Division of Floriculture and Medicinal Crops, ICAR-Indian Institute of Horticultural Research, Hesaraghatta Lake post, Bangalore-89
Corresponding author Email: sindhurangaraju63@gmail.co.in
Article Publishing History
Received: 09/02/2019
Accepted After Revision: 12/03/2019
Jawahar Ashwagandha-20 (JA-20) and Arka Ashwagandha (AA) seeds were raised in in vitro conditions and phenotypic differences between the plants was recorded. The adventitious roots were derived from leaves of the two varieties in in vitro conditions. The effects of the strength of media and concentration and the combination of auxins (IAA and IBA) for adventitious roots multiplication using suspension culture were studied. After 30 days of suspension culture, root biomass was measured and HPLC analysis of major withanolides in leaves and adventitious roots was conducted. Arka Ashwagandha variety had higher total withanolide content of 1.621 mg/g, Withaferin A content of 1.362 mg/g and root yield of 4.066 g from 0.1g inoculum in 30 days compared to JA-20 which had total withanolide content of 1.156 mg/g, Withaferin A content of 0.930 mg/g on dry weight basis and root mass of 3.71g from 0.1g of inoculum in 30 days. The present study thus helps in the identification of an elite cultivar of Ashwagandha and development of a standard protocol for mass multiplication of adventitious root in hormone-free media. This is beneficial in the preparation of health supplements in terms of human health issues due to negligible residual effects of hormones in the final product.
Adventitious Root Culture, Ashwagandha, Ja 20, Iaa- Indole Acetic Acid, Iba- Indole-3-Butyric Acid
Rangaraju S, Lokesha A. N, Aswath C. R. Improved Production of Withanolides in Adventitious Root Cultures of Withania Somnifera by Suspension Culture Method. Biosc.Biotech.Res.Comm. 2019;12 (1).
Rangaraju S, Lokesha A. N, Aswath C. R. Improved Production of Withanolides in Adventitious Root Cultures of Withania Somnifera by Suspension Culture Method. Biosc.Biotech.Res.Comm. 2019;12(1). Available from: https://bit.ly/2HF3dSK
Copyright © Rangaraju et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
Ashwagandha (Withania somnifera Dunal) is a medicinal crop of commercial importance, belongs to family Solanaceae, and is considered as an alternate to Panax ginseng in its therapeutic values (CSIR.,1976). It is used for curing a wide range of diseases in Ayurveda and other indigenous systems of medicine for over 5000 years (Akram et al., 2011). The herb has the highest value for its pharmacological activity in preparation of various Ayurvedic formulations. It is anti-stress, anti aging, and aids in recovering from neurodegenerative disorders (Bhattacharya et al., 2002). It is a small, erect, branched, woody shrub that grows up to 1.50m tall. It is cultivated under rainfed condition in marginal soils by small and marginal farmers of Madhya Pradesh, Rajasthan, Gujarat, Andhra Pradesh, Karnataka, and other states. Apart from chemical constituents like alkaloids and withanolides, it also contains a variety of amino acids including aspartic acid, proline, tryptophan, tyrosine, cysteine, alanine, glycine, and a high amount of iron.
Withanolides are C-28 steroidal lactones (Alfonso et al., 1993). Major withanolides identifi ed include Withanolide A, Withanoside IV and VI (Tohda et al., 2005), Withaferin A (Oh et al., 2008), Withanosides IV and V (Matsuda et al., 2001) and Withanoside B (Pramanick et al., 2008). Ashwagandha is available in the Western world as a dietary supplement. Its also known as “Indian Ginseng” and “Winter Cherry”. Withanolide A has strong neuro pharmacological properties of promoting outgrowth, synaptic reconstruction, and a potential to reconstruct neural networks (Kuboyama et al., 2005; Tohda et al., 2005 a,b). Withaferin A inhibits angiogenesis (Mohan et al., 2004), metastasis (Misico et al., 2002). The major pharmacological activity of Withania somnifera is contributed to two major withanolides, Withaferin and Withaferin D (Gupta et al., 2007, Sindhu et al., 2018).
Arka Ashwagandha is a variety identifi ed at ICARIndian Institute of Horticulture Research, for high dry root yield and high total withanolide content. The variety has double the dry root yield (10 q/ha) than JA-20 (5.27q/ha). The other signifi cant features are early vigor, field tolerance to bacterial wilt, late blight, leaf spot diseases and pests (Epilachna beetle, mites and aphids). It matures in 180 days and is characterized by desired root thickness and depth. The distinguishing features of the variety are lengthy tertiary branch, thick stem which has dense curved pubescence, lanceolate leaves with obtuse leaf tip, bigger fruit capsules and fruits. JA-20 is a released variety from MPKVV, Mandsaur, Madhya Pradesh used as a check in AICRP National trials and it yields about 5 q/ha.
Recent advances in tissue culture methodologies have improved secondary metabolite production across various medicinal plants. Selection of high bioactive producing lines, optimization of culture conditions, metabolic engineering, elicitation strategies and use of bioreactor culture systems has made the production of useful metabolites in vitro at a shorter duration of time (Sarin et al., 2005). Plant-specific metabolites can be effectively obtained from organ and plant culture systems (Verpoorte et al., 2002). Plant cell and organ culture systems are promising methodologies as they aid in rapid proliferation of cells/organs, condensed biosynthetic cycles in comparison to fi eld grown plants (Ramachandra Rao and Ravishankar, 2002, Thanh Tam et al., 2019).
Adventitious roots suspension cultures are found to be ideal for biomass accumulation in Echinacea purpurea (Wu et al., 2007) and P. notoginseng (Gao et al., 2005). Recent studies indicate that explant type and genotype affect the accumulation of bioactive compounds in adventitious root cultures of Polygonum multifl orum (Ho et al.,2019) Lack of post-harvest storage technology for roots (Govil et al., 1993; Singh and Kumar., 1998), excessive exploitation of natural resources, problems in field cultivation as it is dependent on monsoon, time consuming and laborious are reasons enough to multiply adventitious roots of Withania somnifera in suspension culture which meet the global market requirement of Ashwagandha.
Materials and Methods
Seeds of Withania somnifera like JA-20 and Arka Ashwagandha were selected. Seed pretreatment was conducted as per our earlier reports (Sindhu et al., 2018), morphological and phenotypic examination of 30-day old plants was done. The adventitious roots were induced in these two varieties, which were then harvested from 10-day old leaf culture bottles supplemented with auxins. These roots were washed with sterile distilled water two times to remove the small traces of agar followed by treatment for 1 minute with 3% sodium hypochloride, then the traces of sodium hypochlorite from roots were removed by washing again with sterile distilled water. The roots were dried by blotting with sterile tissue paper. The known quantity of roots (100 mg) were weighed and transferred into 100 ml of full strength MS liquid media supplemented with different concentration and a combination of auxins in conical fl asks with 3% sucrose concentration under 16 hours of photoperiod and placed in an orbital shaker at 90 rpm at 25°C. The mass of multiplied roots was observed after 30 days of inoculation and was compared with the control.
Extraction of bioactive principles from W. somnifera: The adventitious roots were extracted from the liquid medium and the in vitro leaves were taken washed using distilled water to remove the traces of medium, dried and powdered using pestle and mortar. Adventitious roots were assessed for total withanolides and also different components that contribute to total withanolides whereas the leaves were assessed only for Withaferin A content. The analysis of bio actives was done using High Performance Liquid Chromatography method. One gram of dry root powder was extracted with methanol at 800C on a water bath and the residue was re-extracted twice with methanol, till the extract was colorless. The extracts were pooled and fi ltered through sample clarifi cation kit and were subjected to analysis by HPLC with Photo Diode Array. Seven standards such as Withanoside IV, Withanoside V, Withaferin A, Withanolide A, Withanolide B, Withanone and Withanostramolide from Natural Remedies Pvt. Ltd, Bangalore were used to quantify the total amount of withaferin A present in leaf samples and withanolides in root samples. The chromatogram was recorded at 227 nm and later the contents of various withanolides were added to estimate the total withanolide and expressed as mg/g on a dry weight basis.
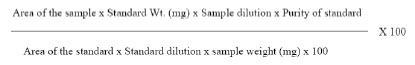
Data Analysis
All experiments were repeated thrice. Mean values of treatments were subjected to ANOVA and significant differences were separated by Duncan’s Multiple Range Test. To determine significance at P<0.05SPSS (Windows version 75.1, SPSS Inc., Chicago) was used.
Results and Discussion
Morphological comparison between two varieties of Ashwagandha
In vitro plants were assessed for plant height using 1 mm ruler from base of the stem to apical meristem. Leaf shape was also observed.
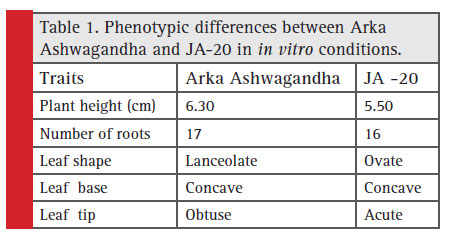 |
Table 1: Phenotypic differences between Arka Ashwagandha and JA-20 in in vitro conditions |
Influence of hormone supplementation on the proliferation of Withania somnifera adventitious roots in suspension culture
For large scale production of useful bioactive metabolites, the use of cell suspension cultures is favored due to its rapid growth cycles over other kinds of cell culture methods. Qualitative and quantitative analysis requires a considerable quantity of cells to determine growth responses and metabolism of phytochemicals, for these studies cell suspension cultures are found to be best suited (Vanishree et al., 2004).
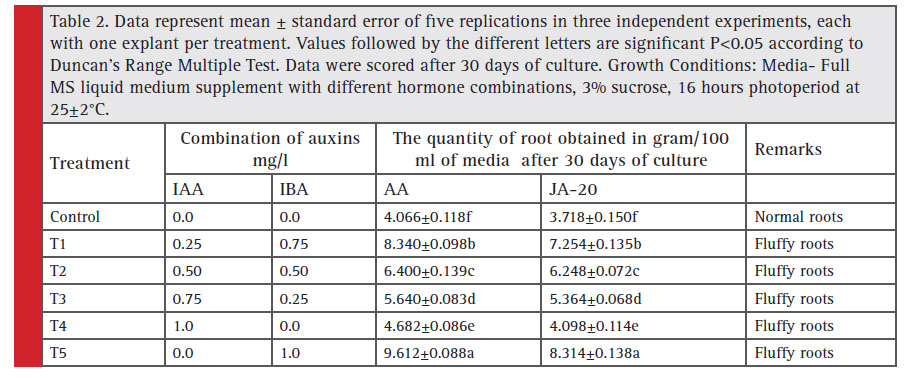
In the current study, the establishment of adventitious root suspension culture of Withania somnifera was done for two varieties Arka Ashwagandha and JA-20 with different hormone supplementation on media and further the biomass accumulation and total withanolides were estimated. Signifi cant phenotypic differences were recorded in the proliferation of root cultures in MS medium, supplemented with hormones and without hormones. In the hormone supplemented medium, the root biomass was higher, but there was no elongation of lateral roots. The suspension culture media with hormones gave rise to fl uffy roots, with callus like exudates formed around the senescent root tissues and subsequently released into the medium which could not be further multiplied in suspension cultures whereas media without supplementation of hormones had normal roots with important factors of proliferation like lateral root formation, elongation of lateral roots which favored further multiplication of adventitious roots. Lateral root formation was essential for rapid growth and higher biomass production in Rauwolfi a serpentine L (Pandey et al., 2010), thus indicating that phytohormone supplementation does not always enhance the regeneration frequency (Roychowdhury et al., 2013).
Genes involved in the synthesis of auxins are expressed in roots, which contribute to normal root growth and maintenance (Petersson et al., 2009). Auxin biosynthesis genes are expressed at root stem cell nice to increase the level of auxins (Stepanova et al., 2008). Signaling pathways for other plant hormones also infl uence the auxin response in roots (Kuppusamy et al., 2008). Though there was higher root mass in media supplemented with hormones, considering lateral root formation, proliferation and morphology of roots, MS media without hormone supplementation was considered best.
Influence of media strength on proliferation of Withania somnifera adventitious roots in suspension cultures
Both half strength MS medium and full strength MS medium were found to be suitable for biomass production of adventitious roots in Panax ginseng, but highest secondary metabolite content was induced in full strength MS medium (Yu et al., 2000). Full strength MS medium supplemented with 2.0mg/l IBA under continuous agitation increased the biomass of root tissue in Vernonia amygdalina (Khalaffala et al., 2009). Several researchers have also reported cell suspension culture studies in Withania somnifera (Sivanandan et al., 2012b and Praveen et al., 2011), hence full strength media without hormone supplementation was considered best suited for adventitious root multiplication in suspension culture as it provides more nutrients and reduces the frequency of subculturing in both varieties of Ashwagandha.
Different types of roots obtained by hormone treatments in Withania somnifera
Withanolides identified and total withanolide content in leaf and adventitious roots by HPLC analysis
HPLC analysis of Withania somnifera using methanolic extraction was reported by many researchers like Ganzera et al., (2003), Sangwan et al., (2004). The present study indicated that the roots had higher total withanolide content i.e., 1.621mg/g on a dry weight basis in Arka ashwagandha, compared to the total withanolide content in JA-20 root was 1.156 mg/g on a dry weight basis. Withanolide A which has a biological activity of sedative and hypnotic was 0.38mg/g on a dry weight basis in Arka Ashwagandha in comparison to JA 20 which has 0.28mg/g on a dry weight basis. Madhavi et al (2012) reported a Withanolide A content of 136 μg/g dry weight in 120 days old hairy root culture and 13μg/g dry weight in 210 days old hairy root culture of Withania somnifera, Dewin et al., (2010) reported Withanolide A content of 0.019 mg/g in in vitro roots of Withania somnifera which is much lesser than the Withanolide A content reported in the present study. The methanolic extract of Withania somnifera has GABA mimetic activity, anti-infl ammatory and anti-stress properties (Mir et al., 2012).
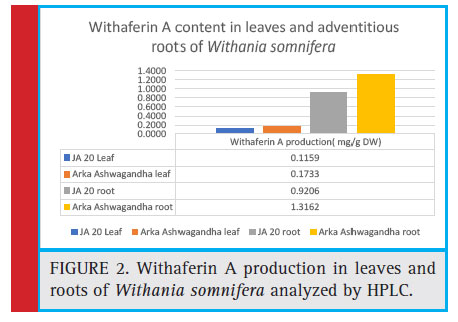 |
Figure 2: Withaferin A production in leaves and roots of Withania somnifera analyzed by HPLC |
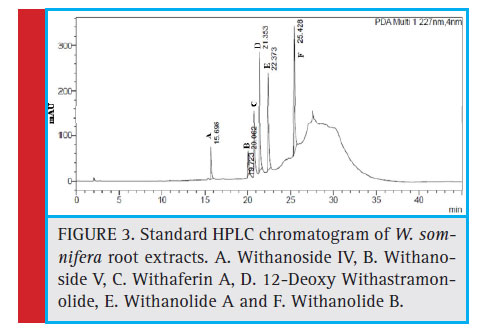 |
Figure 3: Standard HPLC chromatogram of W. somnifera root extracts. A. Withanoside IV, B. Withanoside V, C. Withaferin A, D. 12-Deoxy Withastramonolide, E. Withanolide A and F. Withanolide B. |
Withaferin A contributes to most of the pharmacological activity of Withania somnifera with its antibacterial, antifungal, antiarthritic, antitumor and antibiotic properties. Dalavayi et al., (2006) identifi ed Withaferin A in roots and leaves of Ashwagandha. The Withaferin A content in Arka Ashwagandha roots was identifi ed to be 1.316mg/g on a dry weight basis compared to JA- 20 with a content of 0.926mg/g on a dry weight basis. The Withaferin A content in Arka Ashwagandha leaves was 0.1733mg/g on a dry weight basis compared to JA-20 leaves 0.116 mg/g on a dry weight basis.
Madhavi et al (2012) reported that Withaferin A was not detected in 120 day old hairy root cultures, whereas it was 136μg /g dry weight in 210 day old hairy root cultures of Withania somnifera, Sivanandan et al (2012) reported 0.85mg/g in 40 day old callus cultures of Withania somnifera, which is much lesser than the Withaferin A content reported in the present study. Dewir et al (2010) reported a Withaferin A content of 0.013 mg/g dry weight in in vitro roots, the present study indicates 100 times increased Withaferin A content.
Comparison of Withaferin A content between adventitious roots and in vitro leaves in Withania somnifera
Conclusion
This current study helps in the identification of an elite cultivar of Withania somnifera for mass production of withaferin A and total withanolides. Field cultivation of Withania somnifera is a laborious task, as the crop is prone to diseases like seed rot and blight, harvesting of roots is a tedious task. The provision for alternative sources of Ashwagandha through cell cultures and micropropagation must be encouraged as it reduces the heavy dependence on the wild population to fulfill the global demand of Withania somnifera. Adventitious root multiplication through suspension cultures in hormone free medium provide an easy way for mass production of useful withanolides and can be used by neutraceutical and pharmaceutical industries as there would not be traces of hormones in the end product.
Acknowledgement
This research was conducted at ICAR- Indian Institute of Horticulture Research and was funded by the Department of Science and Technology through DST Inspire fellowship. The first author also acknowledges Centre for postgraduate study, Jain University for conducting this research.
References
Akram M, Mohiuddin E, Hannan A and Usmanghani K (2011) Withania somnifera (L.) Dunal (Pharmacology Activity), Pharmacognosy
Journal, 2(18):77-78.
Alfonso D, Bernardinelle G and Kapetanidisn I (1993) Withanolides from Lochoma coccinaum, Phytochemistry, 34(2):517-521.
Bhattacharya SK, Bhattacharya D, Sairam K and Ghosal S (2002) Effect of Withania somnifera glycol-withanolides on a rat model of tardive dyskinesia, Phytomedicine 9(2):167-170.
Dalavayi S, Kulkarni SM, Itikala RL and Itikala S (2006). Determination of Withaferin A in two Withania species by RP-HPLC method. Indian Journal of Pharmaceutical Sciences. 68:253-256.
Dewir Y.H, Chakrabarty D, Lee S.-H, Hahn E.-J. and Paek K.-Y(2010) Indirect degeneration of Withania somnifera and comparative analysis of Withanolides in in vitro and green house plants. Biologia Plantarum 54(2):357-360,2010.
Sivanandhan G, Arun M, Mayavan S, Rajesh M, Jeyaraj M, Kapil Dev G, Manickavasagam M, Selvaraj N and Ganapathi A (2012b ) Optimization of elicitation conditions with Methyl Jasmonate and Salicylic Acid to improve the productivity of Withanolides in the adventitious root cultures of Withania somnifera(L.)Dunal. Applied Biochem Biotechnol 168:681-696.
Ganzera M, Choudhary MI, Khan IA (2003) Quantitative HPLC analysis of Withanolides in Withania somnifera. Fitoter 74:68- 76.
Gao X, Zhu C, Gao W, Jia W, Qui M, Zhang Y and Xiao P (2005) Induction and characterization of adventitious roots directly from the explants of Panax notoginseng, Biotechnology letters, 27(22):1771-1775.
Gould AR, Everett NP, Wang TL, Street HE (1981) Studies on the control of cell cycle in cultured plant cells: Effects of nutrient limitation and nutrient starvation. Protoplasma, 106 (1-2):1-13.
Govil JN, Singh VK and Shamima H (1993) In: Glimpses in plant research Vol. X medicinal plants: New Vistas of research (Part1) today and tomorrow printers and publishers, New Delhi India. 232-253.
Ho,TT.,Jeonh,CS.,Lee,H. et.al. (2019) Plant Cell Tiss Organ Cult (2019).https://doi.org/10.1007/s11240-018-01556-5.
Kuboyama T, Tohda C, Komastu K (2005)Neuritic regeneration and synaptic reconstruction induced by withanolide A. Br J Pharmacol 144:961-971.
Kuppuswamy KT, Walcher CL and Nemhausar JL (2008) Crossregulatory mechanisms in hormone signaling. Plant Molecular Biology. 9(4)375-381.
Matsuda H, Murakami T, Kishi A, Yoshikawa M (2001) Structures of Withanolides I, II, III, IV, V, VI and VII, new withanolide glycosides from the roots of Indian Withania somnifera Dunal and inhibitory activity of tachyphylaxis to clonidine in isolated guinea pig ileum. Bioorganic and medicinal chemistry, 9(6):1499-1507.
Morano-Risueno MA, Van Norman JM, Moreno A, Zhang J, Ahnert SE and Benfey PN (2010) Oscillating gene expression determines competence for periodic Arabidopsis root branching. Science, 329 (5997): 1306-1311
Oh JH, Lee TJ, Kim SH, Choi YH, Lee SH, Lee JM, Kim YH, Park JW and Kwon TK (2008) Induction of apoptosis by Withaferin A in human leukemia U937 cells through downregulation of AKT phosphorylation. Apoptosis, 13(12):1494-504.
Pandey VP, Cherian E and Pathani G (2010) Effect of Growth Regulators and Culture conditions on Direct Root Induction of Rauwolfi a serpentine L. (Apocynaceae) Benth by leaf explants. Tropical Journal of Pharmaceutical Research, 9(1):2734.
Petersson SV, Johansson AL, Kowalczyk M, Makovychuk A, Wang JY, Moritz T, Grebe M, Benfey PN, Sandberg G and Ljung K(2009) An auxin gradient and maximum in the Arabidopsis root affects shown by high-resolution cell-specifi c analysis of IAA distribution and synthesis. Plant cell 21(6):1659-1668.
Pramanick S, Roy A, Ghosh S, Majumdar HK and Mukhopadhyay S (2008) Withanolide Z, A new chlorinated withanolide from Withania somnifera, Planta Medica, 74(14):1745-48.
Praveen N and Murthy HN (2010) Production of Withanolide- A from adventitious root cultures of Withania somnifera. Acta Physiology Plantarum. 32(5): 1017-1022
Ramachandra Rao S, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotech Adv 20:10-153.
Roy Chowdhary D, Majumdhar A and Jha S (2013) Agrobacterium rhizogenesis mediated transformation in medicinal plants: Prospects and Challenges in biotechnology for medicinal plants. Springer Verlag Berlin Heidelberg, 41. Sarin R (2005) Useful metabolites from plant tissue cultures. Biotechnology 4:79-93.
Sindhu Rangaraju, A.N. Lokesha and Chenna Reddy Aswath (2018) Standardization of various factors for production of adventitious roots in selected varieties of Withania somnifera and estimation of total withanolides content by High Performance Liquid Chromatography, Biosci. Biotech. Res. Comm. 11(3): 451-460.
Singh S and Kumar S (1998) In Withania somnifera: The Indian ginseng, Ashwagandha. Central Institute of medicinal and aromatic plants, Lucknow, India.
Thanh-Tam, Ho Choel-Sueng, Jeong Hyoshin, Lee So-Young Park (2019) Effect of explant- type and genotype on the accumulation of bioactive compounds in adventitious root cultures of Polygonum multifl orum, Plant cell tissue and organ culture:
Tohda C, Kuboyama T and Komatsu K (2005) Search for natural products related to regeneration of the neuronal network. Neurosignals, 14(1-2):34-45.
Vanisree M, Lee C, Lo S, Nalavade SM, Lin CY and Tsay H (2004) Studies on the production of some important secondary metabolites from medicinal plants by plant tissue cultures. Botanical buttelin of Academia Sinica, 45:1-22.
Verpoorte R, Contin A, Memelink J (2002). Biotechnology for the production of Plant secondary metabolites. Phytochem Rev 1:13-25.
Wu CH, Dewir YH, Hahn EJ and Peak KY (2006) Optimization of culturing conditions for the production of biomass and phenolics from adventitious roots of Echinacea angustifolia. Journal of Plant Biology, 49(3):193-199.
Wu CH, Murthy HN, Hahn EJ and Paek KY (2007) Large-Scale cultivation of adventitious roots of Echinacea purpurea in air lift bioreactors for the production of chichoric acid, chlorogenic acid and caftaric acid. Biotechnology Letters 29(8):1179-1182.
Yu KW, Hahn EJ and Paek KY (2000) Production of adventitious ginseng roots using Bioreactor. Korean Journal of Plant Tissue Culture 27:309-315.