, Pakistan, Department of Resources and Environment Microbiology,
College of Land and Environment, Shenyang Agricultural University, Shenyang 110866, Liaoning, P.R. China,
2Department of Plant Pathology, College of Plant Protection, Shenyang Agricultural University, Shenyang 110866, Liaoning, P.R. China,
3College of Pharmacy, University of the Punjab, Lahore 54590, Pakistan4
Corresponding author email: taswarahsan@163.com
Article Publishing History
Received: 15/03/2021
Accepted After Revision: 10/06/2021
Fusarium oxysporum f. sp. Cubense is causal agent of Fusarium wilt disease in Banana. The activity of antagonistic bacterial strain was studied for the low-cost and eco-friendly management of F. oxysporum in banana. Streptomyces S128 was identified by 16S rRNA sequence analysis and resulted as Streptomyces albidoflavus. Bioassay activity showed inhibition diameter of 22.5mm against Fusarium oxysporum f. sp. Cubense. Optimum combination of factors in the fermentation medium was obtained as: soluble starch 4%, peanut flour 2%, (NH4)2SO4 0.2%, CaCO3 0.8%, NaCl 0.8%. Identification of active compounds were performed via GC/MS chromatographic techniques. There were total of nineteen compounds identified in the extract. On the base of area percent (18.10) Phenol, 2, 4-bis (1, 1-dimethylethyl) and (18.71) morphine were the major constituents of extract. This study concluded that Streptomyces albidoflavus effective against Fusarium oxysporum f. sp. Cubense, which might be introduced as an effective biocontrol agent for sustainable agriculture.
Banana Fusarium Wilt, Streptomyces Albidoflavus, GC-MS, Morphine
Ahsan T, Yuanhua W, Rehman S. In-vitro Biocontrol Activity of a Novel Soil Streptomyces Strain Albidoflavus Against Fusarium Oxysporum as Causal Agent of Fusarium wilt in Banana Plants. Biosc.Biotech.Res.Comm. 2021;14(2).
Ahsan T, Yuanhua W, Rehman S. In-vitro Biocontrol Activity of a Novel Soil Streptomyces Strain Albidoflavus Against Fusarium Oxysporum as Causal Agent of Fusarium wilt in Banana Plants. Biosc.Biotech.Res.Comm. 2021;14(2). Available from: <a href=”https://bit.ly/2QNVhGb“>https://bit.ly/2QNVhGb</a>
Copyright © Ahsan et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Over 400 million people rely on Banana (Musa spp.) as major subsistence (Dale et al. 2017). Fusarium wilt or Panama is one of the destructive diseases of banana. In the past century in the early days, it has caused heavy economic loss (Ploetz 2015). The production of Banana (Musa spp.) is severely in danger because of the infection of the soil-borne fungus q f. sp. cubense (Foc) and is commonly referred to as Panama disease (Dita et al. 2018).
Due to complex nature of soil zone, it’s quite challenging to combat soil-borne diseases. Soil-borne fungus had severe effects on crops which leads to heavy economic loss (Jayaprakashvel et al. 2019). Chemical practices in disease management of Fusarium wilt can make the soil unfit (Siamak and Zheng 2018). It also caused negative effects on health, so biocontrol is an alternative measure (Rajaofera et al. 2019). In several mechanisms of disease management, this approach has gained significant value, (Bubici et al. 2019).
Currently, the demand for natural bioactive substances has increased because of the potential effects in clinical practices and as well as in crop protection (Singh et al. 2017). In recent years, bioactive compounds are in high demand in the pharmaceuticals and naturopathy, due to their health benefits to human and plants. Actino bacteria are a major source to obtain novel compounds, which could be utilized in clinical practices, pharmaceutical industry and agricultural applications, (Barka, et al. 2016; Chater 2016). Mostly Actinomyces obtained have been from the soil (Guo et al. 2015) as production of natural compounds from microbes requires optimal growth conditions besides the nutrient medium (Rajnisz et al. 2016).
Nowadays different statics models are applied to optimize the fermentation mechanisms to extend the production of bioactive compounds, (Latha et al. 2017). There are several approaches in use, such as GC-MS, LC-MS, and NMR, for identification and structural characterization of the bioactive compounds (Tiwari et al. 2015). GC-MS is an innovative approach to identify the compound (Awla et al. 2016). In the current study, we have identified a novel potent Streptomyces strain for the biological control of banana Fusarium wilt. This study was aimed to produce secondary metabolites by optimizing the nutrient medium through Orthogonal Array Design, extraction and identification of bioactive compounds by GC-MS.
MATERIAL AND METHODS
Microorganisms: All the microbes, Banana Fusarium wilt pathogen and bio-agent were obtained from plant pathology lab, College of Plant Protection, Shenyang Agricultural University, China. Streptomyces strain 128 and other comparative antagonistic microbes (Bacillus subtilis, Trichoderma harzianum, Paenibacillus polymyxas, Bacillus licheniformis, Streptomyces cacaoi, Bacillus laterosporus, and Bacillus mucilaginosus), were maintained on nutrient and Gausses medium. Fusarium oxysporum was maintained on PDA.
Identification of Streptomyces strain: PCR amplification of the 16S rRNA gene of strain S128 was performed using the universal primer: 27(5’AGTTTGTCMTGGCTCAG-3) and 1492R (5′-GGTTACCTTACGACTT-3) (verity TM 96-well PCR, Applied Biosystems, Singapore). The PCR products were sent to Sangon Biotech (Shanghai, China) Co., Ltd for sequence determination. Phylogenetic analysis was conducted by using Mega version 6 (Ahsan et al. 2017).
Inoculum development: Fermentation was performed in two stages, seed growth and production of the active antifungal substance. Strain S128 was grown on plates of gausses medium (Gao et al. 2016) at 28oC for 5 days after spore production in the liquid fermentation medium. Two spore cakes (5 mm) of Strain 128 were used to inoculate 40ml medium in a 250 mL flask volume and incubated at 28oC with a shaking speed of 160 rpm for 48 h.
Fermentation process: From seed culture, 5% (v/v) were inoculated aseptically into 250 mL flask containing 40 mL of fermentation medium The medium comprised of [47 g soluble starch, 3 g yeast extract, 22 g peanut meal, 2.7 g (NH4)2SO4, 2.7 g NaCl, 2.7 g CaCO3 dissolved in 1 L distilled water and pH were adjusted to 6.8–7.2] and incubated at 28 oC in a rotatory shaker (HZQ-F16 Harbin Dong Lian Electronic Technology Production. Co., Ltd., China) at the speed of 160 rpm for 96 h. After that, the fermented culture was centrifuged the supernatant was stored at 4 oC for further study. The antifungal activity was determined by measuring the diameter of inhibition zones (Ahsan et al. 2017).
Experimental design for optimization of nutrients: The main nutrient factors affecting the fermentation of the strain and its concentration range were determined by a single factor test. On the basis of this, the nutritional formula of the strain was optimized by orthogonal design test. Based on the average value, the optimal fermentation medium formulation was determined by orthogonal analysis.
Effect of KH2PO4 on antibiotic production:KH2PO4 was added in the optimized medium in the amounts of 0.01%, 0.02%, 0.03%, and 0.04%, respectively, without KH2PO4 as control, and cultured at 28 ° C, 140 r / min constant temperature shaker for 96 h. The Oxford cup plate method was used to determine the antifungal activity of the fermentation broth.
Figure 1: Phylogenetic tree of Streptomyces albidoflavus

Effect of inorganic salts and trace elements on bioactive compound production: On the basis of optimizing the medium, a certain amount of inorganic salts and trace elements were added respectively, that is, K2SO4, ZnSO4, MnSO4, MgSO4, CuSO4, and FeSO4 were added with a concentration of 0.01%, 0.02%, 0.04%, and 0.08%, respectively, at 28 °C and 140r/min shaking speed for 96 hours. The antifungal activity of the fermentation broth was determined by Oxford cup plate method and without adding trace elements was considered as a control value.
Biochemical profiling for stability test of fermentation broth: Using the dinitrosalicylic acid, reducing sugar in the fermentation batch was measured and total sugars were measured by Phenol–sulphuric acid method. Amino nitrogen was determined using ninhydrin reagent. pH values of the fermentation batch were determined at different interval of time using pH meter. Dry cell weight analysis was done by the method of (Ahsan et al. 2017).
Figure 2: Bioassay effects of different solvent extracts against Fusarium oxysporum. All the inhibition zone values are mean of 3 replicates.
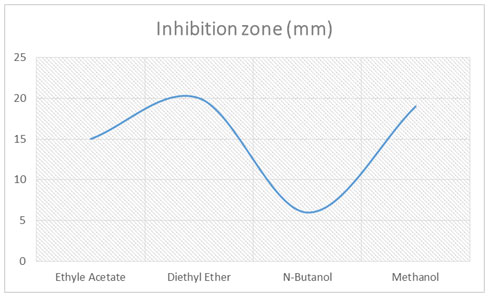
Separation, extraction, and identification of active compounds: The fermented broth was further treated with different organic solvents by the solvent extraction method. Four different solvents were used to separate fermented broth, i.e. Ethyl acetate, Diethyl ether, Ethanol, Methanol, and N-Butanol, with 1:1ratio and check the activity. Potent separated extract further purified by Silica gel column chromatography. The column was packed with silica gel (60–120 mesh). The sample to be separated was loaded on the packed column and eluted with the ethanol solvent at the flow rate of one drop per 30 seconds. Collected the fractions in test tubes and check the antifungal activity of the fractions. Most potent fraction selected to identify the active compound. GC-MS was used to identify the active compound (Ahsan set al. 2017).
Figure 3: Antifungal bioassay of purified fraction by silica gel column chromatography against Fusarium oxysporum f. sp. Cubense. In the graph antifungal values are the mean of 3 replicates
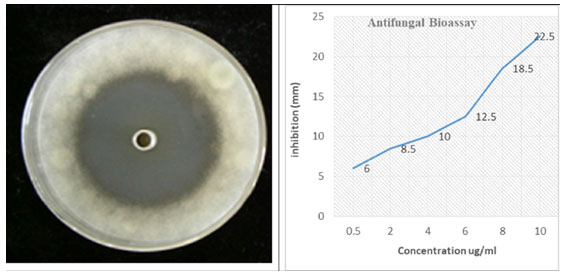
Antifungal assay: Purified Silica gel column chromatography fractions were utilized for antifungal activity of Streptomyces against the Fusarium oxysporum f. sp. Cubense. Make a serial dilution concentration of most potent fraction, i.e., 0.5, 2, 4, 6, 8, 10 µg/ml. Oxford cup method was used.
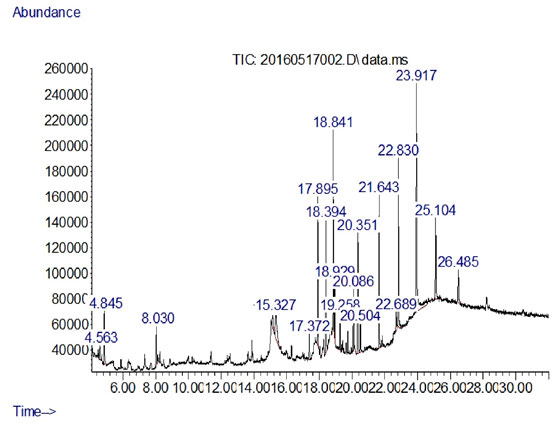
Figure 4: GC-MS Profile from the extract of Streptomyces albidoflavnous
Effects of inhibition rate among S. albidoflavous and other biocontrol agents against Fusarium oxysprum: A comparative antifungal activity was conducted against Fusarium. S. albidoflavnous and other test biocontrol agents (Bacillus subtilis, Trichoderma harzianum, Paenibacillus polymyxas, Bacillus licheniformis, Bacillus laterosporus, and Streptomyces cacaoi were evaluated against Fusarium wilt. Antifungal activity was performed by oxford cup plate method.
Statistical analysis: Statistical analysis was performed with Minitab software version 0.7.
RESULTS AND DISCUSSION
Identification of Streptomyces strain: A partial 16S rRNA gene sequence (1435 nucleotides) of strain S128 was determined and deposited in the Gene Bank database (Waiting for Accession number). Comparative 16S rRNA gene sequence analysis using BLAST showed that the strain could be classified as a member of the genus Streptomyces and shared sequence identity (99%) with Streptomyces albidoflavus Eu257268. A 16sRNA gene-based phylogenetic tree was constructed with the maximum likelihood method with the different Streptomyces reference species available in the Gen Bank database (Fig-1). Phylogenetic analysis indicated that strain S128 closely clustered with the strain Streptomyces albidoflavus (Eu257268).
Submerged fermentation: Based on the single factor optimization, the number of influencing factors and the different levels of each factor was determined. In order to eliminate the interaction between various factors and the differences between different test batches, and determine the optimal ratio of various factors in the fermentation medium, soluble starch, peanut cake powder, sodium chloride, yeast extract, ammonium sulfate and carbonic acid Calcium was the most influencing factor. Orthogonal design test L25 (56), results were mentioned in (Table 1). Based on the results of 3 trials, the optimal fermentation medium formulation was determined by orthogonal analysis.
Table 1. Results of L25(56)orthogonal test for the production of antifungal compounds
| Run No. | Peanut flour (%) | Soluble starch (%) | Sodium chloride (%) | Yeast extract (%) | (NH4)2SO4 (%) | CaCO3 (%) | Diameter of inhibition zone (mm) |
| 1 | 0 | 0 | 0.2 | 0.4 | 0.6 | 0.2 | 9.51 |
| 2 | 2 | 0 | 0. 6 | 0.8 | 0.8 | 0.8 | 19.21 |
| 3 | 4 | 0 | 0 | 0.6 | 0 | 0.6 | 22.20 |
| 4 | 6 | 0 | 0.4 | 0 | 0.4 | 0 | 10.51 |
| 5 | 8 | 0 | 0. 8 | 0.2 | 0.2 | 0.4 | 25.55 |
| 6 | 0 | 2 | 0.6 | 0.6 | 0.4 | 0.4 | 22.01 |
| 7 | 2 | 2 | 0 | 0 | 0.2 | 0.2 | 31.11 |
| 8 | 4 | 2 | 0.4 | 0.2 | 0.6 | 0.8 | 31.10 |
| 9 | 6 | 2 | 0.8 | 0.4 | 0.8 | 0.6 | 25.09 |
| 10 | 8 | 2 | 0.2 | 0.8 | 0 | 0 | 22.81 |
| 11 | 0 | 4 | 0 | 0.2 | 0.8 | 0 | 0 |
| 12 | 2 | 4 | 0.4 | 0.4 | 0 | 0.4 | 21.98 |
| 13 | 4 | 4 | 0.8 | 0.8 | 0.4 | 0.2 | 25.00 |
| 14 | 6 | 4 | 0.2 | 0.6 | 0.2 | 0.8 | 23.90 |
| 15 | 8 | 4 | 0.6 | 0 | 0.6 | 0.6 | 25.10 |
| 16 | 0 | 6 | 0.4 | 0.8 | 0.2 | 0.6 | 21.76 |
| 17 | 2 | 6 | 0.8 | 0.6 | 0.6 | 0 | 20.45 |
| 18 | 4 | 6 | 0.2 | 0 | 0.8 | 0.4 | 23.95 |
| 19 | 6 | 6 | 0.6 | 0.2 | 0 | 0.2 | 17.00 |
| 20 | 8 | 6 | 0 | 0.4 | 0.4 | 0.8 | 17.50 |
| 21 | 0 | 8 | 0.8 | 0 | 0 | 0.8 | 15.14 |
| 22 | 2 | 8 | 0.2 | 0.2 | 0.4 | 0.6 | 11.80 |
| 23 | 4 | 8 | 0.6 | 0.4 | 0.2 | 0 | 11.00 |
| 24 | 6 | 8 | 0 | 0.8 | 0.6 | 0.4 | 10.00 |
| 25 | 8 | 8 | 0.4 | 0.6 | 0.8 | 0.2 | 8.00 |
| k1 | 17.510 | 13.780 | 18.630 | 16.800 | 18.852 | 17.880 | |
| k2 | 26.467 | 20.910 | 18.413 | 19.801 | 14.976 | 22.398 | |
| k3 | 18.824 | 22.400 | 16.400 | 19.087 | 19.818 | 20.395 | |
| k4 | 20.150 | 17.160 | 18.775 | 21.274 | 17.042 | 12.989 | |
| k5 | 10.756 | 19.912 | 21.896 | 16.898 | 23.356 | 20.875 | |
| kmax-kmin | 15.654 | 8.616 | 5.431 | 4.980 | 8.340 | 9.547 |
In the table, ki (i = 1, 2, 3, 4, 5) represents the average value of the diameter of the inhibition zone of the fermentation broth when the i-th level of a factor is combined with other factors, and the highest ki value is selected as the optimal level of the factor, that is, if a factor k2>k1, k3, k4 and k5, the second level of the factor is selected as the optimal fermentation level. So the best combination of factors in the fermentation medium was obtained as: soluble starch 4%, peanut flour 2%, (NH4)2SO4 0.2%, CaCO3 0.8%, NaCl 0.8%. Kmax-kmin represents the extreme difference between the average values of different factors, and its size represents the degree of influence of different factors on the antibacterial activity of the fermentation broth. An increase in the values; cause an increase the degree of influence.
Table 2. Effect of mineral salt and trace element on yield of S. albidoflavus
| Concentration (%) | Diameter of inhibition zone(mm) | |||||
| K2SO4 | ZnSO4 | MnSO4 | MgSO4 | CuSO4 | FeSO4 | |
| 0(CK) | 30.54 | 29.10 | 28.54 | 28.98 | 25.58 | 29.14 |
| 0.01 | 28.38 | 27.3 | 28.62 | 27.92 | 0 | 28.70 |
| 0.02 | 27.60 | 26.62 | 28.82 | 27.78 | 0 | 28.24 |
| 0.04 | 27.36 | 0 | 30.24 | 27.12 | 0 | 28.00 |
| 0.08 | 27.36 | 0 | 30.24 | 28.88 | 0 | 28.00 |
Conversely, the smaller the values decrease the degree of influence. The smaller, so it can be seen that the degree of influence of the six factors on the activity of the fermentation broth is: peanut flour > calcium carbonate > soluble starch > ammonium sulfate > sodium chloride > yeast powder. The shake flask fermentation test was carried out with the best medium formula and the original medium formula under the same culture conditions. The results showed that the diameter of the inhibition zone of the optimized fermentation broth was higher than that of the original medium %.
Effect of inorganic salts and trace elements on the yield of active compound
Microorganisms require certain inorganic salts, and trace elements such as iron, magnesium, zinc, manganese and potassium during growth and reproduction and secondary metabolite synthesis. The effect of many metal ions on the physiological activity of microorganisms is related to their concentration, low concentrations tend to be stimulating, and high concentrations show inhibition. In order to further determine whether the trace elements contained in the fermentation medium of Streptomyces S128 can meet the needs of the synthesis of secondary metabolites, six different inorganic salts such as K2SO4, ZnSO4, MnSO4, MgSO4, CuSO4, and FeSO4 were added to the optimized medium to study the pair of elements and impact of compound production.
Table 3. Metabolism of S. albidoflavus during fermentation in shaking flasks
|
Parameter |
Culture time (h) | ||||||||||
| 0 | 12 | 24 | 36 | 48 | 60 | 72 | 84 | 96 | 108 | 120 | |
| Total sugar (mg/mL)
|
84.7 | 78.5 | 76.1 | 64.2 | 50.7 | 40.3 | 34.3 | 27.6 | 19.8 | 14.2 | 11.3 |
| Reducing sugar (mg/mL)
|
0 | 3.2 | 4.8 | 5.6 | 15.7 | 18.4 | 20.3 | 17.5 | 14.3 | 8.9 | 8.3 |
| Amino nitrogen (mg/mL)
|
0.63 | 0.61 | 0.62 | 0.59 | 0.56 | 0.50 | 0.38 | 0.15 | 0.12 | 0.11 | 0.09 |
|
pH value |
6.4 | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 | 6.1 | 5.9 | 5.9 | 5.3 | 5.3 |
| Dry mycelium weight (mg/mL)
|
0 | 4.1 | 6.3 | 14.5 | 19.3 | 20.9 | 20.9 | 22.4 | 21.7 | 19.5 | 18.1 |
The results showed that the yield of the bioactive compounds was increased after adding proper amount of MnSO4 in the medium; while the yield of agricultural anti-SN03 was decreased after adding ZnSO4, when the content exceeded 0.02%, the fermenting cells could not produce the bioactive compound. After adding CuSO4, the bacteria could not produce compound; after adding FeSO4 and K2SO4, the capacity of the cells was reduced to some extent; after adding MgSO4, there was no effect on the yield of extract (Table 2).
Biochemical changes during fermentation: Biochemical analysis during fermentation process showed a stable production of secondary metabolites. Results showed in (Table 3), after inoculation to the fermentation medium, the reducing sugar content increases with the degradation of the starch. After that, the content of reducing sugar decreased due to the growth of mycelium and energy activities such as metabolism. At the same time, carbon sources and nitrogen sources were continuously consumed, and the total sugar content and amino nitrogen content also showed a downward trend.
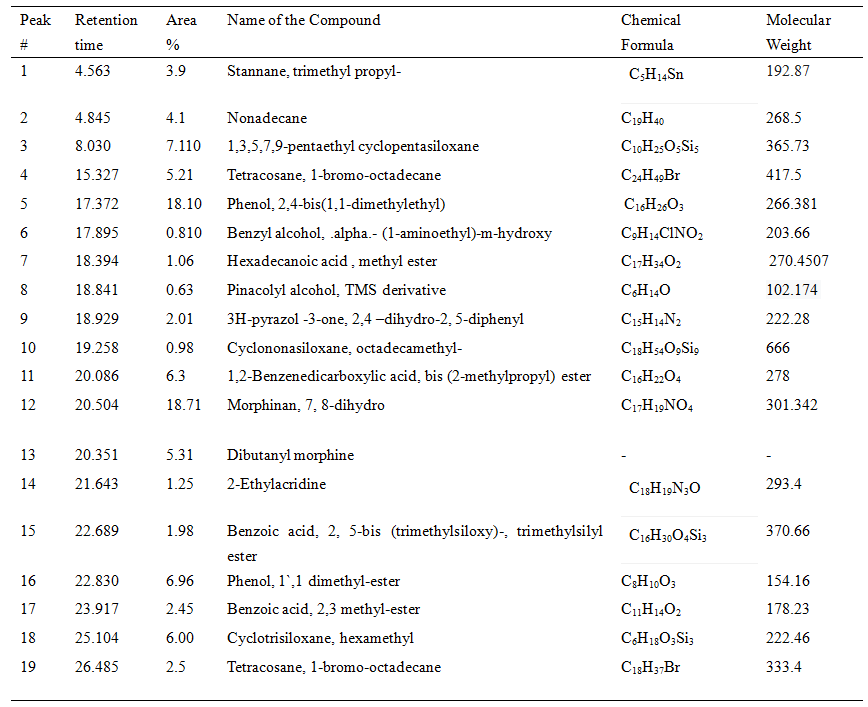
Table 4. GC-MS chromatograph of identified compounds from the extract of S. albidoflavus
The concentration of the bacteria increased continuously, and the growth of the bacteria reached a peak at 84 hours. After that, due to the large consumption of nutrients, the products inhibiting the metabolic activity of the bacteria continued to accumulate, the growth rate of the cells decreased, and the death of the growth phase was entered. From the utilization of the nitrogen source and the carbon source, both can satisfy the growth and metabolism requirements of the bacteria throughout the fermentation process, and therefore, no intermediate feed was required. During the fermentation process, the pH value decreases with the accumulation of secondary metabolites and other metabolites. So the results indicted a stable fermentation broth was manufactured.
Table 5. Inhibition Effects of S. albidoflavus and Test Antagonistic agents against Fusarium oxysporum f. sp. cubense
| Antifungal Biocontrol Agents | Concentration (Cfu/ ml) | Colony diameter (mm) | Inhibitory rate (%) | Significance of
Differences |
| 5% 1% | ||||
| Bacillus subtilis
|
1.0×10 9 | 9.92 | 92.65 | a A |
| Streptomyces albidoflavus
|
1.0×10 9 | 10.79 | 89.53 | a A |
| Trichoderma harzianum
|
1.0×10 9 | 29.88 | 71.42 | b B |
| Paenibacillus polymyxas
|
1.0×10 9 | 22.97 | 83.09 | bc BC |
| Bacillus licheniformis
|
1.0×10 9 | 26.76 | 74.97 | bcd BCD |
| Streptomyces cacaoi
|
1.0×10 9 | 13.07 | 91.83 | cd CD |
| Bacillus laterosporus
Bacillus mucilaginosus |
1.0×10 9
1.0×10 9
|
19.56
13.00 |
80.11
91.00 |
cd CD
d D |
Separation, Purification, and identification of compound: Fermented broth was separated by solvent extraction with the 1:1 ratio. Among four solvents (Ethyl acetate, Diethyl ether, Ethanol, Methanol, and N-Butanol) Diethyl ether had significant antifungal effects as in (Fig-2). The inhibition zone was 20 mm while all other low inhibition effects. Selected this potent extract for Silica gel column chromatography. Silica gel column chromatography purified fractions were analyzed by antifungal activity and selected the most potent for further analysis. At different concentrations of the potent fraction (0.5, 2, 4, 6, 8, 10 µg/ml) antifungal activity was varies.
As the concentration of fraction increased from 0.5 to 10 there was increased in the activity as shown in (Fig-3). At 0.5 10 µg/ml the inhibition zone was 6mm and later on 10 10 µg/ml inhibition zone was 22.5mm. The data presented in the graph was mean of three replicates. Later on Silica gel column chromatography purified potent fraction was selected for biochemical profile identification by GC-MS technique. There were 19 compounds identified in GC-MS spectrometer profile as shown in (Fig-4). On the base of area percent, there were two compounds considered as a major composite in this extract. Morphinan, 7, 8-dihydro and Phenol, 2, 4-bis (1, 1-dimethylethyl) with the area percent of 18.71 and 18.10 respectively (Table 4).
Inhibition Effects of S. albidoflavus and Test Antagonistic agents against Fusarium: Among these seven agents (including S. albidoflavus) of biocontrol in the inhibition test, almost all of them exhibited potent inhibitory effects against F. oxysporum, while Bacillus subtilis had exhibited the most significant effect at the inhibiting rate of 94.26 %, whereas S. albidoflavus displayed the effects of inhibition rate of 90%. As it was observed the second most potent antagonistic agent (Table 5). From the results it indicated this novel strain have potent effects against Fusarium oxysporum f. sp. cubense by comparison to other test antagonists.
In this study, a soil bacterial isolate were screened against Fusarium oxysporum f. sp. Cubense, the causal agent of Fusarium wilt in Banana. Molecular analysis indicated the strainS128 belongs to Streptomyces bacteria identified as S. albidoflavus. It indicated from the results (Fig-1), concerned strain had potent antifungal effects against F. oxysporum. In previous studies indicated that, Actinomycetes are the major source of bioactive compounds (Hug et al. 2018).
Several studies founded that Streptomyces can control the fungus phytopathogens likewise Rhizoctonia solani (tobacco target spot) (Ahsan et al. 2017), Ginseng damping-off (Van et al. 2017) and Streptomyces plicatus on the oomycete Phytophthora capsici (Chen et al. 2016). So this novel Streptomyces strain could be potent biocontrol antagonist against Fusarium oxysporum f. sp. Cubense. Recently reported that Streptomyces sp. AC-19 and Bacillus sp. BS-20 were successfully controls the Banana Fusarium wilt (Anusha, et al. 2019).
For the production of active compounds in fermentation batch the best combination of factors in the fermentation medium was as followed, soluble starch 4%, peanut flour 2%, (NH4)2SO40.2%, CaCO3 0.8%, and NaCl 0.8%. The results indicated that it is necessary to make a well-balanced combination to develop the bioactive compounds. Well, balanced combination of nutrients helps out to produce maximum yield of active compounds. Optimization of nutrients not only caused in an increased of efficiency but also give the understating of nutrient components (Gao et al. 2016).
From the results, it was indicated that orthogonal design helps out to optimize the best nutrient parameter in less experiment for the production of active compounds. Experimental designs decrease the labor and cost to produce active substance from submerged fermentation. During the process of fermentation statistical designs, not only reduced the cost and work but also improved the quality and production (Elibol 2004). Solvent extracts from fermented broth showed efficacy against the pathogen. The strong activity exhibited by Diethyl ether. As diethyl ether are virtuous solvents for a comprehensive range of polar and nonpolar organic compounds (Ouellette and Rawn 2015).
Potent extract of Diethyl ether from fermented broth of strain further purified by silica gel column chromatography. Several fractions assayed against the pathogen F. oxysporum then selected the most potent fraction. The purified fraction exhibited inhibition zone against the pathogen, which indicated from the results that the strain have the potential to control the pathogen. In earlier reports investigated that Streptomyces strain could control the soil borne fungus pathogens (Anusha, et al. 2019).
The current study indicated that purified fractions from S. albidoflavnous produced 19 compounds. Production of antibiotic substances greatly depends on natural resources. In natural resources, Streptomyces is a major source of bioactive compounds for pharmaceutical products (Jakubiec et al. 2018). Among 19 different compounds, there are 2 compounds of phenol were identified, one of them had a high area of percentage (18.10) Phenol, 2,4-bis(1,1-dimethylethyl) 18.0 while other had low (6.96) Phenol, 1`,1 dimethyl-ester. Phenol compounds have potent antimicrobial effects (Al-Youssef and Hassan 2015). GC-MS analysis revealed that Phenol, 2,4-bis(1,1-dimethylethyl) could be an active compound as it constitutes the major portion of this extract.
This compound previously reported as antimicrobial to combat biofilm formation (Padmavathi et al. 2014). From the GC-MS analysis, there was another compound Morphinan, 7, 8-dihydro with the highest 18.71 area percentage was investigated. As previously reported that derivatives of morphine have no antimicrobial effects, but caused mammalian seizures (Jalodia et al. 2018). From the results, it indicated that S. albidoflavus had significant effects on the pathogen. In conclusion, S. albidoflavus is a strong antagonistic agent against Fusarium oxysporum fungus and could have broad-spectrum capability to control Fusarium wilt in banana.
CONCLUSION
This study concluded that Streptomyces albidoflavus identified a novel antagonistic agent against soil borne fungus pathogen Fusarium oxysporum f. sp. Cubense. This strain might be introduced as an effective biocontrol agent for sustainable agriculture. The results indicated that, extract from the Streptomyces strain would be a substitute to chemical substances for the disease management of Banana Fusarium wilt. S. albidoflavus strain have broad spectrum potential against fungus pathogens.
Competing Interest : There is no Competing interest.
Funding: Supported by Plant Pathology lab, College of Plant Protection, Shenyang Agricultural University, Shenyang 110866, Liaoning, China
Authors’ contributions: TA; did the experiments, write, analyze and drafting of the manuscript, WY; plan the work, supervise the work, provide funds, analyze, and proof read. All the authors have read and approved the manuscript”, and ensure that this is the case.
ACKNOWLEDGEMENTS
A special thanks to Professor Dr. Wu Yuanhua, College of Plant Protection, Shenyang Agricultural University, Shenyang 110866, Liaoning China for providing the Pathogens and Antagonistic agents.
REFERENCES
Ahsan, T., J. Chen, Y. Wu and M. Irfan (2017). Application of response surface methodology for optimization of medium components for the production of secondary metabolites by Streptomyces diastatochromogenes KX852460. AMB Express 7(1): 96.
Ahsan, T., J. Chen, Y. Wu, M. Irfan and J. Shafi (2017). Screening, identification, optimization of fermentation conditions, and extraction of secondary metabolites for the biocontrol of Rhizoctonia solani AG-3. Biotechnology & Biotechnological Equipment 31(1): 91-98.
Ahsan, T., J. Chen, X. Zhao, M. Irfan and Y. Wu (2017). Extraction and identification of bioactive compounds (eicosane and dibutyl phthalate) produced by Streptomyces strain KX852460 for the biological control of Rhizoctonia solani AG-3 strain KX852461 to control target spot disease in tobacco leaf. AMB Express 7(1): 54.
Al-Youssef, H. M. and H. Hassan (2015). Antimicrobial and antioxidant aculeata and chemical compo.
Anusha, B., S. Gopalakrishnan, M. Naik and M. Sharma (2019). “Evaluation of Streptomyces spp. and Bacillus spp. for biocontrol of Fusarium wilt in chickpea (Cicer arietinum L.) Archives of Phytopathology and Plant Protection 52(5-6): 417-442.
Awla, H. K., J. Kadir, R. Othman, T. S. Rashid and M.-Y. Wong (2016). Bioactive compounds produced by Streptomyces sp. isolate UPMRS4 and antifungal activity against Pyricularia oryzae. American Journal of Plant Sciences 7(7): 1077-1085.
Barka, E. A., P. Vatsa, L. Sanchez, N. Gaveau-Vaillant, C. Jacquard, H.-P. Klenk, C. Clément, Y. Ouhdouch and G. P. van Wezel (2016). Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 80(1): 1-43.
Bubici, G., M. Kaushal, M. I. Prigigallo, C. Gómez-Lama Cabanás and J. Mercado-Blanco (2019). Biological control agents against Fusarium wilt of banana. Frontiers in microbiology 10: 616.
Chater, K. F. (2016). Recent advances in understanding Streptomyces.F1000 Research 5.
Chen, Y.-Y., P.-C. Chen and T.-T. Tsay (2016). The biocontrol efficacy and antibiotic activity of Streptomyces plicatus on the oomycete Phytophthora capsici. Biological control 98: 34-42.
Dale, J., A. James, J.-Y. Paul, H. Khanna, M. Smith, S. Peraza-Echeverria, F. Garcia-Bastidas, G. Kema, P. Waterhouse and K. Mengersen (2017). Transgenic Cavendish bananas with resistance to Fusarium wilt tropical race 4. Nature communications 8(1): 1-8.
Dhayakaran, R. and S. Neethirajan (2017). Microscopic methods in biofilm research. Biofilms: Emerging Concepts and Trends, Murthy, S.(Ed.) Narosa Publishers, İndia.
Dita, M., M. Barquero, D. Heck, E. S. Mizubuti and C. P. Staver (2018). Fusarium wilt of banana: current knowledge on epidemiology and research needs toward sustainable disease management.Frontiers in plant science 9: 1468.
Elibol, M. (2004). Optimization of medium composition for actinorhodin production by Streptomyces coelicolor A3 (2) with response surface methodology Process Biochemistry 39(9): 1057-1062.
Gao, X., Q. He, Y. Jiang and L. Huang (2016). Optimization of nutrient and fermentation parameters for antifungal activity by Streptomyces lavendulae Xjy and its biocontrol efficacies against Fulvia fulva and Botryosphaeria dothidea. Journal of Phytopathology 164(3): 155-165.
Gomathi, A. and K. M. Gothandam (2019). Investigation of anti‐inflammatory and toxicity effects of mangrove‐derived Streptomyces rochei strain VITGAP173 Journal of cellular biochemistry 120(10): 17080-17097.
Gomes, F., P. Teixeira, N. Cerca, J. Azeredo and R. Oliveira (2011). Effect of farnesol on structure and composition of Staphylococcus epidermidis biofilm matrix. Current microbiology 63(4): 354.
Hug, J. J., C. D. Bader, M. Remškar, K. Cirnski and R. Müller (2018). Concepts and methods to access novel antibiotics from actinomycetes. Antibiotics 7(2): 44.
Jakubiec-Krzesniak, K., A. Rajnisz-Mateusiak, A. Guspiel, J. Ziemska and J. Solecka (2018). Secondary metabolites of actinomycetes and their antibacterial, antifungal and antiviral properties.Pol. J. Microbiol 67(3): 259-272.
Jalodia, R., J. Meng, M. Sharma, S. Ramakrishnan and S. Roy (2018). Morphine dysregulates Paneth cell antimicrobial peptide secretion in a TLR2 dependent manner, Am Assoc Immnol.
Jayaprakashvel, M., C. Chitra and N. Mathivanan (2019). Metabolites of Plant Growth-Promoting Rhizobacteria for the Management of Soilborne Pathogenic Fungi in Crops. Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms, Springer: 293-315.
Latha, S., G. Sivaranjani and D. Dhanasekaran (2017). Response surface methodology: A non-conventional statistical tool to maximize the throughput of Streptomyces species biomass and their bioactive metabolites. Critical reviews in microbiology 43(5): 567-582.
Neu, T. R. and J. R. Lawrence (1999). Lectin-binding analysis in biofilm systems. Methods in enzymology, Elsevier. 310: 145-152.
Padmavathi, A. R., B. Abinaya and S. K. Pandian (2014). Phenol, 2, 4-bis (1, 1-dimethylethyl) of marine bacterial origin inhibits quorum sensing mediated biofilm formation in the uropathogen Serratia marcescens. Biofouling 30(9): 1111-1122.
Ploetz, R. C. (2015). Management of Fusarium wilt of banana: A review with special reference to tropical race 4. Crop Protection 73: 7-15.
Rajaofera, M. J. N., Y. Wang, G. Y. Dahar, P. Jin, L. Fan, L. Xu, W. Liu and W. Miao (2019). Volatile organic compounds of Bacillus atrophaeus HAB-5 inhibit the growth of Colletotrichum gloeosporioides. Pesticide biochemistry and physiology 156: 170-176.
Rajnisz, A., A. Guśpiel, M. Postek, J. Ziemska, A. Laskowska, D. Rabczenko and J. Solecka (2016). Characterization and optimization of biosynthesis of bioactive secondary metabolites produced by Streptomyces sp. 8812. Polish journal of microbiology 65(1): 51-61.
Siamak, S. B. and S. Zheng (2018). Banana Fusarium wilt (Fusarium oxysporum f. sp. cubense) control and resistance, in the context of developing wilt-resistant bananas within sustainable production systems Horticultural Plant Journal 4(5): 208-218.
Singh, M., A. Kumar, R. Singh and K. D. Pandey (2017). Endophytic bacteria: a new source of bioactive compounds. 3 Biotech 7(5): 315.
Tiwari, V., R. Roy and M. Tiwari (2015). Antimicrobial active herbal compounds against Acinetobacter baumannii and other pathogens. Frontiers in microbiology 6: 618.
Van, N. M., E.-E. Woo, G.-S. Lee, D.-W. Ki, I.-K. Lee, S.-Y. Lee, K. Park, J. Song, J. E. Choi and B.-S. Yun (2017). Control Efficacy of Streptomyces sp. A501 against Ginseng Damping-off and Its Antifungal Substance. Mycobiology 45(1): 44-47.


