Department of Biotechnology, Maharaja Sriram Chandra Bhanja
Deo University, Baripada, Odisha, India
Corresponding author email: brath_2000@yahoo.com
Article Publishing History
Received: 26/01/2022
Accepted After Revision: 29/03/2022
Lichens appear to be a promising source of antioxidants due to presence of numerous metabolites that can reduce free radicals. The Lichen species were obtained from Similipal biosphere Reserve (SBR). The antioxidant activity was carried out by DPPH, H2O2, FRAP scavenging assay and further TPC and TFC was also estimated. Among the lichens tested, Dirinaria applanata exhibited strong antioxidant activities than Parmotrema andium. The methanol and acetone extracts of D. applanata showed DPPH radical scavenging activities (IC50 value) as 471.16±0.85μg/ml and 519.79±1.29μg/ml whereas in P. andium IC50 value was 534.77±0.75μg/ml and 600.77±0.95μg/ml respectively. Similar result was also observed in H2O2 scavenging assay and FRAP. An interesting strong relationship between total phenolic and flavonoid contents and their antioxidant activities in both the Lichen species was marked as determined with respect to gallic acid and quercetin equivalents. The results indicates that the selected lichen species possess significant antioxidant activity which may be utilizedas novel sources of natural antioxidant compounds.
Lichens, Parmotrema andium,Antioxidant.
Sahoo B, Dash S, Parida S, Pradhan S, Rath B. In vitro Antioxidant Activities of Lichen Species Dirinaria applanata and Parmotrema andium Collected from Similipal Biosphere Reserve, India. Biosc.Biotech.Res.Comm. 2022;15(1).
Sahoo B, Dash S, Parida S, Pradhan S, Rath B. In vitro Antioxidant Activities of Lichen Species Dirinaria applanata and
Parmotrema andium Collected from Similipal Biosphere Reserve, India. Biosc.Biotech.Res.Comm. 2022;15(1). Available from: <a href=”https://bit.ly/3KqQbWi“>https://bit.ly/3KqQbWi</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Lichen is a symbiotic organism that consists of a fungus (mycobiont) and a photosynthetic partner (photobiont) and are important constituents of many ecosystems (Obohv and Ademosun 2006; Bates et al. 2011; Hawksworth et al. 2020). Lichens are unanimously distributed in diverse climatic conditions spanning from the plains to the high mountains and from polar regions to the tropics and are susceptible to a variety of environmental stress (Felczykowska et al. 2017).
As a result, they produce different secondary metabolites including some specific such as terpenes, depsides, depsidone, dibenzofuran, and xanthone which are unique to lichen species (Olivier-Jimenez et al. 2019; Stanojković 2019; Sabarwati et al. 2020). The secondary metabolites of lichen contain bioactive substances which have manifold biological activities such as antimicrobial, anticancer anti-inflammatory and antioxidant properties (Maulidiyah et al. 2016; Mohammadi et al. 2020; Mohan et al. 2020; Nugraha et al. 2020; Studzińska-Sroka et al. 2021).
Antioxidants are compounds that can retain the quality of foods by delayed process of oxidation and protect from damage caused by free radical induced oxidative stress (Souri et al. 2008). Synthetic antioxidants such as butylated hydroxyl anisole (BHA), butylated hydroxyl toluene (BHT), tertbutylhydroquinone (TBHQ) and propyl gallate (PG) are widely accepted, but their use is being restricted nowadays due to their toxic and harmful side effects (Zhang et al. 2009; Maulidiyah et al. 2021). Thus, at present there is a growing interest towards natural antioxidants. So in the search for novel natural antioxidant sources, our target is aimed on lichens found in Similipal Biosphere Reserve (SBR) as it harbours a large variety of lichen species. Thus, the purpose of the present work is to assess the antioxidant activity of acetone and methanol extract of the lichens obtained from this region. Our investigation is the first report describing the evaluation of Similipal Biosphere Reserve lichen species as a source of natural antioxidant.
MATERIAL AND METHODS
For the collection of lichen samples, two lichen species, Dirinaria applanata and Parmotrema andium as shown in fig.1 were collected from various localities within Similipal Biosphere Reserve (SBR). Herbarium specimen of each species was maintained in research laboratory, Department of Biotechnology, Maharaja Sriram Chandra Bhanja Deo University, Baripada, Odisha for experimental purpose. The lichen species were identified following available monographs and standard methods (Awasthi 1988; Awasthi 1991; Orange et al. 2001). For the preparation of lichen extract, dried lichen samples were milled into fine powder using a sterile mortar and pestle. The powered form of material was moved through the sieve (75 microns).10 g of dry fine powder of sample was mixed with 100 ml of solvent (acetone and methanol) on an orbital shaker and filtered using Whatman no. 1 filter paper. Then it was vaporised in a rotary evaporator at 45°C and preserve in deep freeze for subsequent use.
For the DPPH radical scavenging activity, the antiradical activity of two test lichen extracts was evaluated by DPPH (1,1-diphenyl-2-picryl-hydrazil) assay (Kosanic et al. 2011). One ml of DPPH solution (0.1mM) was added to 3 ml of different concentration (100-1000 µg/ml) of lichen extract and the mixture was kept for 30 min at room temperature and the absorbance was recorded at 517 nm using UV-Vis spectrophotometer (Systronics-119). Ascorbic acid was used as positive control. For the hydrogen peroxide (H2O2) scavenging assay, the spectrophotometric analysis of H2O2 scavenging activity was determined following the method of Ruch et al. (1989). H2O2 solution was prepared in PO4 buffer (1 M, pH 7.4) and 0.6 ml was added to the lichen extract of various concentrations (100-1000μg/ml).
The optical density was recorded at 230 nm after 10 min. Ascorbic acid was used as standard against the test extract. The DPPH/ H2O2 radical scavenging activity was calculated by the following equation:
% activity = [(control Abs – sample Abs) / control Abs] x100
IC50 values were derived from the percentage of inhibition versus concentration plot and expressed in μg/ml.A lower IC50 meant better radical scavenging activity.
For the ferric reducing antioxidant power assay (FRAP), the ferric reducing antioxidant power of the tested lichen species was estimated according to the method of Oyaizu (1986). Various concentration of lichen extract (100, 250, 500, 750, 1000μg/ml) was added with 2.5 ml of PO4 buffer (pH 6.6, 0.2M) and potassium ferricyanide (1%) and was kept at 50℃ for 25 minutes. Then it was mixed with trichloro acetic acid (10%) and centrifuged at 3000g for 20 minutes. Finally, the obtained supernatant was thoroughly mixed with 2.5 ml distilled water alongwith 0.5 ml FeCl3 (0.1%) and recorded at 700nm. Butylated hydroxyl toluene is used as positive control for the experiment. Total phenolic content (TPC) was estimated by Folin-Ciocalteu reagent following the method of Taga et al. (1984).
The lichen extract (100µl) was mixed with 2ml of Sodium carbonate (2%). After 10 min, 500 μl of Folin’s reagent was supplemented and then the reaction mixture was kept under dark for 20 minutes and the absorbance was measured at 650 nm. The result was expressed as µg GAE /g dry extract. Total flavonoid content (TFC) was determined according to the method of Zhishen et al. (1999). One ml of the lichen extract was mixed with 500 μl of Sodium nitrite and Aluminum chloride (10%c 300 μl). After 10 min, Sodium hydroxide (1M, 1ml) was mixed and the volume was made up to 5ml with distilled water. Then the mixture was incubated for 30 min and the absorbance was read at 510nm. The result was expressed as quercetin equivalent i.e µg QE /g dry extract.
RESULTS AND DISCUSSION
DPPH and H2O2 scavenging assay: The scavenging of DPPH and H2O2 radicals by the lichen extracts was determined and shown in table1.The test lichen species extracts exhibited differential scavenging ability. However, methanol extract of D. applanata has strong DPPH and H2O2 scavenging activity with IC50 i.e., 471.16±0.85 µg/ml and 554.57±0.90 µg/ml respectively. The lowest IC50 of DPPH and H2O2 was recorded in acetone extract of both the species. These results are in agreement with the literature where it was reported that many lichen extracts has their ability to scavenge free radicals (Behera et al. 2009; Manojlović et al. 2012; Hawrył et al. 2020).
Table 1. DPPH radical and H2O2 scavenging activity of methanol and acetone extract of Dirinaria applanata and Parmotrema andium
| Lichen species | Solvent | DPPH radical scavenging IC50(µg/ml) | H2O2 scavenging activity IC50 (µg/ml) |
| Dirinaria applanata | Methanol | 471.16±0.85 |
554.57±0.90
|
| Acetone | 519.79±1.29 |
707.74±0.79
|
|
| Parmotrema andium
|
Methanol | 534.77±0.75 |
592.25±0.93
|
| Acetone | 600.77±0.95 |
755.34±0.86
|
|
| Gallic acid (standard) | 326.61±0.94 |
341.52±0.87
|
|
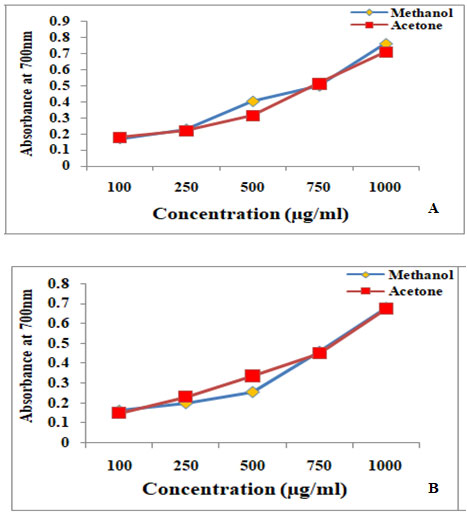
Ferric reducing antioxidant power assay (FRAP): The experimental result on assay of Ferric reducing power of two lichen species was represented in fig 1.
Figure 1: (A-B) Lichens collected from Similipal Biosphere Reserve (SBR)
The results infer that the higher absorbance (700nm) of lichen extracts results in higher reducing power. Accordingly, more reducing power was recorded in methanolic extract of Dirinaria applanata (absorbance: 0.762±0.0008) as compared to Parmotrema andium (absorbance:0.673±0.0008). Comparing this result it was found that Dirinaria applanata showed more promising antioxidant activity than Parmotrema andium. Likewise, Smitha et al. (2016) reported comparative reducing power activity with ethanol and methanol extracts of the Ramalina pacifica which was less when compared with Rocella montagnei. Similarly, the maximum reducing power was detected in methanol extract of P.centrifuga (Ranković and Kosanić, 2019; Aoussar et al. 2020; Hawrył et al. 2020).
Figure 2: Ferric reducing antioxidant power assay of A. Dirinaria applanata and B. Parmotrema andium
Total Phenolic and Flavonoid content (TPC and TFC): TP and TF content of methanol and acetone extracts in both the lichen species was determined in terms of gallic acid equivalent (µg GAE/ g of extract) and quercetin equivalent (µg QE/ g of extract) respectively and the findings was summarized in table 2. Maximum phenolic content was recorded in methanolic extract of Dirinaria applanata (68.96±0.60 μg/ml) while acetone extracts of Parmotrema andium showed the lowest phenolic content (48.56±0.97μg/ml) (Manojlović et al. 2021).
Similarly high flavonoid content was also found in Dirinaria applanata, 38.22±0.89 μg/ml as compared to Parmotrema andium (27.57±1.03 μg/ml). Both Phenolic and flavonoid compounds have antioxidant characteristics due to their redox properties, which can assist in the absorption and neutralisation of free radicals quenching of singlet and triplet oxygen and the decomposition of peroxides. Thus, total phenolic compounds in Dirinaria applanata were found to be more encouraging with higher level of TPC and TFC (Manojlović et al. 2021).
Table 2. TPC and TFC of Dirinaria applanata and Parmotrema andium
| Lichen Species | Methanol | Acetone | |
| TPC(µg/ml) | |||
| Dirinaria applanata | 68.96±0.60 | 57.24±0.96 | |
| Parmotrema andium | 59.82±0.85 | 48.56±0.97 | |
| TFC(µg/ml) | |||
| Dirinaria applanata | 38.22±0.89 | 30.79±0.82 | |
| Parmotrema andium | 32.54±0.53 | 27.57±1.03 | |
It was understood and established that the antioxidant activity might be cumulative effect of different natural components and not solely of its fractions. In our result, the antioxidant activity of the Dirinaria applanata and Parmotrema andium extract might be the resultant synergistic effect of various compounds present within the extract and more interestingly encouraging antioxidant potential of Dirinaria applanata is due to the associated action which contributes a higher antioxidant activity in its extracts (Sargsyan et al. 2021).
CONCLUSION
The findings of the present study demonstrate that methanol extract of Dirinaria applanata has impressive antioxidant activities in vitro. In summary, the experimental results conclude that Dirinaria applanata and Parmotrema andium may act as an agent of potential natural sources of antioxidants. Results indicated that probably the higher amount of phenolic compounds in the lichen extracts are responsible for encouraging antioxidant properties that would be of interest in food and pharmaceutical industry. Hence further work needs to be carried out to isolate and purify the active components from these species to determine their antioxidant activity.
ACKNOWLEDGEMENTS
The laboratory facilities were provided by the Department of Biotechnology, Maharaja Sriram Chandra Bhanja Deo University.
Conflict of Interests: Authors declare no conflicts of interests to disclose.
Data Availability Statement: The database generated and /or analysed during the current study are not publicly available due to privacy, but are available from the corresponding author on reasonable request.
REFERENCES
Aoussar, N., Laasri, F.E., Bourhia, M., et al. (2020). Phytochemical analysis, cytotoxic, antioxidant, and antibacterial activities of lichens. Evidence-Based Complementary and Alternative Medicine, 2020. https://doi.org/10.1155/2020/8104538.
Awasthi, D.D., (1988). A key to the macro lichens of India and Nepal, The Journal of the Hattori Botanical Laboratory, 65, pp.207-302. https:// eurekamag .com/ research / 006/ 944/ 006944869. php.
Awasthi, D.D., (1991). Key to the microlichens of India, Nepal and Sri Lanka, J. Cramer,40, pp.1-336.http://prr.hec.gov.pk/jspui/bitstream/123456789/11989/1/Asmatullah%20botany %202019%20hazara%20uni%20prr.pdf.
Bates, S.T., Cropsey, G.W., Caporaso, J.G., et al. (2011). Bacterial communities associated with the lichen symbiosis, Applied and environmental microbiology, 77,(4), pp.1309-1314. https://doi.org/10.1128/aem.02257-10.
Behera, B.C., Verma, N., Sonone, A., et al. (2009). Optimization of culture conditions for lichen usneaghattensis G. Awasthi to increase biomass and antioxidant metabolite production, Food Technology and Biotechnology, 47, (1), pp.7-12. https://hrcak.srce.hr/file/52602.
Felczykowska, A., Pastuszak-Skrzypczak, A., Pawlik, A., et al. (2017). Antibacterial and anticancer activities of acetone extracts from in vitro cultured lichen-forming fungi. BMC complementary and alternative medicine, 17, (1), pp.1-12. https://dx.doi.org/10. 1186%2Fs12906-017-1819-8.
Hawksworth, D.L., and Grube,M., (2020). Lichens redefined as complex ecosystems. New Phytol, 227, (5), pp.1281. https://doi.org/10.1111/nph.16630.
Hawrył A, Hawrył M, Hajnos-Stolarz A, et al. (2020). HPLC fingerprint analysis with the antioxi- dant and cytotoxic activities of selected lichens combined with the chemometric calculations. Molecules 25, (18), pp. 4301. https://doi.org/10.3390/molecules25184301.
Kosanić, M., Ranković, B., and Vukojević, J., (2011). Antioxidant properties of some lichen species, Journal of food science and technology, 48, (5), pp.584-590. https://dx.doi.org/10.1007%2Fs13197-010-0174-2.
Manojlović, N., Ranković, B., Kosanić, M., et al. (2012). Chemical composition of three Parmelia lichens and antioxidant, antimicrobial and cytotoxic activities of some their major metabolites, Phytomedicine, 19, (13), pp.1166-1172, doi: 10.1016/j.phymed.2012.07.012.
Manojlović, N.T., Rančić, A.B., Décor, R., et al. (2021). Determination of chemical composition and antimicrobial, antioxidant and cytotoxic activities of lichens Parmelia conspersa and Parmelia perlat, Journal of Food Measurement and Characterization, 15, (1), pp.686-696. https://doi.org/10.1007/s11694-020-00672-1.
Maulidiyah, I., Watu, M., and Nurdin, M., (2016). Secondary metabolites identification from Lichen Usnea longissima Ach: Bioactivity test of antibacterial, international journal of applied chemistry, 12, (3), pp.347-357. https://www.ripublication.com/ijac16/ijacv12n3_16.pdf.
Maulidiyah, M., Akhmad, D., Usman, U., et al. (2021). Antioxidant activity of secondary metabolite compounds from lichen Teloschistes flavicans,11, (6), pp.13878–13884. https://doi. org/10. 33263/BRIAC116.1387813884.
Maurya, S.K., Gupta, Y.K., Smitha, T.T., et al. (2016). A new exact anisotropic solution of embedding class one, The European Physical Journal A, 52, (7), pp.1-12, http://dx.doi.org/10.1007/s12648-020-01884-3.
Mohammadi, M., Zambare, V., Malek, L., et al. (2020). Licheno chemicals: extraction, purification, characterization, and application as potential anticancer agents, Expert opinion on drug discovery, 15, (5), pp. 575-60. https://doi.org/10.1080/17460441.2020.1730325.
Mohan, C.D., Rangappa, S., Preetham, H.D., et al. (2020). Targeting STAT3 signaling pathway in cancer by agents derived from Mother Nature, In Seminars in cancer biology, Academic Press, 8, (9), 368. https://doi.org/10.1016/j.semcancer.2020.03.016.
Nugraha, A.S., Untari, L.F., Laub, A., et al. (2020). Anthelmintic and antimicrobial activities of three new depsides and ten known depsides and phenols from Indonesian lichen: Parmelia cetrata Ach, Natural product research, 35, (23), pp.1-10. https:// doi.org/10.1080/ 14786419. 2020.1761361.
Oboh, G., and Ademosun, A.O., (2006). Comparative studies on the ability of crude polyphenols from some Nigerian citrus peels to prevent lipid peroxidation—In vitro, Asian J Biochem, 1, pp.169-177. https://dx.doi.org/10.3923/ajb.2006.169.177.
Olivier-Jimenez, D., Chollet-Krugler, M., Rondeau, D., et al. (2019). A database of high-resolution MS/MS spectra for lichen metabolites, Scientific data, 6, (1), pp.1-11. https://www.nature.Com/articles/s41597-019-0305-1.
Orange, A., James, P.W. and White, F.J., (2001). Microchemical methods for the identification of lichens, British Lichen Society, 34, (2), pp.181. https://doi.org/10.1006/lich.2002.0376.
Oyaizu, M., (1986). Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine, The Japanese journal of nutrition and dietetics, 44, (6), pp. 307-315. http://dx.doi.org/10.5264/eiyogakuzashi.44.307.
Ranković, B., and Kosanić, M., (2019). Lichens as a potential source of bioactive secondary metabolites, In Lichen secondary metabolites, pp. 1-29. Doi: 10.1007/978-3-030-16814-8_1.
Ruch, R.J., Cheng, S.J., and Klaunig, J.E., (1989). Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea, Carcinogenesis, 10, (6), pp.1003-1008. https://doi.org/10.1093/carcin/10.6.1003.
Sabarwati, S.H., Harjuliarto, R., Watoni, A.H., et al. (2020). Antibacterial activity of usnic acid from Usnea longissima Ach, Pakistan Journal of Pharmaceutical Sciences, 33, (4), pp.1631-1639, Doi: 10.36721/PJPS.2020.33.4.REG.1631-1639.1.
Sargsyan, R., Gasparyan, A., Tadevosyan, G., et al. (2021). Antimicrobial and antioxidant potentials of non-cytotoxic extracts of Corticolous lichens sampled in Armenia, AMB Express, 11, (1), pp.1-11. https://doi.org/10.1186/s13568-021-01271-z.
Souri, E., AMIN, G.R., Jalalizadeh, H., et al. (2008). Screening of thirteen medicinal plant extracts for antioxidant activity, Iranian Journal of Pharmaceutical Research, 7, (2), pp.149-154.
Stanojković, T., (2019). Investigations of lichen secondary metabolites with potential anticancer activity. In Lichen secondary metabolites, pp. 155-174. Doi:10.1007/978-3-030-16814-8_5.
Studzińska-Sroka, E., Majchrzak-Celińska, A., Zalewski, P., et al. (2021). Lichen-Derived Compounds and Extracts as Biologically Active Substances with Anticancer and Neuroprotective Properties, Pharmaceuticals, 14, (12), p.1293. https://doi.org/10.3390/ph14121293.
Taga, M.S., Miller, E.E. and Pratt, D.E., (1984). Chia seeds as a source of natural lipid antioxidants, Journal of the American Oil Chemists’ Society, 61, (5), pp.928-931. https://doi.org/10.1007/BF02542169.
Zhang, Q., Streets, D.G., Carmichael, G.R., et al. (2009), Asian emissions in 2006 for the NASA INTEX-B mission, Atmospheric Chemistry and Physics, 9, (14), pp.5131-5153. https://doi.org/10.5194/acp-9-5131-2009.
Zhishen, J., Mengcheng, T. and Jianming, W., (1999), The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals, Food chemistry, 64, (4), pp.555-559. https://doi.org/10.1016/S0308-8146(98)00102-2.


