1Department of Life Sciences, Kristu Jayanti College, Bangalore, Karnataka, India.
2Department of Biotechnology, Sri Kaliswari College, Sivakasi, Tamil Nadu, India.
Corresponding author email: ramidster@Gmail.com
Article Publishing History
Received: 13/07/2021
Accepted After Revision: 18/09/2021
The aim of the present study is to analyse the growth of Zea mays L. supplemented with different concentrations (20 ppm, 40 ppm and 60 ppm) of copper nanoparticles. Green synthesis of copper nanoparticles was obtained from the leaf extract of Zea mays L. The synthesized nanoparticles were characterized and confirmed using UV-Vis spectrometric analysis, SEM, EDAX and FTIR analysis. Copper nanoparticles were conformed based on the colour change (sea green) and the peak at 600 nm using UV Visible spectrophotometer is due to Surface Plasmon resonance of copper nanoparticles. Scanning electron microscope analysis of copper nanoparticles reveals that the size ranges between 150-200 nm. The present investigation involves a novel method of synthesizing the nanoparticles from the leaf extract of Zea mays L. and utilizing the same for the growth analysis of the same plant.
Seed germination of Zea mays L. supplemented with copper nanoparticles showed maximum growth in root and shoot length at 20 ppm concentration of nanoparticles. In vivo growth analysis of Zea mays L. supplemented with copper nanoparticles at one time exhibited maximum growth at 20 ppm concentration. Similarly In vivo growth analysis of Zea mays L. supplemented with copper nanopartilces continuously for 15 days also revealed that 20 ppm concentration of copper nanoparticles with optimal growth characteristics in Zea mays L. However, with increasing concentrations (40 ppm and 60 ppm) of copper nanoparticles resulted in the decrease of growth and protein content. This reveals the toxicity of copper nanoparticles in Zea mays L. Accumulation of silver nanoparticle in the plant was measured using atomic absorption spectroscopy.
Copper Nanoparticles, In Vivo, Seed Germination, Zea Mays L.
Thiruvengadam S, Ganesan M, Varadharajaperumal P. Impact of Foliar Application of Copper Nanoparticles on growth of Zea mays. Biosc.Biotech.Res.Comm. 2021;14(3).
Thiruvengadam S, Ganesan M, Varadharajaperumal P. Impact of Foliar Application of Copper Nanoparticles on growth of Zea mays. Biosc.Biotech.Res.Comm. 2021;14(3). Available from: <a href=”https://bit.ly/2VUlll6“>https://bit.ly/2VUlll6</a>
Copyright © Thiruvengadam et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Nanotechnology opens an oversized scope of novel application within the fields of agricultural industries and biotechnology, as a result of their distinctive physiochemical properties, i.e., tunable pore size, high extent, high reactivity, and particle morphology. Although fertilizers are very important for plant growth and development, most of applied fertilizers are rendered unavailable due to many factors such as leaching, degradation by photolysis, hydrolysis and decomposition. Hence, it’s necessary to reduce the nutrient losses in fertilization and increase the crop yield through exploitation of latest applications with facilitate of applied science and nanomaterials (Siddiqui et al. 2015; Milewska et al. 2016).
Nanotechnology has the ability to increase the yield of nutrient values and also plays a vital role in developing improved systems for monitoring ecological conditions and increasing the capacity of crops to absorb nutrients. There are majority of nano-material which is known for its plant growth promoting effects. Recent researches on effects of nanoparticles on various plant crops have reported with increased germination and growth of seeds (Farooqui et al. 2016).
In recent years, numerous studies have been conducted to analyze and describe the influence of nanoparticles on plant growth. Many studies have been performed on the impact of nanoparticles on the physiological and metabolic processes that directly influence plant growth and development. An investigation of the response of six crop species: barley, maize, rice soybean, switchgrass, tomato and tobacco showed that nanomaterials accelerate seed germination and enhance the growth (Milewska et al. 2016; Patil et al. 2018; Rajkumar et al. 2019).
The elements including Copper, Magnesium, Nickel and Zinc play vital functions in plant cells. Micronutrients are essential for biosynthesis and function of nucleic acids, growth substances, chlorophyll and secondary metabolites, as well as for growth and stress resistance, on the other hand they are required in a small trace, whereas turns toxic at higher concentrations. Lower concentrations of copper sulphate and copper sulphate nanoparticles induced increasing in shoot length, root length and fresh weight of Verbena bipinnatifida Nutt.
Copper enhanced the seedling growth of Vigna radiate at low level supplementation. High level retarded the growth of Vigna radiate by interfering the normal cellular metabolic events (Genady et al. 2016). The effect of copper nanoparticles on germination and growth of soybean and chickpea seeds revealed that lower concentration of nanoparticles increased the growth, however high levels become phytotoxic for morphological, physiological and biochemical changes in plants (Mustafa et al. 2017; Patil et al. 2018; Rajkumar et al. 2019).
The Cu deficiency in plants is expressed as curled leaves, petioles bent downwards and light chlorosis along with permanent loss of turgor in the young leaves. Chronic Cu deficiency develops a rosette form of growth. Diagnosis of Cu deficiency in plants is an important as it results in yield losses, with little evidence of the characteristic symptoms. Cu deficiency may become more prevalent in coming future, the applications made 10 to 30 years ago would be running out and increased use of nitrogenous fertilizers will lead to severity of Cu deficiency (Shobha et al. 2014).
Copper nanoparticles possess broad range of applications like antimicrobial materials, heat transfer systems, sensors, catalysts and super strong materials. They are very reactive because of their high surface to volume ratio and can easily interact with other particles. Copper nanoparticles possess a strong antibacterial activity and were able to decrease the microorganism concentration by 99.9% (Shobha et al. 2014; Sriram and Pandidurai 2017; Patil et al. 2018).
For the synthesis of copper nanoparticles, both the precursor (plant extract) and the reducing agent (copper sulphate) were mixed in a clean tube in 1:1 proportion. For the reduction of copper ions, 5ml of freshly prepared aqueous plant extract was mixed with 5 ml of freshly prepared 0.001M aqueous copper sulphate solution. It was then kept for incubation for 1hour. After the incubation period color change to sea green from dark brown was noted.
This color change indicates synthesis of copper nanoparticles (Sriram and Pandidurai 2017). To investigate the size, shape and composition of copper nanoparticles Scanning electron microscopy and X ray diffraction studies were employed. The outcome of these studies will take a step closer in designing simple, environmentally friendly and low-cost synthesis method of copper nanoparticles (Patil et al. 2018; Rajkumar et al. 2019).
Zea mays L. (Maize or Corn) is a cereal belonging to the Poaceae family. The plant has rich source of carbohydrate, fat, vitamins, minerals and protein. Reports reveal that biomolecules from maize are responsible for the formation nanoparticles. Maize is considered as the most emerging versatile crop possessing wider adaptability under varied agro-climatic condition. Maize is the third most important cereal in the world with wide market potential (Sriram and Pandidurai 2016; Rajkumar et al. 2019). Although soil and climatic conditions of Pakistan favour successful production of maize but inappropriate planting methods significantly reduce the maize production.
Planting method is an important agronomic practice for enhancing crop yield (Tanveer et al. 2014; Rajkumar et al. 2019). The present investigation involves a novel method of synthesizing the nanoparticles from the leaf extract of Zea mays L. and utilizing the same for the growth analysis of the same plant. Seed germination and in vivo growth of Zea mays L. supplemented with various concentrations (20 ppm, 40 ppm and 60 ppm) of copper nanoparticles was analysed based on several parameters like root, shoot leaf length, leaf surface area and whole plant weight. Chlorophyll content of the leaf was also measured.
MATERIAL AND METHODS
Maize leaves were collected from Tamil Nadu Agricultural University, Madurai. The collected leaves were washed with double distilled water and air dried. Then it was cut into small pieces and homogenized with the help of mortar and pestle and dispensed in 100 ml of distilled water and heated for 5 minutes at 70-80oC. The extract was then filtered using Whatman’s No.1 filter paper. The filtrate was collected in a clean and dried conical flask by standard sterilized filtration method and was stored at 30º C. The copper nanoparticles were synthesized by mixing the precursor (Zea mays L. leaf extract) and the reducing agent (copper sulphate) in 1:1 proportion in a clean conical flask. For this reaction 50 ml of freshly prepared 0.01 M aqueous copper sulphate solution was mixed with 50 ml of filtered plant extract. The resultant solution was observed for its color change after 1 hour at room temperature (Rajput et al. 2018).
For the purification of synthesized copper nanoparticles, the solution was centrifuged for 10 minutes at 10, 000 rpm after the color change. Pellet were collected and concentrated. It was mixed with equal volume of distilled water. Centrifugation process was repeated many times to get better separation of other entities of the copper nanoparticles. For UV-Visible spectra analysis, UV-Visible spectrophotometer was used for monitoring the reduction of copper to nanoparticles. The sample was mixed with distilled water and UV-Visible spectral analysis was done between the range of 340-600 nm. The analysis was done in every 1, 3, 6, 12 and 24 hours) (Sriram and Pandidurai 2017; Rajput et al. 2018).
Fourier transform infrared spectroscopy (FTIR) spectrophotometer is used to analyze the functional groups present in the sample. For this analysis the sample and potassium bromide were mixed in the ration of 1:100 respectively. It was incubated for overnight at 110oC. The mixture was cooled and compressed with hydraulic press. The resultant was scanned with FTIR in the range of 500-4000cm-1. The elemental composition of sample was determined by Energy Dispersive X-ray analysis (EDAX). The EDAX analysis system works as an integrated feature of a scanning electron microscope (SEM) and cannot operate on its own without the latter (Ghosh et al. 2020).
The embedded copper nanoparticles in the filtrate were subjected to Scanning Electron Microscope (SEM) analysis after drying under vacuum. Scanning Electron Microscope was used to examine the powdered sample of copper nanoparticles. The surface and it internal structures of copper nanoparticles are visualized using this study (Wu et al. 2020). For the seed germination analysis, seeds of Zea mays L. were obtained from Tamil Nadu agricultural College, Madurai. Seed germination study was carried out in a Petri dish placed with a water porous filter paper.
To each 5ml of copper nanoparticles with various concentrations (20ppm, 40ppm and 60ppm) was added. The seeds were incubated in dark and germination was monitored during 6th and 12th day. A total of 50 seeds were used for studying the seed germination analysis (Almutairi and Alharbi 2015). The parameters used are Root length, Shoot length, Fresh weight (12th day), Dry weight (12th day).
For the in vivo growth analysis of Zea mays L, the experimental soil for raising the cultivars was sandy loam. The soil was sterilized by solar sterilization method for 5 days. It was then analyzed for its physio chemical properties. The analyzed soil was taken in earthen pots of size 30´33 cm and filled in for about two-third of their height (5 kg of soil per pot). In vivo growth of Zea mays L. supplemented with nanoparticles by foliar spray was carried out by two different methods
(I) Different concentration of nanoparticles (20 ppm, 40 ppm and 60 ppm) supplemented at one time.
(II) Different concentration of nanoparticles (20 ppm, 40 ppm and 60 ppm) supplemented continuously for 15 days. On 15th day the following parameters were analyzed for the growth of Zea mays L. using nanoparticles. The parameters used are Root length, Shoot length, Leaf length, Leaf surface area, Fresh weight & Dry weight (Tanveer et al. 2014). For the analysis of chlorophyll content, 1 gram of finely cut leaf was homogenized using a mortar and pestle. The leaf material was added with 0.5 grams of Magnesium carbonate and 20 ml of 80% acetone. Grinding was continued (Tanveer et al. 2014; Wu et al. 2020).
The leaf material was then refrigerated for 4 hours at 4°C. Centrifugation was carried out at 500 rpm for 5 minutes. After centrifugation in a volumetric flask the supernatant was alone transferred. 80% of acetone was used to made up the final volume to 100 ml. The solution was estimated using a spectrophotometer with absorbance at 663 and 645 nm. Acetone (80%) was used as blank (Plaksenkova et al. 2019).
Chlorophyll content was measured using the formula
Chlorophyll a = 11.75 x A662 -2.35xA645 Chlorophyll b= 18.61 x A645 -3.96xA662
Where A662 is Absorbance at 662 nm, A645 is Absorbance at 645 nm.
For statistical analysis, each experiment was repeated three times and each treatment had 10 replicates. All data obtained were subjected to Standard deviation and one way analysis of variance (ANOVA). For the analysis of copper nanoparticles in plant material, the nanoparticles accumulated in experimental plants were assayed after 15 days. Copper concentrations in plants were analyzed using the method of Baker et al. (1994). The plant sample as a whole was washed, dried in oven at 1600C for 40 minutes and digested in a mixture of nitric acid and perchloric acid (10:1).
Then the solution was centrifuged at 5000 rpm for 5 minutes and double filtered with Whatmann filter paper no.4 and the filtrate were used for analysing the concentration by Atomic Absorption Spectrometry (Shimadzu Model AA–6300), available in the Science Instrumentation Centre of Ayya Nadar Janaki Ammal College (Autonomous), Sivakasi, Tamil Nadu.
The Accumulation Factor (AF) was considered to determine the quantity of nanoparticles absorbed by the plant from soil. This is an index of the plant to accumulate a particular nanoparticle with respect to its concentration in the soil and is calculated using the formula (Ghosh and Singh 2005).
![]()
RESULTS AND DISCUSSION
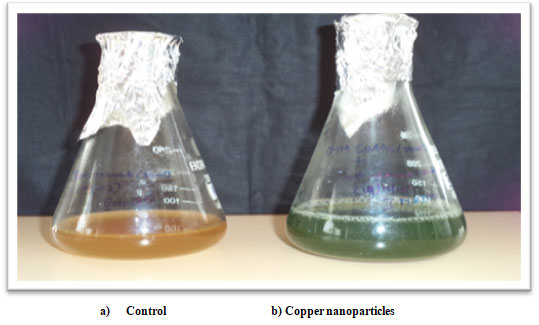
Synthesis of copper nanoparticles from maize leaves: The reduction of copper sulphate using Zea mays L. leaf extract was viewed by the color change from dark brown to sea green (Figure 1). Due to the excitation of surface Plasmon vibration in nanoparticles, it exhibits sea green colour. Copper nanoparticles synthesized from Zea mays L. involve 5.0 ml of leaf extract and 5.0 ml of 0.001M aqueous copper sulphate solution. After 1 hour of incubation the colour change was found to be sea green. (Sriram and Pandidurai 2017; Plaksenkova et al. 2019; Wu et al. 2020).
Figure 1: Synthesis of copper Nanoparticles from maize leaves.
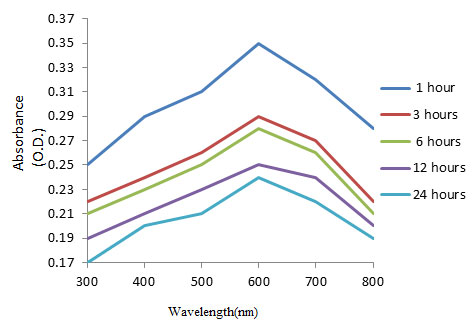
Characterization of synthesized copper nanoparticles: UV-Visible spectra analysis of copper nanoparticles: UV-Visible spectrophotometer recorded the reduction of copper sulphate in the leaf extract of Zea mays L. Copper nanoparticles exhibited maximum absorbance at 600 nm in various time intervals (Figure 2). The peak at 600 nm is due to Plasmon resonance of copper nanoparticles
Figure 2: UV-Vis Absorption Spectrum of copper nanoparticles synthesized by using Zea mays L. leaf extract
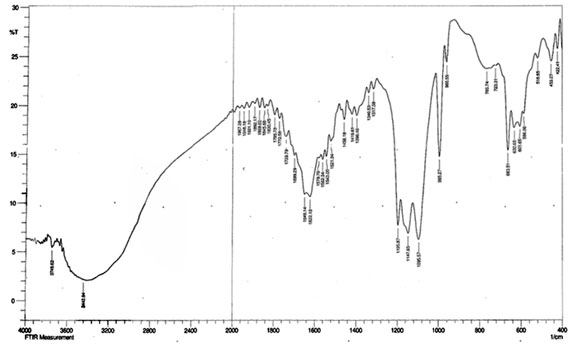
Fourier transform infrared spectroscopy (FTIR) of copper nanoparticles: FTIR spectrum of synthesized copper nanoparticles was shown in Figure 3. Functional groups involvement between metal particles and biomolecules is analyzed by Fourier-Transform Infrared spectroscopy. Identification of biomolecules responsible for capping, reduction and stabilization of metal nanoparticles were carried out using FTIR. The spectrum showed the band at 1192.01cm-1 corresponds to O=C-O-C stretching of ester, which are very strong bonds. The supernatant with the active functional groups results in swift reduction of copper nanoparticles from Cu ions (Ghosh et al. 2020).
Figure 3: FTIR spectrum of copper nanoparticles synthesized by using Zea mays L. extract.
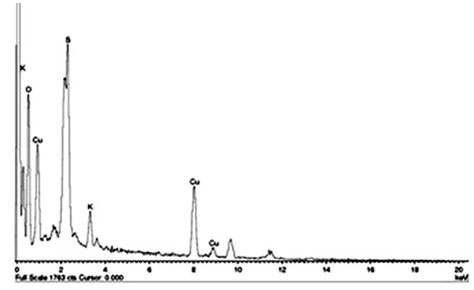
Energy Dispersive X-ray analysis (EDAX) of copper nanoparticles: EDAX spectra were recorded from the synthesized copper nanoparticles and showed a peak in the Cu region, confirmed the copper nanoparticle formation (Figure 4). The horizontal axis displays the energy in electron volts while the vertical axis represents the number of X-ray counts. The lines displayed with major emission energies for copper correspond with the peaks of the spectrum that ensures the identification of copper nanoparticles (Wu et al. 2020).
Figure 4: EDAX analyses of copper nanoparticles synthesized by using Zea mays L. leaf extract
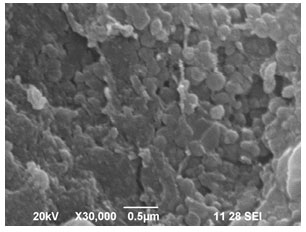
Scanning Electron Microscope (SEM) analysis of copper nanoparticles: Copper nanoparticles size and shape were clearly visualized using Scanning Electron Microscope. Figure 5 shows the presence of copper nanoclusters in panoramic view with the size ranging between 150-200 nm. The copper nanoparticles with higher magnification reveals that these nanoparticles are in the form of small nanoclusters with average diameter of 40 nm which has good uniformity. The SEM observations reveal that the size of copper nanoparticles is about 40 to 45 nm (Wu et al. 2020).
Figure 5: SEM image of copper nanoparticles synthesized by using Zea mays L. leaf
Effect of copper nanoparticles on root and shoot length of Zea mays L.: Figure 6 shows the seed germination studies of Zea mays L. supplemented with various concentrations of copper nanoparticles. On 6th day different concentrations (20 ppm, 40 ppm and 60 ppm) of copper nanoparticles supplemented to Zea mays L. showed maximum growth in root length with 20 ppm (7.30 ± 0.20 cm) (Table 1) The shoot length was higher in 20 ppm (2.17 ± 0.15 cm). On 12th day also the root length was found to be maximum with 20 ppm (14.77 ± 0.31 cm) (Table 1).
The shoot length was higher in 20 ppm (4.70 ± 0.10 cm). It is similar to the results obtained by (Rajput et al. 2018). Seed germination analysis of the present study reveals those 20 ppm concentrations of copper nanoparticles increases the root and shoot length on 6th and 12th day when compared to the other concentrations (40 ppm&60 ppm) and control. Similar results were observed by (Almutairi and Alharbi 2015; Rajput et al. 2018; Wu et al. 2020).
Figure 6: Seed germination analyses of Zea mays L. treated with copper Nanoparticles (6th day)

Table 1. Effect of copper nanoparticles on root and shoot length of Zea mays L. seeds (6th and 12th day)
| Concentration of copper nanoparticles (ppm) | 6th day | 12th day | ||
| Root length(cm) | Shoot length(cm) | Root length(cm) | Shoot length(cm) | |
| Control | 2.23±0.15 | 0.50±0.10 | 4.53±0.25 | 1.07±0.06 |
| 20 | 7.30±0.20 | 2.17±0.15 | 14.77±0.31 | 4.70±0.10 |
| 40 | 4.30±0.10 | 1.53±0.15 | 8.67±0.21 | 3.20±0.10 |
| 60 | 2.90±0.20 | 0.73±0.06 | 5.87±0.15 | 1.80±0.10 |
Values represent the mean (±) standard error of three independent experiments. All the experiments were statistically analyzed by One-way Anova using SPSS interpretation. The results were significant at p < .05
Effect of copper nanoparticles on fresh and dry weight of Zea mays L.: Total fresh weight of Zea mays L. on 12th day was higher in 20 ppm (3.00 ± 0.10 grams) (Table 2). Similarly, the total dry weight of Zea mays L. was higher in 20 ppm (2.90 ± 0.10 grams). It is similar to the results obtained in the past studies (Kolenčík et al. 2019).
Table 2. Effect of copper nanoparticles on fresh and dry weight of Zea mays L. seeds on 12th day
| Concentration of copper nanoparticles | Fresh weight of Zea mays L.(grams) | Dry weight of Zea mays L. (grams) |
| Control | 1.83± 0.06 | 1.27±0.06 |
| 20ppm | 3.00± 0.10 | 2.90±0.10 |
| 40ppm | 2.10± 0.06 | 2.07±0.10 |
| 60ppm | 1.83±0.15 | 1.73±0.06 |
Values represent the mean (±) standard error of three independent experiments. All the experiments were statistically analyzed by One-way Anova using SPSS interpretation. The results were significant at p < .05
In vivo growth analysis of Zea mays L. supplemented with copper nanoparticles at one time: Figure 7 shows the in vivo growth of Zea mays L. supplemented with various concentrations (20 ppm, 40 ppm and 60 ppm) of copper nanoparticles. The root length of Zea mays L. was higher in 20 ppm (11.73 ± 0.21 cm), (Table 3). Similarly, the shoot length was higher in 20 ppm (12.27 ± 025 cm). The leaf length of Zea mays L. was higher in 20 ppm (45.23 ± 0.25 cm). Similarly, the leaf surface area was higher in 20 ppm (39.67 ± 0.21 cm2) (Rajput et al. 2018; Wu et al. 2020).
Table 3. Root and shoot length of Zea mays L. supplemented with copper nanoparticles at one time
| Concentration of copper nanoparticles
(ppm) |
Root length(cm) | Shoot length(cm) | Leaf length(cm) | Leaf surface area(cm2) |
| Control | 8.90±0.10 | 10.97±0.15 | 35.83±1.26 | 28.80±0.26 |
| 20 | 11.73±0.21 | 12.27±025 | 45.23±0.25 | 39.67±0.21 |
| 40 | 8.83±0.15 | 7.30±0.20 | 37.00±0.10 | 29.50±0.30 |
| 60 | 8.47±0.06 | 7.10±0.10 | 32.37±0.47 | 24.77±0.23 |
Values represent the mean (±) standard error of three independent experiments. All the experiments were statistically analyzed by One-way Anova using SPSS interpretation. The results were significant at p < .05
Biomass analyses of Zea mays L. supplemented with copper nanoparticles at one time: The root weight of Zea mays L. was higher in 20 ppm (0.636 ± 0.004 grams). Similarly, the shoot weight was higher in 20 ppm (0.802 ± 0.003 grams). The weight of the leaf was higher in 20 ppm (0.906 ± 0.003 grams). Similarly, the whole plant weight was higher in 20 ppm (2.344 ± 0.005 grams) (Table 4). In the present study we analyzed the role of copper nanoparticles in Zea mays L. and it was revealed that 20 ppm concentration proved best for the growth and yield of Zea mays L. based on the parameters like root, shoot length, leaf surface area and leaf length. Similar results are observed in past studies (Hafeez et al. 2015; Wu et al. 2020).
Figure 7: In vivo growth analysis of Zea mays supplemented with copper nanoparticles at one time.
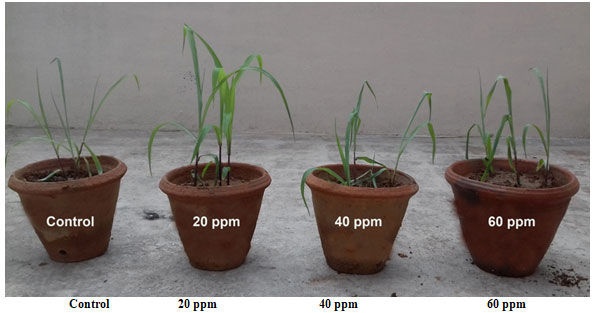
Table 4. Root, shoot, leaf and whole plant weight of Zea mays L. supplemented with copper nanoparticles at one time.
| Concentration of copper nanoparticles
(ppm) |
Root weight(grams) | Shoot weight(grams) | Leaf weight(grams) | Whole plant weight(grams) |
| Control | 0.545±0.003 | 0.612±0.003 | 0.666±0.008 | 1.810±0.009 |
| 20 | 0.636±0.004 | 0.802±0.003 | 0.906±0.003 | 2.344±0.005 |
| 40 | 0.549±0.001 | 0.613±0.002 | 0.665±0.005 | 1.818±0.008 |
| 60 | 0.540±0.001 | 0.607±0.003 | 0.662±0.004 | 1.807±0.005 |
Values represent the mean (±) standard error of three independent experiments. All the experiments were statistically analyzed by One-way Anova using SPSS interpretation. The results were significant at p < .05
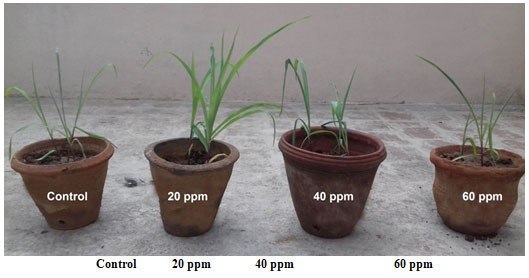
In vivo growth analysis of Zea mays L. supplemented with copper nanoparticles continuously for 15 days: Figure 8 shows the in vivo growth of Zea mays L. supplemented with various concentrations (20 ppm, 40 ppm and 60 ppm) of copper nanoparticles continuously for 15 days. The root length was higher in 20 ppm (12.37 ± 0.15 cm) (Table 5). The shoot length was higher in 20 ppm (13.23 ± 0.25 cm). Based on measuring the leaf length and leaf surface area it was revealed that the leaf length was higher in 20 ppm (46.97 ± 006 cm) (Table 5). Similarly, the leaf surface area was higher in 20 ppm (41.17 ± 0.29 cm2). The results were similar to results obtained in past studies (Margenot et al. 2018; Wu et al. 2020).
Table 5. Root and shoot length of Zea mays L. supplemented with copper nanoparticles continuously for 15 days.
| Concentration of copper nanoparticles
(ppm) |
Root length(cm) | Shoot length(cm) | Leaf length(cm) | Leaf surface area(cm2) |
| Control | 8.90±0.10 | 10.97±0.15 | 35.83±0.06 | 28.80±0.29 |
| 20 | 12.37±0.15 | 13.23±0.25 | 46.97±006 | 41.17±0.29 |
| 40 | 10.93±0.06 | 10.10±0.10 | 37.97±0.06 | 30.33±0.49 |
| 60 | 8.77±0.25 | 10.03±0.06 | 34.80±0.26 | 26.17±0.29 |
Values represent the mean (±) standard error of three independent experiments. All the experiments were statistically analyzed by One-way Anova using SPSS interpretation. The results were significant at p < .05
Biomass analyses of Zea mays L. supplemented with copper nanoparticles continuously for 15 days: The root weight was higher in 20 ppm (0.637 ± 0.003 grams), (Table 6). The shoot weight was also higher in 20 ppm (0.804 ± 0.001 grams). The leaf weight was higher in 20 ppm (0.923±0.002 grams). Similarly, the whole plant weight was higher in 20 ppm (2.367±0.20grams). In a study the effect of silver nanoparticles on plant growth parameters such as root length, fresh weight dry weight, and germination percentage of fenugreek were analyzed.
The result of this experiment showed that use of silver nanoparticles increased the germination in Fenugreek (Hojjat 2015). These results correlate with the present study as we supplemented different concentration of copper nanoparticles (20 ppm, 40 ppm and 60 ppm) for 15 days in Zea mays L .and lower concentrations i.e., 20 ppm concentration of copper nanoparticles had significant positive influence on root, shoot and leaf length, leaf surface area, root, shoot leaf and whole plant weight (Hojjat 2015; Plaksenkova et al. 2019; Wu et al. 2020).
Table 6. Root, shoot, leaf and whole plant weight of Zea mays L. supplemented with copper nanoparticles continuously for 15 days
| Concentration of copper nanoparticles
(ppm) |
Root weight(grams) | Shoot weight(grams) | Leaf weight(grams) | Whole plant weight(grams) |
| Control | 0.545±0.003 | 0.612±0.003 | 0.666±0.008 | 1.810±0.009 |
| 20 | 0.637±0.003 | 0.804±0.001 | 0.923±0.002 | 2.367±0.20 |
| 40 | 0.546±0.004 | 0.615±0.005 | 0.671±0.001 | 1.824±0.006 |
| 60 | 0.541±0.002 | 0.605±0.003 | 0.661±0.001 | 1.805±0.006 |
Values represent the mean (±) standard error of three independent experiments. All the experiments were statistically analyzed by One-way Anova using SPSS interpretation. The results were significant at p < .05
Fig. 8 In vivo growth of Zea mays L. supplemented with copper nanoparticle continuously for 15 days
Analysis of chlorophyll: Copper nanoparticles supplemented at one time on day 1 in Zea mays L. possess increase in the chlorophyll content from control (Total chlorophyll 18.5 mg) to 40 ppm (Total chlorophyll 26.2 mg) concentration of copper nanoparticles. Chlorophyll content decreased with 60 ppm (16.5 mg) concentration of copper nanoparticles. Copper nanoparticles supplemented continuously for 15 days in Zea mays L. possess increase in the chlorophyll content from control to 20ppm (Total chlorophyll 19.2 mg).
Further increase in the concentration of copper nanoparticles 40 ppm (15.2 mg) and 60 ppm (14.5 mg) decreases the total chlorophyll content. The results reveal that 20ppm concentration of copper nanoparticles is considered as the optimum level in increasing chlorophyll content as the increase in the chlorophyll content with 40 ppm (26.2) concentration copper nanoparticles supplemented at one time in Zea mays L. is negligible. It is similar to the results obtained in past studies (Plaksenkova et al. 2019).
Analysis of copper nanoparticles in plant material: The results of the atomic absorption spectrometry reveal that among the various concentration (20 ppm, 40 ppm and 60 ppm) of copper nanoparticles supplemented to Zea mays L. at one time, 60 ppm (1.5067 ± 0.0021), followed by 40ppm (0.0114 ± 0.0006) and 20 ppm (0.0044 ± 0.0004 ppm). The copper nanoparticle in control was 0.0025 ± 0.0033. Similarly, among the various concentration (20 ppm, 40 ppm and 60 ppm) of copper nanoparticles supplemented to Zea mays L. continuously for 15 days, 60 ppm (1.6110 ± 0.0036) has more copper particles followed by 40 ppm (0.0212 ± 0.0003 ppm) and 20 ppm (0.0084 ± 0.0002) The copper nanoparticles in control were 0.0025 ± 0.003.
Accumulation Factor (AF): To evaluate the copper nanoparticle accumulation in the plant tissue, the accumulation factor (AF) was calculated on the effect of copper on Zea mays L. and tabulated in Table 7. The accumulation factor was significantly increased with the increasing concentrations of copper nanoparticles. Accordingly, the accumulation factor in Zea mays L. was ranging from 0.004 ppm to 1.40 ppm with copper nanoparticles supplemented at one time. Similarly, the accumulation factor in Zea mays L. was ranging from 0.007 ppm to 1.50 ppm with copper nanoparticles supplemented continuously for 15 days. Many studies on accumulation of nanoparticles in plants reveals that they influence crop improvement, yield, plant advancement and huge numbers have aggregated in various plant tissues (Javed et al. 2019; Melusi et al. 2021).
The accumulation factor was significantly increased with the increasing concentrations of copper nanoparticles in the present study. However, increasing concentration of nanoparticles in plants causes toxicity in molecular and cellular level. In a study by Melusi et al. (2021), accumulation of silver nanoparticles in plants were relatively higher with large sized particles compared to the small one. Since copper nanoparticles are usually large in size accumulation could be less when compared to accumulation of small sized nanoparticles (Melusi et al. 2021).
Table 7. Accumulation factor of copper nanoparticle
| Nanoparticles | Control (ppm) | 20 ppm | 40 ppm | 60 ppm |
| Copper
nanoparticles supplemented at one time to Zea mays L. |
0.002 | 0.004 | 0.0106 | 1.40 |
| Copper nanoparticles supplemented continuously for 15 days to Zea mays L. | 0.002 | 0.007 | 0.019 | 1.50 |
CONCLUSION
The findings of the present study reveal that copper nanoparticles have potential to enhance the growth of Zea mays L. Among the various concentrations (20 ppm, 40 ppm and 60 ppm) of copper nanoparticles, 20 ppm is considered as the optimum level for the growth of Zea mays L. However higher concentration (40 ppm, 60 ppm and more) of nanoparticles affect the plant growth. The outcome of the present study will be useful in finding the potential of nanoparticles in crop improvement and other agricultural applications.
ACKNOWLEDGEMENT
This study was financially supported by the Secretary, Sri Kaliswari College, Sivakasi, Tamil Nadu, India.
Conflict of Interests: Authors declare no conflicts of interests to disclose.
REFERENCES
Almutairi, Z.M. and Alharbi, A., (2015). Effect of silver nanoparticles on seed germination of crop plants. Journal of Advances in Agriculture, 4(1):283-288.
Farooqui, A.R.E.E.B.A., Tabassum, H.E.E.N.A., Ahmad, A.S.A.D., et al. (2016). Role of nanoparticles in growth and development of plants: A review. Int J Pharma Bio Sci, 7(4):22-37.
Genady, E.A., Qaid, E.A. and Fahmy, A.H., (2016). Copper sulfate nanoparticales in vitro applications on Verbena bipinnatifida Nutt. Stimulating growth and total phenolic content increments. Int. J. Pharm. Res. Allied Sci, 5, 196-202.
Ghosh, M.K., Sahu, S., Gupta, I. et al. (2020). Green synthesis of copper nanoparticles from an extract of Jatropha curcas leaves: characterization, optical properties, CT-DNA binding and photocatalytic activity. RSC Advances, 10(37), 22027-22035.
Hafeez, A., Razzaq, A., Mahmood, T. et al. (2015). Potential of copper nanoparticles to increase growth and yield of wheat. J Nanosci Adv Technol, 1(1), 6-11.
Hojjat, S.S., (2015). Impact of silver nanoparticles on germinated fenugreek seed. Int J Agric Crop Sci, 8(4), 627-30.
Javed Z, Dashora K, Mishra M, et al. (2019). Effect of accumulation of nanoparticles in soil health-a concern on future. Frontiers in Nanoscience and Nanotechnology. 5(1), 1-9.
Kolenčík, M., Ernst, D., Komár, M., et al. (2019). Effect of foliar spray application of zinc oxide nanoparticles on quantitative, nutritional, and physiological parameters of foxtail millet (Setaria italica l.) under field conditions. Nanomaterials, 9(11), 1559.
Margenot, A.J., Rippner, D.A., Dumlao, M.R., et al. (2018). Copper oxide nanoparticle effects on root growth and hydraulic conductivity of two vegetable crops. Plant and Soil, 431(1), 333-345.
Milewska-Hendel, A., Gawecki, R., Zubko, M., et al. (2016). Diverse influence of nanoparticles on plant growth with a particular emphasis on crop plants. Acta Agrobotanica. 69(4), 1-9.
Mustafa, H.S., Oraibi, A.G., Ibrahim, K.M. et al. (2017). Influence of silver and copper nanoparticles on physiological characteristics of Phaseolus vulgaris L. in vitro and in vivo. Int J Curr Microbiol Appl Sci, 6, 834-843.
Patil, S.A., Ryu, C.H. and Kim, H.S., (2018). Synthesis and characterization of copper nanoparticles (Cu-Nps) using rongalite as reducing agent and photonic sintering of Cu-Nps ink for printed electronics. International Journal of Precision Engineering and Manufacturing-Green Technology, 5(2), 239-245.
Plaksenkova, I., Jermaļonoka, M., Bankovska, L., et al. (2019). Effects of Fe3O4 nanoparticle stress on the growth and development of rocket Eruca sativa. Journal of Nanomaterials. 2019, 1-11.
Rajkumar, T., Sapi, A., Das, G., et al. (2019). Biosynthesis of silver nanoparticle using extract of Zea mays (corn flour) and investigation of its cytotoxicity effect and radical scavenging potential. Journal of Photochemistry and Photobiology B: Biology, 193, 1-7.
Rajput, V.D., Minkina, T., Suskova, S., et al. (2018). Effects of copper nanoparticles (CuO NPs) on crop plants: a mini review. BioNanoScience, 8(1), 36-42.
Shobha, G., Moses, V. and Ananda, S., (2014). Biological synthesis of copper nanoparticles and its impact. Int. j. pharm. sci. Invent, 3(8), 6-28.
Siddiqui, M.H., Al-Whaibi, M.H. and Mohammad, F., (2015). Nanotechnology and plant sciences. Springer International Publishing Switzerland. DOI, 10, 978-3.
Sriram, T. and Pandidurai, V., (2017). In vitro growth analysis of Zea mays L. using silver nanoparticles. Int J pharma Bio Sci, 8(3b), 30-37.
Sriram, T. and Pandidurai, V., (2016). Degradation of atrazine using copper nanoparticles synthesized from the leaf extract of Zea mays L. Journal of Science. 6(5), 235-238.
Tanveer, M., Anjum, S.A., Zahid, et al. (2014). Growth and development of maize (Zea mays L.) in response to different planting methods. Journal of Agricultural Research, (03681157), 52(4).
Thwala, M., Klaine, S. and Musee, N., (2021). Exposure Media and Nanoparticle Size Influence on the Fate, Bioaccumulation, and Toxicity of Silver Nanoparticles to Higher Plant Salvinia minima. Molecules, 26(8), 2305.
Wu, S., Rajeshkumar, S., Madasamy, M. et al. (2020). Green synthesis of copper nanoparticles using Cissus vitiginea and its antioxidant and antibacterial activity against urinary tract infection pathogens. Artificial Cells, Nanomedicine, and Biotechnology, 48(1), 1153-1158.


