1Pests and Plant Protection Department 2Molecular Biology Department,
National Research Center, 33-El Bohouth St. Dokki, Giza P.O.12622 EGYPT
Corresponding author email: abzs9999@yahoo.com
Article Publishing History
Received: 11/07/2024
Accepted After Revision: 25/09/2024
This study was targeted to test four vegetable oils namely (Ruta chalepensis, Azadirachta indica, Simmondsia chinensis, Nigella sativa K.L.) against tomato leaf miner Tuta absoluta under laboratory and field conditions, in addition to record its effect on their enzymatic activities. The data illustrated that N. sativa and R. chalapensis caused the highest reduction in eggs hatchability, mortality larval instars and moderate effect in larval penetration under laboratory condition.
On other side, the field evaluation was noticed that the most effective one was S. chinensis followed by A. indica and R. chalepensis which recorded reduction in insect infestation reach to ≥ 72 % on sprayed green part. On other hand, the least fruit infestation was recorded in case of treatments by R. chalepensis followed by S. chinensis, A. indica and N. sativa. The enzymatic analysis was given spot light on the reason of the variation in the effect among the tested oils toward 4th larval instars. Data was showed that the compounds reduced the enzymatic activities, which might suggest a poor defense mechanism in the detoxification of the used oils.
Tuta Absoluta – Vegetable Oils – Enzymatic Activities- Eggs- Larval Mortality- Crop Production- Fruit Infestation.
Moawad S.S, Ebadah I.M.A, Ghanem M.M.E. Impact of Some Natural Resources on Biological Aspect of Tuta absoluta M. Under Laboratory and Green House Conditions with Reference to its Enzymatic Activity. Biosc.Biotech.Res.Comm. 2024;17(3).
Moawad S.S, Ebadah I.M.A, Ghanem M.M.E. Impact of Some Natural Resources on Biological Aspect of Tuta absoluta M. Under Laboratory and Green House Conditions with Reference to its Enzymatic Activity. Biosc.Biotech.Res.Comm. 2023;17(3). Available from: <a href=”https://shorturl.at/ExkFS“>https://shorturl.at/ExkFS</a>
Introduction
Tomato, Lycopersicon esculentum Mill, is the most important and lucrative vegetable crop around the world which is planted in both outdoors and under green houses. The tomato crop yield has been faced different factors leading to reduce their productivity including pests and diseases (Materu et al., 2016 and Kandil et al., 2020). The tomato yield productivity reduced up to 100% in different governorate of Egypt due to the invasion with a newly dangerous insect pest namely tomato leaf minor Tuta absoluta (Meyrick) (Moussa et al., 2013; Soares et al., 2019; Mansour & Biondi, 2021 and Ahmed et al., 2022). In early infestation, newly emerged neonates penetrate tomato leaf into the mesophyll layer and feed between the lower and upper surfaces of the leaf to form small and transparent mines. The larvae attack all other parts of the tomato plant except only the root (Kandil et al., 2020 and Ahmed et al., 2022). The application of chemical insecticides is the most effective method for management T. absoluta. However, such strategy has a number of disadvantages including development of insect resistance towards conventional insecticides, environmental pollution, and potential toxicity to non-target organisms (Maneno et al., 2015; Abouelfadal, 2016, Campolo et al., 2018 and Ahmed et al., 2022).
Along the late decades all over the world, a variety of botanical extracts as alternatives to chemical insecticides for controlling different insect species have been examined (Campolo et al., 2018; Fergani and Yehia., 2020). The insecticidal activities of various plant species against T. absoluta have been proven (Nadia et al., 2014; Moawad et al. 2013 and Esther et al., 2019 Al-Solami, 2021; Erbas and Altuntas, 2021; Moawad and Ebadah 2022 and Moawad et al., 2022).
The ability of plant extracts to reduce or suppress antioxidant and detoxifying enzymes activities may improve the insecticidal efficacy of the botanical extract-based formulation, as well as exploited as synergistic ingredient to enhance the efficacy of other insecticides (Campolo et al., 2018). Therefore, estimation the biochemical effects of T. absoluta toward insecticidal plant extracts are critical to develop new options for their control (Ayil-Gutierrez et al., 2018). Additionally, estimation of such enzymes could help to propose or find new biological control agents. The present study aims to evaluate the efficiency of some natural resource oils against tomato leaf miner T. absoluta for developing the management strategy for such pest. In addition, the biochemical changes in some antioxidant and detoxifying enzymes will be investigated.
Materials and Methods
The experiments were carried out to test four types of natural oils namely (Ruta chalepensis, Azadirachta indica, Simmondsia chinensis, Nigella sativa K.L.) which were obtained from Luna Company Egypt. The preparation of tested oils concentration 10% was followed methods (Katoune et al., 2011 and Moawad and Sadek, 2018). 10% oil concentration was done by dissolved 10 ml of tested oil in 80 ml distilled water + 8 ml Arabic gum (20%) + 2 ml tween (20%) with add two drop from glycerin.
Laboratory experiments: Insect culture: To test oils on immature stages of T. absoluta (eggs and larvae) the infested tomato leaves were collected from field to start rearing. The larvae were reared and maintained on tomato leaves, cultivated in plastic pots inside a glass cage (50x 50x100cm3). The culture was provided by infested tomato leaves harboring T. absoluta pre-imagine stages collected from the field for isolation of eggs or outer larvae and pupae to maintain it. Newly emerged adults were collected and transferred to another glass cage (50x50x100 cm3) containing untreated plastic pots of tomato. The experiments were carried out on the 1st generation of tomato leaf miner
Treatment of egg stage: Leaves from the maintained culture were examined under Stereo-binocular to isolate deposited eggs by fine brush and keeping them in a Petri-dish. Eggs of one day old were used in the experiments. The tested oils was sprayed on eggs and let till dry. Each test was used 30 eggs and it was replicated five times. Percentages of reduction in eggs hatchability were calculated as follows:
Reduction of eggs hatchability % = a – b/a x 100 Where; a= number of eggs hatched in the control, b= number of eggs hatched in the treatment.
Treatment of larval stages: Couples of males and females were placed in glass tubes (10 cm.) for egg deposition and for facilitating obtain of 1st instar larvae. While other larval stages (3rd and 4th instar) were collected directly from the infested tomato leaves. In case of exposure 1st instar to treatment was investigated leaves daily to calculated penetration percentage and follow up till record pupation %.On other target the treatment of last larval stage (3rd and 4th) was observed and recorded their mortality and pupation %. Mortality % in all treatments was corrected by Abbott’s formula (Abbott, 1925).
Mortality % = (T – C) / (100 – C) x 100, Where: T=Mortality in the treatment C= Mortality in the control
Green house experiments: The present study was carried out in a plastic green house (9 x 40 m2) in reclaimed desert sandy soil in Nubaria region, Egypt and cultivated with tomato variety at winter plantation. The green house area 360 m2 was randomly divided into six experimental blocks, each block (5 rows, 7 plants/ row i.e. 35 plants/block) was specified for each treatment and two block were specified for the control +additive and control (without any treatment). The whole tested area was followed normal agricultural practices. Each block was divided to three replicates. Each one was sprayed twice interval time by tested oil. The first spraying was done after one month while second one was done after two months of tomato plantation. Tested oils were sprayed by using a manual sprayer (10 litter / plot). To evaluate the effect of tested oils on population of T. absoluta the randomly sample were collected from each replicate before spraying followed by subsequently samples after spraying (5,7,10,and 15 days). Examination of tomato leaflets were done under stereomicroscope to count number of deposited eggs and tunnel were targeted to calculate the reduction percentage in insect population. The reduction percentage of population density of T. absoluta was calculated according Henderson and Tilton (1955) equation as follows:
R % = 1 – (no. of individuals in control before treatment X no. of individuals in treatment after treatment / no. of individuals in control after treatment X no. of individuals in treatment before treatment) X100.To evaluate the effect of treatments on crop production and infestation percentage of tomato fruits were done once for first spray (by let 100 plant without 2nd spraying) and other one for 2nd spray by pick up the whole fruits to investigate and weight.
Enzyme assays:Polyphenol oxidase: Polyphenol oxidase (PPO) activity was conducted using L-3,4-dihydroxyphenylalanine (DOPA) as substrate according to Leonard (1971) and modified by Taleh et al. (2014) . The reaction mixture was contained in 1.0 ml: 100 mM potassium phosphate buffer, pH 7.0, 10 mM DOPA and enzyme crude extract ranged from 10.0-50.0 μl. The increase in the absorbance was recorded for 5 min at 470 nm. One unit of PPO activity was defined as the amount of enzyme that cause changes of 0.1 O.D./min under standard assay conditions.
Peroxidase: Peroxidase activity (PO) activity was determined according to Lee (1973) and modified by Aydinz and Kadioglu (2001) using guaiacol as substrate. The reaction mixture was contained in 1.0 ml: 100 mM sodium acetate buffer, pH 5.6, 20 mM guaiacol, 30 mM hydrogen peroxide (H2O2) and enzyme crude extract ranged from 5.0-20.0 μl. The increase in the absorbance was recorded for 3 min at 470 nm. One unit of PO activity was defined as the amount of enzyme that cause changes of 1.0 O.D./min under standard assay conditions.
Catalase: Catalase (CAT) activity was routinely assayed according to the method described by Aebi (1984). The reaction mixture contained in 2 ml; 20 mM H2O2 and 50 mM phosphate buffer, pH 7.0. The reaction was started after addition of suitable amount of enzyme solution (0.15 mg protein). The decomposition of H2O2 was followed as a decline in absorbance at 240 nm for 1 min at 27oC. One unit of CAT activity was defined as the amount of enzyme capable of catalyzing the decomposition of 1 µmol of H2O2/min at 27oC using an extension coefficient of 43.6 M-1 cm-1.
Carboxylesterase: Carboxylesterase (CaE) activity was measured using P-NPA as substrate according to Galliard and Dennis (1974). The reaction mixture contained in 1.0 ml: 2 mM of P-NPA and 100 mM phosphate buffer, pH 7.5. The change in absorbance was recorded at 407 nm for 10 min. One unit of CaE activity was defined as µmoles of P-nitrophenol produced per hour under standard assay conditions.
Acetylcholinesterase: Acetylcholinesterase (AChE) activity was measured using AcSChI as substrate according to Ellman et al. (1961). The reaction mixture contained in 1ml: 60 mM Tris-HCl buffer, pH 8.5, 1 mM AcSChI, 1 mM DTNB. The reaction mixtures were incubated at 37oC and the increase in the absorbance was recorded at 412 nm. One unit of AChE activity was defined as the amount of enzyme that catalyses hydrolysis 1µmol of substrate per hour under standard assay conditions.
Acid phosphatase: Acid phosphatase (AcP) activity was measured using P-NPP as substrate for according to the method described by Dinan et al., (1983). The reaction mixture contained in 0.5 ml: 2 mM of P-NPP and 100 mM acetate buffer, pH 5.5. The reaction mixture was incubated at 37 for 30 min and terminated by adding of 1.0 ml of 0.1M NaOH. The increase in the absorbance was recorded at 410 nm. One unit of AcP activity was defined as µmoles of P-nitrophenol produced per hour under standard assay conditions.
Protein determination: Protein contents were determined according to Bradford (1976) using bovine serum albumin as a standard.
Statistical analysis: All data were subjected to analysis of variance (ANOVA) and the means were compared by LSD test at 0.05 levels, using SAS computer program (SAS, 2009).
Results
Effect of tested oils on different stages of T. absoluta under laboratory condition:
On Eggs stage: Table1 illustrated that N. sativa and R. chalapensis caused the highest reduction in eggs hatchability reached to 94 and 86.7% but embryonic development was varied from 0.0 to 93.3%. While S.chinensis and A. indica were recorded 78.8 and 65.9% reduction in eggs hatchability and 36.7 and 95.8% embryonic development compare to control 0.0%. The result indicated that the most of tested oils didn’t cause direct toxicity its effect might be attributed to endocrine disturbance; except in case of treatment by N. sativa which had toxicity effect.
On larval instars: Table 2 illustrated that the tested oils were moderately effect on percentage of 1st larval penetration but the most of them failed to complete cycle till pupation. Treatments by A. indica were recorded 48.6 % larval penetration and 0.0% pupation. On other treatments by N. sativa, S.chinensis and R. chalepensis were recorded penetration percentage ranged between (48.6 to 60%) and pupation % (0.0 to 22.2%).
Table 1. Effect of 10% concentration of tested natural plant oils on eggs hatchability
and embryonic development of T. absoluta.
| Embryonic development % | Reduction in eggs hatching % | Treatment |
| 93.3 | 86.7 | Ruta chalepensis |
| 36.7 | 78.8 | Simmondsia chinensis |
| 95.8 | 65.9 | Azadirachta indica |
| 0.0 | 94 | Nigella sativa |
| 100 | 5.9 | Control+additive |
| 100 | 0.0 | control |
Table 2. Effect of treatment leaflets by 10% concentration of tested natural plant oils
on 1st larval stage penetration and pupation percentage of T. absoluta.
| Pupation % | Penetration % | Treatment |
| 22.2 | 60 | Ruta chalepensis |
| 4.5 | 53.3 | Simmondsia chinensis |
| 0.0 | 48.6 | Azadirachta indica |
| 11 | 77.6 | Nigella sativa |
| 87 | 92.6 | Control+additive |
| 96 | 100 | control |
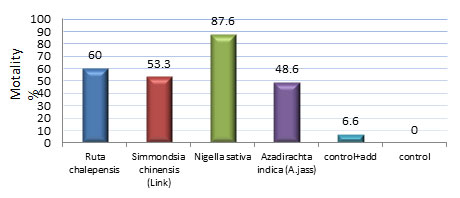
While Treatment 2nd, 3rd and 4th larval instar by tested oils were recorded mortality percentage reached to 87.6% in case of treatment by N. sativa (Fig.1). The remaining tested oils were recorded mortality % more than 48% and not more 60% compare to control + additives were record 6.6% mortality %.
Figure 1: Effect of tested oils on mortality percentage of 3rd and
4th instar larvae of T. absoluta.
II- Effect of tested oils on control T. absoluta infestation under field condition:
– On Deposited eggs: The table3 cleared that all of tested oils were recorded reduction percentage in eggs deposition compare to control. the highest reduction percentage in case of treatment by S. chinensis which reached to 70.6, followed by N. sativa, R. chaepensis and A. indica which ranged from 58 to 48%.
– On infestation rate of green part of tomato: Data in table 4 gave indication on reduction percentage of Tuta infestation due to spray leaflets by tested oils. The most effective one was S. chinensis followed by A. indica and R. chalepensis which recorded reduction % in insect infestation reach to 78.4, 72.4 and 71.4 compare to control + add were recorded 13.8%.
Table 3. Effect of tested oils on percentage reduction in eggs deposition of T. absoluta at
different time intervals under greenhouse conditions
| control | Control+ additive | Nigella sativa | Azadirachta indica | Simmondsia chinensis | Ruta chalepensis | Test
oils
Date Of Examination
|
|||||||||||||||
| Deposited Eggs / 50 leaflets | |||||||||||||||||||||
| reduction% | number | reduction% | number | reduction% | number | reduction% | number | reduction% | number | reduction% | number | ||||||||||
| For 1st spray | |||||||||||||||||||||
| … | 47 | … | 65 | …. | 64 | …. | 52 | …. | 61 | …. | 49 | pre-treatment | |||||||||
| … | 43 | 4.4 | 51 | 34.9 | 48 | 15.9 | 40 | 42.7 | 32 | 44.2 | 25 | 5th day | |||||||||
| …. | 37 | 37.5 | 32 | 58 | 31 | 38.9 | 25 | 75.01 | 12 | 42.9 | 22 | 7th days | |||||||||
| …. | 59 | 41.2 | 48 | 74.3 | 19 | 60.2 | 26 | 69.9 | 23 | 41.5 | 36 | 10 th days | |||||||||
| …. | 85 | 41.3 | 69 | 78.3 | 16 | 54.3 | 43 | 87.3 | 14 | 61.6 | 34 | Two weeks | |||||||||
| …. | 54.2 | 31.1±8.9 | 53 | 63.8±9.9 | 35.6 | 42.3±9.9 | 37.2 | 68.7±9.4 | 28.4 | 47.6±4.7 | 33.2 | General mean | |||||||||
| L.S.D.0.05=23.5
L.S.D.0.01=32.54 |
Statistical analysis | ||||||||||||||||||||
| 2nd spray | |||||||||||||||||||||
| …. | 98 | …. | 112 | …. | 159 | … | 94 | … | 144 | … | 106 | pre-treatment | |||||||||
| …. | 131 | 16.5 | 125 | 60.5 | 84 | 43.5 | 71 | 67.8 | 62 | 53.4 | 66 | 5th day | |||||||||
| …. | 118 | 17.8 | 111 | 60.3 | 76 | 62.01 | 43 | 73.5 | 46 | 65.5 | 44 | 7th days | |||||||||
| …. | 92 | 1.1 | 104 | 53.1 | 70 | 44.5 | 49 | 70.4 | 40 | 57.7 | 42 | 10 th days | |||||||||
| …. | 102 | 4.7 | 122 | 61.9 | 63 | 42.8 | 56 | 70.7 | 44 | 48.3 | 57 | Two weeks | |||||||||
| 108.2 | 9.98±4.2 | 114.8 | 58.9±1.98 | 90.4 | 48.2±4.6 | 62.6 | 70.6±1.2 | 67.2 | 56.3±3.6 | 63 | General mean | ||||||||||
| L.S.D.0.05=8.91
L.S.D.0.01=12.54 |
Statistical analysis | ||||||||||||||||||||
Table 4. Effect of tested oils on the reduction percentage of T. absoluta infestation
at different time intervals under greenhouse conditions
| control | Control+ additive | Nigella sativa | Azadirachta indica | Simmondsia chinensis | Ruta chalepensis | Test
oils
Date Of Examination
|
||||||
| No. of larvae /50 leaflets | ||||||||||||
| reduction% | Alive
larvae |
reduction% | Alive
larvae |
reduction% | Alive
larvae |
reduction% | Alive
larvae |
reduction% | Alive
larvae |
reduction% | Alive
larvae |
|
| For 1st spray | ||||||||||||
| … | 84 | … | 91 | … | 75 | …. | 69 | ….. | 76 | …. | 93 | pre-treatment |
| … | 87 | 8.8 | 86 | 24.6 | 47 | 38.4 | 44 | 54.3 | 36 | 20.1 | 77 | 5th day |
| …. | 96 | 14.4 | 89 | 64.7 | 22 | 30.1 | 52 | 79.3 | 18 | 39.8 | 64 | 7th days |
| …. | 49 | 37 | 85.6 | 9 | 62.7 | 15 | 84.2 | 7 | 66.8 | 18 | 10 th days | |
| …. | 33 | 29 | 80.7 | 12 | 52.1 | 13 | 66.5 | 10 | 34.3 | 24 | Two weeks | |
| …. | 69.8 | 8.9±2.01 | 60.3 | 63.9±13.8 | 33 | 45.8±13.8 | 38.6 | 71.1±6.7 | 29.4 | 40.2±9.8
|
45.8 | General mean |
| L.S.D.0.05=23.6
L.S.D.0.01=32.7 |
Statistical analysis | |||||||||||
| 2nd spray | ||||||||||||
| …. | 80 | … | 54 | …. | 94 | …. | 65 | … | 89 | … | 102 | pre-treatment |
| …. | 82 | 13.3 | 48 | 57.5 | 41 | 59.5 | 27 | 61.6 | 35 | 58.9 | 43 | 5th day |
| …. | 76 | 23.9 | 39 | 64.2 | 32 | 77.3 | 14 | 90.5 | 8 | 68.0 | 31 | 7th days |
| …. | 68 | 8.5 | 42 | 71.2 | 23 | 78.3 | 12 | 77.5 | 17 | 80.4 | 17 | 10 th days |
| …. | 73 | 9.6 | 54 | 28.5 | 15 | 74.7 | 15 | 83.9 | 13 | 78.5 | 20 | Two weeks |
| …. | 75.8 | 13.8±3.5 | 47.4 | 55.4±9.4 | 41 | 72.4±4.4 | 26.6 | 78.4±6.2 | 32.4 | 71.4±4.99 | 42.6 | General mean |
| L.S.D.0.05=16.13
L.S.D.0.01=22.36 |
Statistical analysis | |||||||||||
– On fruit infestation and crop production: The rate of fruit infestation in all treatment were recorded ratio less than control or control + add as described in Table 5. the least fruit infestation was recorded in case of treatments by R. chalepensis followed by S. chinensis, A.indica and N. sativa which recorded infestation ranged between 14.2 to 36.7% compare to control were recorded 71,6% infestation. On other view the crop production was affected and recoded highly significance variation between treatments and control (Table 5). The crop weight/kg//plot/35 plot were arranged descending as follow: S.chinensis > N.sativa> R.chalepensis > A. indica which were recorded (15.8, 15.3, 13.6 and 12.7) compared to contol+add or control were recorded 10.5 and 8.9 mean crop weight/35 plant.
Table 5. Effect of different treatments on the fruit infestation and crop production
| Mean of crop weight/kg/plot/35 plant
(X±S.E) |
Fruits Infestation % | Treatment |
| 13.6±1.01cc. | 14.2 | Ruta chalepensis |
| 15.8±0.5cc. | 25.8 | Simmondsia chinensis |
| 12.7±1.1cb. | 36.7 | Azadirachta indica |
| 15.3±0.74cc. | 36.7 | Nigella sativa |
| 10.5±0.38aa. | 53.1 | Control+additive |
| 8.9±0.7a | 71.6 | control |
| L.S.D.0.05=2.03
L.S.D.0.01=2.8 |
Statistical analysis | |
Effects of botanical extracts on enzymatic activities: Antioxidant enzymes
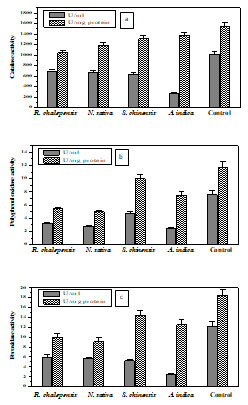
Catalase: A variation in CAT activity of 4th instar larvae of tomato leaf miner has been demonstrated before and after treatment with tested botanical oils. CAT activity ranged from 275-1022 U/ml with specific activity ranged from 1047-1556 U/mg protein (Fig.2a).
Polyphenol oxidase: In the 4th instar larvae of tomato leaf miner, PPO activity has been determined before and after treatment with tested botanical oils. The activity ranged from 2.5-7.6 U/ml with specific activity ranged from 5.0-11.7 U/mg protein (Fig.2b).
Peroxidase : A variation in PO activity has been detected in 4th instar larvae of T. absoluta. The PO activity ranged from 2.5-12.2 U/ml with specific activity 9.14-18.5 U/mg protein (Fig.2c). The results showed that R. chalepensis and N. sativa had the most effect on the activity of measured antioxidant enzymes.
Figure 2: Antioxidant enzymes activity in 4th instar larvae of T. absoluta before
and after treatment with tested botanical oils. Each result represents the average
of three separate experiments ± SE. (a) CAT, (b) PPO and (c) PO.
Detoxifying enzymes: Carboxylesterase: The activity of carboxylesterase (CaE) was determined in 4th instar larvae of tomato leaf miner before and after treatment with tested botanical oils. The CaE activity ranged from 30-110 U/ml with specific activity 89.5-167 U/mg protein (Fig.3a).
Acetylcholinesterase: A variation in AChE activity of 4th instar larvae of tomato leaf miner has been demonstrated before and after treatment with tested botanical oils. AChE activity ranged from 275-1022 U/ml with specific activity ranged from 146-569 U/mg protein (Fig.3b).
Figure 3: Detoxifying enzymes activity in 4th instar larvae of T. absoluta before and
after treatment with tested botanical oils. Each result represents the average of
three separate experiments ± SE. (a) CaE, (b) AChE and (c) AcP.
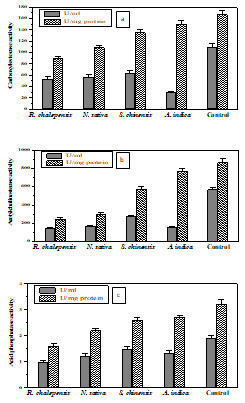
Acid phosphatase: In the 4th instar larvae of T. absoluta, AP activity has been determined before and after treatment with tested botanical oils. The activity ranged from 1.0-1.9 U/ml with specific activity ranged from 1.6-3.2 U/mg protein (Fig.3c). R. chalepensis and N. sativa exhibited the greater inhibitory effect on the activity of the tested detoxifying enzymes.
Discussion
The present data was cleared that vegetable oils might be used in decrease population of tomato leaf miner to gain good fruits quality production. The laboratory evaluation was made focus on the effect of tested oils on egg and different larval stages which indicated to its hormonal and toxicity effect toward eggs and larval stage. The tested plant oils were also investigated on the activities of two groups of enzymes, antioxidant and detoxifying enzymes. Data was showed that the compounds reduced the enzymes activity, which might suggest a poor defence mechanism in the detoxification of the used oils.
CAT worked in concert to reduce the oxidative stress by detoxifying O2– to molecular O2 and H2O, also PO acted as H2O2 scavenger enzyme. CAT can remove H2O2 only at high cellular level and is inefficient for scavenging H2O2 at low concentration. However, PO acts as a scavenger under all conditions (Mathews et al., 1997 and Jia et al., 2011).
Insects consume plant phenolic compounds which are toxic if ingested at high amounts. Insects have the ability for detoxifying these compounds. PPO has important role in insect’s immunity mechanisms (Wu et al., 2015 and Mohamed et al., 2022). PPO has major role for detoxifying the toxicity of plant pro-oxidant allelochemicals, so it can be interpreted that reduced PPO activity in the treated T. absoluta resulted in the death of the larvae.
CaEs are vital detoxifying enzymes which hydrolyzes the esteric bond in synthetic chemicals. The response decreases of CaE enzymes to botanical extracts were concurrence with Mojarab-Mahboubkar et al., 2015 and Abdel-Razi, 2018 revealed a decreased amount of esterases. AchE has a key role in neurotransmission by hydrolyzing the neurotransmitter acetylcholine in cholinergic synapses of the nervous system and is the target site of several neurotoxic insecticides (Mohamed et al., 2020 and 2021).
The essential oils exhibited a neurotoxic action resulting in spasms, lack of mechanical coordination and tremors (Abdellaoui et al., 2018). Several previous studies recorded that rapid action of essential oils against pests is an indicative of neurotoxic actions. Bessette et al., 2013 reported that in direct contact, essential oils can penetrate through insect’s cuticle and contact the nerve endings, and cause neurotoxic activity and rapid death. The neurotoxic modes of action on insects are mainly related to AChE levels. Many reports have demonstrated the interference of essential oils or its constituents with AChE enzyme activity in insects (Yeom et al. 2013).
AcP is hydrolytic enzyme, which hydrolyze phosphomonoesters under acidic conditions. Changes in AcP activities after treatments indicate that changing the physiological balance of the midgut might affect these enzymes (Ayil-Gutiérrez et al. 2018).Many researchers searched on the effect of botanical materials on control of T. absoluta. Nadia et al., 2014 reported that application of four concentrations of neem (Azadirachta indica) seeds ethanolic extract and Jatropha (Jatropha curcas) seeds petroleum ether extract on young larvae of T. absoluta resulted in larval mortalities that ranged between 33%-46.7% and 23.5%-48.5%, respectively, obtained after 24 h. Also, higher larval mortalities, up to 100%, were obtained with the two extracts after 4 d of treatments.
Esther et al., (2019) tested four plant extract (Commiphora swynnertonii, Synadenium glaucescens and Allium sativum) and found that all plant extracts were effective and controlled adult T. absoluta under laboratory condition. While, Commiphora extracts were highly effective and controlled T. absoluta in screen house. Foliar application reduced T. absoluta population, improved quality and yield of tomato. The results confirmed that inhibition in the enzymes may be the reason explained why N. sativa and R. chalapensis showed higher mortality than S.chinensis and A. indica. The mechanism of resistance of T. absoluta toward natural products is critical to develop new options for their control. Additionally, an analysis of this mechanism could help to propose or find new biological targets.
Conflict od Interest: Authors declare no conflict of interest
Acknowledgments: This work was supported by Pests and Plant Protection department and Molecular Biology departments at the National Research Center in Egypt.
Data Availability: Data are available with the corresponding author on reasonable request
References
Abbott, W.S., (1925). A method for computing the effectiveness of an insecticides. J. Econ. Entmol., 18:265-267.
Abdellaoui, K., Acheuk, F., Miladi, M., Boughattas, I. and Omri, G., (2018). Phytochemistry, biochemical and insecticidal activities of Ruta chalepensis essential oils on Tribolium confusum. Agriculture and forestry. 64: 31-45.
Abdel-Razi, M.R., (2018). Toxicity of traditional, novel and bio-insecticides and their mixtures against house fly Musca domestica in relation to some biochemical activities. Res. J. Environ. Toxicol. 12: 1–10.
Abouelfadal, M.A., (2016). Biological, Ecological, and bio-control studies on some insects attacking tomato plants. Menoufia J. Plant Prot., (1): 37 – 38
Aebi, H., (1984). Catalase in vitro. Methods in Enzymol. 105, 121-126.
Ahmed, D.M., Mohsem, A.M.A., El-Deeb, M.A., Alkhedaide, A., El-Tahan, A.M.and Metwally, E.M., (2022). The larvicidal effect of neemazal T/S, clove oil and ginger oil on tomato leafminer, Tuta absoluta compared to coragen. Saudi J Biol Sci 29:1447–1455.
Al-Solami, H.M., (2021). Larvicidal activity of plant extracts by inhibition of detoxification enzymes in Culex pipiens. J. King Saud Univ. Sci., 33, 101371.
Aydin, N. and Kadioglu, A., (2001). Changes in the chemical composition, polyphenol oxidase and peroxidase activities during development and ripening of medlar fruits (Mespilus germanica L.). Bulg. J. Plant Physiol. 27, 85-92.
Ayil-Gutiérrez, B.A., Sánchez-Teyer, L.F., Vazquez-Flota, F., Monforte-González, M., Tamayo-Ordóñez, Y., Tamayo-Ordóñez, M.C. and Rivera G., (2018). Biological effects of natural products against Spodoptera spp. Crop Prot. 114, 195-207.
Bessette, S., Lindsa, A., Enan, E., (2013). Pesticidal compositions containing rosemary oil and wintergreen oil. US Patent. US 20130142893 A1.
Bradford, M.M., (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248-254.
Campolo, O., Giunti, G., Russo, A., Palmeri, A., and Zappala L., (2018). Essential oils in stored product insect pest control. Journal of Food Quality. 6906105.
Dinan, L., Glansener, G. and Emmerich, H., (1983). Characterization of the acid phosphatase and arylsulphatase activities in a tumorous blood cell line of Drosophila melanogaster. Insect Biochem. 13, 411-419.
Ellman, G. R., Courtney, K. D., Andres, V. and Featherstone, R. M., (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7, 88-95.
Erbaş, D.E. and Altuntaş, H., (2021). Effects of Juglone on the Antioxidant Metabolism in the Larval Hemolymph of the Greater Wax Moth Galleria mellonella L. (Lepidoptera: Pyralidae). Karadeniz Fen Bilimleri Dergisi, 11, 18-28.
Esther, M.R., Emmanuel, N.H. George, B.G. Bintu, N.N. Walad, M.M. and Petro, M.A., (2019). Controlling Tuta absoluta (Meyrick) by selected crude plant extracts in the laboratory and in the screen house. J Agricul Sci Technol A 9, 227-239.
Fergani, Y.A. and Yehia, R.S., (2020). Isolation, molecular characterization of indigenous Beauveria bassiana isolate, using ITS-5.8 s rDNA region and its efficacy against the greatest wax moth, Galleria mellonella L. (Lepidoptera: Pyralidae) as a model insect. Egypt J Biol Pest Control 30, 96.
Galliard, T. and Dennis, S., (1974). Isoenzyme of lipolytic acyl hydrolase and esterase in potato tuber. Phytochemistry 13, 2463-2468.
Henderson, C.F. and Tilton, E.W., (1955). Tests with acaricides against the brown wheat mite. J. Econ. Entomol., 48: 157-161.
Jia, F.X., Dou, W., Hu, F. and Wang, J.J., (2011). Effects of thermal stress on lipid peroxidation and antioxidant enzyme activities of oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Florida Entomologist 94, 956-963.
Kandil, M.A.H., Sammour, E.A., Abdel-Aziz, N.F., Agamy, E.A.E., El-Bakry, A.M. and Abdelmaksoud, N.M., (2020). Comparative toxicity of new insecticides generations against tomato leafminer Tuta absoluta and their biochemical effects on tomato plants. Bull Natl Res Cent 44, 126.
Katoune, H.I., Lafia, D.M., Salha, H., Doumma, A., Drame, A.Y., Pasternak, D. and Ratnadass A., (2011). Physic nut (Jatropha curcas) oil as a protectant against field insect pests of cowpea in Sudano-Sahelian cropping systems. J. SAT Agric. Res. 9, 1–6.
Lee, T.T., (1973). On extraction and quantitation of plant peroxidase isoenzymes. Physiologia Plantarum. 29, 198-203.
Leonard, T.J., (1971). Phenoloxidase activity and fruiting body formation in Schizophyllum commune. J. Bacteriol., 106, 162-167.
Maneno, C., Secilia, M., and Christopher, L. M., (2015). Knowledge and Practices of agricultural extension officers in management of the invasive Tuta absoluta Meyerick (Gelechiidae) in Tanzania. International Journal of Science and Research 5 (5): 428-30.
Mansour, R. and Biondi, A., (2021). Releasing natural enemies and applying microbial and botanical pesticides for managing Tuta absoluta in the MENA region. Phytoparasitica 49, 179–194.
Materu, C.L., Shao, E.A., Losujaki, E., and Chidege, M., (2016). Farmer’s Perception Knowledge and Practices on Management of Tuta Absoluta Meyerick ( Lepidotera: Gelechiidae ) in Tomato Growing Areas in Tanzania.;3(2):1-5.
Mathews, M.C., Summers, C.B., Felton, G.W., (1997). Ascorbate peroxidase: a novel antioxidant enzyme in insects. Arch. Insect Biochem. Physiol. 34, 57–68.
Moawad, S. S. and Ebadah I. M. A., (2022). Evaluation efficacy and persistence of some volatile plant oils on immature stages of Galleria mellonella (L.). Journal of Agricultural Science; 14 (1): 104-108.
Moawad S. S., Ebadah I.M.A. and Mahmoud Y.A., (2022). Action of eco-friendly natural oils fumes on control greater wax moth, Galleria mellonella (L.). J. ent. Res., 46 (1): 67-72.
Moawad, S. S., Ebadah, I. M. A. and Mahmoud, Y. A., (2013). Biological and histological studies on the efficacy of some botanical and commercial oils on Tuta absoluta Meyrick (Lepidoptera: Gelechiidae). Egyptian journal of biological pest control, 23(2), 2013, 301-308.
Moawad, S.S. and Sadek, H.E. (2018). Evaluation of two eco friendly botanical oils on cotton leaf worm, Spodoptera littoralis (Boisd) (Lepidoptera/ Noctuidae). Ann. Agric. Sci., 63: 141-44.
Mohammad MA (2022). Defense status in larval stage of red palm weevil Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae). Biocat. Agricul. Biotechnol., 44: 10246.
Mohamed, M.A., Shaalan, S., Ghazy, A.M., Ali, A.A., Abd-Elaziz, A.M., Ghanem, M.M. and Abd-Elghany, S.A., (2020). Purification and characterization of acetylcholinesterases in Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae), Int. J. Biol. Macromol. 147, 1029-1040.
Mohamed, M.A., Shaalan, S., Ghazy, A.M., Ali, A.A., Abd-Elaziz, A.M., Ghanem, M.M. and Abd-Elghany, S.A., (2021). Susceptibility of purified acetylcholinesterases from Rhynchophorus ferrugineus towards insecticides and botanical extracts. Middle East J. Applied Sci. 11, 165-187.
Mojarab-Mahboubkar, M., Sendi, J.J. and Aliakbar, A., (2015). Effect of Artemisia annua L. essential oil on toxicity, enzyme activities, and energy reserves of cotton bollworm Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). J. Plant Prot. Res. 55: 371–377.
Moussa, S., Sharma, A., Baiomy, F. and El-Adl, F. E., (2013). The Status of Tomato Leafminer; Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in Egypt and Potential Effective Pesticides. Academic Journal of Entomology 6 (3): 110-115, 2013.
Nadia, E. M. K., Awad, K. T., and Mohammed, E. E. M., (2014). “Effects of Botanical Extracts of Neem (Azadirachta indica) and Jatropha (Jatropha curcus) on Eggs and Larvae of Tomato Leaf Miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae).” Persian Gulf Crop Protection 3 (3): 41-6.
SAS (2009). Statistical analysis system software. Ver 9.1. SAS Institute Tnc.carry.NG.
Soares, M.A., Passos, L.C., Campos, M.R. Collares, L.J., Desneux, N. and Carvalho, G.A., (2019). Side effects of insecticides commonly used against Tuta absoluta on the predator Macrolophus basicornis. J Pest Sci 92, 1447–1456.
Taleh, M., Saadati, and M., Farshbaf, R., (2014). Partial characterization of phenoloxidase enzyme in the hemocytes of Helicoverpa armigera Hübner (Lepidoptera: Noctuidae). J. King Saud Univ. Sci. 26, 285-289.
Wu, K., Zhang, J., Zhang, Q., Zhu, S., Shao, Q., Clark, K.D., Liu, Y. and Ling, E., (2015). Plant phenolics are detoxified by prophenoloxidase in the insect gut. Scientific Reports. 5, 16823.
Yeom, H.J., Jaesoon, J., Kim, S.W. and Park, I.K., (2013). Fumigant and contact toxicity of Myrtaceae plant essential oils and blends of their constituents against adults of German cockroach (Blattella germanica) and their acetylcholinesterase inhibitory activity. Pesticide Biochemistry and Physiology, 107, 200-206.


