Patna University, Department of Botany, Patna-800 005
*Head of the Department Biochemistry (Retd.), Patna University, Patna- 800 005, India
Corresponding author email: abhaykumar.kr@gmail.com
Article Publishing History
Received: 14/07/2020
Accepted After Revision: 20/09/2020
The study revealed the impact of various concentration of arsenic chloride on the biochemical characteristics of Abelmoschus esculentus L. The results have shown that photosynthetic pigment such as total chlorophyll shows declines trend form stress of 2mM to 10mM. The total soluble sugar and the protein content in the leaves were found to decrease with increase in the short concentration of heavy metal treatment. The most abundant forms of arsenic in the environment are the inorganic As (V) and As (III) species, and only the organic species monomethyl arsenic acid (MMAA) and dimethyl arsenic acid (DMAA) can be found in detectable concentrations. The form in which As is present in the environment influences its chemical behaviour and its toxicity.
Concentrations of arsenic in uncontaminated soils range from 0.2 to 40 ppm. In uncontaminated soils, the level of arsenic is not sufficiently high to cause phytotoxicity and does not, therefore, represent a health hazard. Soils from land repeatedly treated with inorganic arsenicals contain levels of arsenic often 10-100 folds those of untreated areas. Use of arsenicals as insecticides usually results in higher concentrations of arsenic in the soil than when they are used as defoliants. Under strongly reducing conditions elemental arsenic (AS4) and arsine (AsH3) (-III) are the stable forms. Species in the cells causing cell damage. Research studies revealed that Arsenic could inhibit 200 Arsenic can exert its toxic effects through impairment of cellular respiration by inhibition of various mitochondrial enzymes and uncoupling of oxidative phosphorylation. The As (III) species can react with the SH group of protein and enzymes, thereby make them inactive and increase reactive oxygen enzymes in the body.
Abelmoschus esclulentus, Arsenic Chloride, MMAA (monomethyl arsenic chloride), DMAA (Dimethyl arsenic Acid)
Kumar A, Sinha R, Kumar A. Impact of Arsenic on Biochemical Component of Abelmoschus esculentus Arsenic And Abelmoschus esculentus. Biosc.Biotech.Res.Comm. 2020;13(3).
Kumar A, Sinha R, Kumar A. Impact of Arsenic on Biochemical Component of Abelmoschus esculentus Arsenic And Abelmoschus esculentus. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/3g2V0G4
Copyright © Kumar et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Arsenic pollution in groundwater has become a major public concern in many countries and potentially impacting millions of people since more and more groundwater withdrawal are taking place due to human usage and agricultural irrigation. The most abundant forms of arsenic in the environment are the inorganic As (V) and As (III) species, and only the organic species monomethyl arsenic acid (MMAA) and dimethyl arsenic acid (DMAA) can be found in detectable concentrations (Rahman et al., 2008; Tlustos et al., 2002). The form in which As is present in the environment influences its chemical behaviour and its toxicity. Generally, As (III) is more mobile and more toxic than As (V) and inorganic arsenicals are more toxic than organic arsenicals (Chung et al., 2006; Inskeep et al., 2002, Chakarborti et al., 2018).
Concentrations of arsenic in uncontaminated soils range from 0.2 to 40 ppm. In uncontaminated soils, the level of arsenic is not sufficiently high to cause phytotoxicity and does not, therefore, represent a health hazard. Soils from land repeatedly treated with inorganic arsenical contain levels of arsenic often 10-100 folds those of untreated areas. Use of arsenicals as insecticides usually results in higher concentrations of arsenic in the soil than when they are used as defoliants. Under strongly reducing conditions elemental arsenic (AS4) and arsine (AsH3) (-III) are the stable forms (Wan et al., 2019).
In a less reduced environment such as those in flooded soils, the relatively toxic arsenite (MAsO2) (+III) can be formed. However, in aerated soils, arsenate (MAsO4) (+V) predominates. In reduced through arsenite to dimethyl arsenic acid, which is extremely toxic. Soluble arsenic has been observed to increase in flooded rice soils (Wan et al., 2019). Arsenic can exert its toxic effects through impairment of cellular respiration by inhibition of various mitochondrial enzymes and uncoupling of oxidative phosphorylation. The As (III) species can react with the SH group of protein and enzymes, thereby make them inactive and increase reactive oxygen species in the cells causing cell damage. Research studies revealed that Arsenic could inhibit 200 enzymes in the body. It has been regarded that multi-systemic non-cancerous could be due to deactivation of essential enzymatic functions by trivalent Arsenic compounds and subsequent oxidative stress to cell (Wan et al., 2019).
More recent studies have detected all the four species [As (III), As (V), MMA (V), DMA (V)] and also the presence of MMA (III) and DMA (III) in the urine. It is also considered that inorganic As (III) and the reduced forms of MMA (III) and DMA (III) formed during methylation are highly reactive and contribute to the observed toxicity of inorganic Arsenic. So far, no evidence has been found that inorganic Arsenic directly causes genetic mutations affecting cancerous cells. Inorganic Arsenic indirectly enhances susceptibility to cancer-inducing chromosomal alterations, inhibition of DNA repair process, oxidative stress and cell proliferation. Arsenate (AsO43 –) has a similar structure as phosphate (PO43 –) and thus can substitute for PO43 – in adenosine diphosphate (ADP). This substitution prevents the conversion of ADP to ATP which produces energy to the cell (Wan et al., 2019).
MATERIAL AND METHODS
Collection of Plant Material: Seeds of Abelmoschus esculentus, L., was procured from local seed centre, Patna, Bihar, India.
Abelmoschus esculentus, L. Var. S7 (Family; Malvaceae) was chosen as an experimental plant. The effects of various concentrations of arsenic chloride on biochemical features were analyzed on the selected plant.
Preparation of the Experimental Soil: The experimental soil for raising the cultivars was prepared by mixing red soil, black soil and sandy soil in the ratio of 1:1:1. The prepared soil was sterilized by solar sterilization method for 5 days (Handiseni et al., 2010). It was then analysed for its physicochemical properties. The analysed soil medium was taken in earthen pots of size 30×33 cm and filled in about two-third of its height (5 kg of soil per pot).
Heavy Metals used for the Study: To investigate the effect of heavy metals on Abelmoschus esculentus L., Arsenic was applied in the form of arsenic chloride (AsCl2).
Experimental Design :Heavy Metals Stress on Abelmoschus esculentus: The heavy metal arsenic was treated separately in the experimental plant with different concentrations viz., 2 mM, 4 mM, 6 mM, 8 mM and 10 mM (w/v) in five replicates. The aqueous solutions of heavy metal were applied to the soil after the development of first leaves in the seedlings. Then the plant was watered with the respective concentration of metals on every alternate day. A set of plants without heavy metal treatment was maintained as control. The surface-sterilized seeds of Abelmoschus esculentus, L., was sown uniformly in the pots for the experimental purpose. The biochemical parameters and metal concentration in plants were analysed on the 35th day after planting (DAP).
Impact assessment of Arsenic chloride on biochemical characteristics :Estimation of Chlorophyll: For extracting total chlorophyll from leaves, fresh leaves were deveined and cut into small bits. From the pooled leaf bits, a sample of 100 mg was weighed. The leaf bits were homogenized in 100% acetone using a mortar and pestle. The homogenate was centrifuged at 4000 rpm for 5 minutes at room temperature. Extraction with 100% acetone was repeated till the pellet becomes pale-yellow or white. The supernatant was used for the estimation of photosynthetic pigments. The absorbance was measured at 662nm, 645nm and 470nm for chlorophyll a, b and carotenoids, respectively using a spectrophotometer. The amount of chlorophyll a, chlorophyll b and total chlorophyll was calculated using the formulae of Wellburn and Lichtenthaler 1984.

Estimation of Total Soluble Sugar: Exactly 100 mg of leaf sample was ground in 20 mL of distilled water using a mortar and pestle. The homogenate was filtered through two layers of cheesecloth and the filtrate was spun at 3000 rpm for 5 minutes. The pellet was discarded and the supernatant was taken. Three mL of Trichloroacetic acid (TCA) was added to the supernatant. It was thoroughly mixed and kept in ice for 10 minutes. This mixture was centrifuged at 3000 rpm for 5 minutes. The pellet was discarded and the supernatant was used as a test solution. An aliquot of 0.1 mL of test solution was taken in a test tube and to this, 0.9 mL of distilled water and 4 mL of anthrone reagent were added. The solution was mixed thoroughly and the tubes were kept in boiling water for 10 minutes. Glucose content was measured using a standard value (Jayaraman, 1981).
Estimation of Protein: The total soluble protein was estimated by Lowry’s method (Lowry et al., 1951). Fresh leaf samples were ground in 10 mL of distilled water using mortar and pestle. The homogenate was spun at 3000 rpm for 5 minutes.
The supernatant was taken and the pellet was discarded. To the supernatant, 1 mL of ice-cold 10% (w/v) TCA was added and kept in ice for 10 minutes. The extract was centrifuged at 5000 rpm for 10 minutes. The pellet was dissolved in 0.1N NaOH and used as the test solution.

The mixture of 0.5 mL of A and 0.5 mL of B with 4.9 mL of solution C is known as an alkaline copper reagent. An aliquot of 0.1 mL of test solution was taken in a test tube and 0.4 mL of distilled water, 0.5 mL of freshly diluted (1:1) folin-phenol reagent and 5.5mL of alkaline copper reagent were added. Contents in the test tube were mixed immediately and left undisturbed for 10 minutes for the development of blue colour. The absorbance was measured at 650 nm with a spectrophotometer with alkaline copper reagent as blank. The protein content was calculated from a standard graph of protein constructed with bovine serum albumin (BSA) as marker protein.
RESULTS AND DISCUSSION
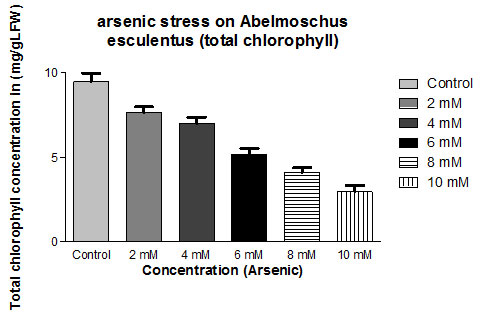
Total Chlorophyll Content: The results of the effect of arsenic chloride and nickel chloride on the photosynthetic pigment contents of co-cultivated Abelmoschus esculentus, L. have been represented in Table 1. The total chlorophyll content under arsenic chloride treatment was found reduced to 19% (at 02 mM Conc.; Mean ± SD 7.65 ± 0.311) to 69% (at 10 mM Conc.; Mean ± SD 2.95 ± 0.364) in Abelmoschus esculentus, L.
Table 1
| Arsenic stress on Abelmoschus esculentus (Total Chlorophyll) | |||
| Sample | mean±SD | %Change | |
| Control | 9.45±0.492 | ||
| 2mM | 7.65±0.311 | -19.0476 | |
| 4mM | 6.99±0.354 | -26.0317 | |
| 6mM | 5.16±0.359 | -45.3968 | |
| 8mM | 4.11±0.261 | -56.5079 | |
| 10mM | 2.95±0.364 | -68.7831 | |
Figure 1: Impact of arsenic chloride on the total chlorophyll content (mg/gLFW) of (Abelmoschus esculentus L.) (p ≤ 0.05)
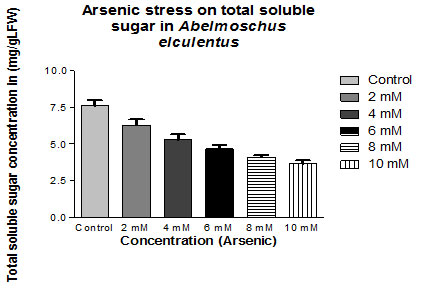
Total Soluble Sugar: The reduction in total Soluble Sugar and arsenic chloride treatment in Abelmoschus esculentus was 18% (at 02 mM Conc.; Mean ± SD 6.278 ± 0.394) to 52% (at 10 mM Conc.; Mean ± SD 3.638 ± 0.233) represented in table 2.
Table 2
| Arsenic stress on Total Soluble Sugar in A. E | ||
| Sample | mean±SD | %Change |
| Control | 7.60±0.393 | |
| 2mM | 6.278±0.394 | -17.5 |
| 4mM | 5.270±0.389 | -30.65789474 |
| 6mM | 4.652±0.270 | -38.81578947 |
| 8mM | 4.082±0.144 | -46.31578947 |
| 10mM | 3.638±0.233 | -52.23684211 |
Figure 2: Impact of arsenic chloride on the total soluble sugar content (mg/gLFW) of (Abelmoschus esculentus L.) (p ≤ 0.05)
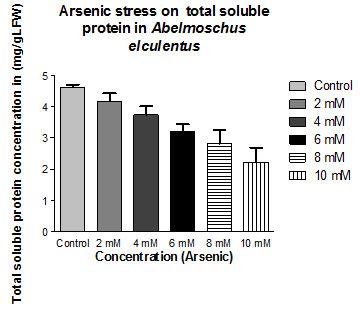
Total Soluble Protein: Under Arsenic Chloride treatment the percentage change in same plant was 09% (at 02 mM Conc.; Mean ± SD 4.19 ± 0.244) to 52% (at 10 mM Conc.; Mean ± SD 2.22 ± 0.461) represented in table: – 3
Table 3
| Arsenic stress on total soluble Protein in A. E | ||
| Sample | mean±SD | %Change |
| Control | 4.62±0.0873 | |
| 2mM | 4.19±0.244 | -9.307359307 |
| 4mM | 3.74±0.276 | -19.04761905 |
| 6mM | 3.20±0.248 | -30.73593074 |
| 8mM | 2.82±0.432 | -38.96103896 |
| 10mM | 2.22±0.461 | -51.94805195 |
Figure 3: Impact of arsenic chloride on the total protein content (mg/gLFW) of (Abelmoschus esculentus L.) (p ≤ 0.05)
Discussion
Inhibition of the photosynthetic pigment biosynthesis is one of the primary events in plants during heavy metal stress (Prasad and Prasad 1987). As a consequence, a delay in the assembly of the photosynthetic apparatus, lower photosynthetic efficiency, slower plant growth and decreased biomass production occur. Thus, heavy metal pollution could be a serious agricultural problem as it decreases the yield of crop plants and lowers the quality of plant products due to increased content of toxic metals (Melo et al. 2009). The present study revealed the impact of various concentrations of arsenic on the biochemical characteristics, of Abelmoschus esculentus, L. where photosynthetic pigments such as chlorophyll and carotenoid also showed a similar significant declining trend. However, the anthocyanin accumulates in the leaves with an increase in the concentration of metal indicating its antioxidant property and protective function against heavy metal pollutants (Melo et al. 2009).
The total soluble sugar and the protein content in the leaves were found to decrease with the increase in the concentration of heavy metals treatment. Reduction in protein level can be directly correlated to the observed increase in the accumulation of free amino acids. Proline accumulation was more in the stressed plants than in the control. Under the heavy metal treatment, there was a considerable reduction in the growth and photosynthetic pigments, which could be due to the disturbance in photosystem I and induced activity of chlorophyllase enzyme. This disturbance paralleled with the reduction in sugar content could be attributed to the reduction in chlorophyll contents of the leaf and also a decline in protein. This change might have already affected the photosynthetic activity in the plant and hence the reduction in carbohydrate contents (Dowton, 1977; Swaminathan et al., 1998).
The major total soluble protein in the leaf is RUBPCase. A reduction in leaf protein indicated the reduction in RUBPCase, which caused a reduction in photosynthetic activity, which in turn, affects the total soluble sugar level (Goodwin and Mercer, 2005). Reduction in the protein contents in the roots, leaves and petioles of water hyacinth and lettuce plants after chromium treatment and suggested that metal ions seem to interfere with protein synthesis which is one of the major components of biochemical activities. In the present study, a reduction in protein content observed in both arsenic and nickel treated plants, could be attributed to the decrease in the synthesis of protein macromolecules under metal toxicity and the denaturation of protein by protease activity resulting in increasing level of protein degradation. The reduction in sugar content could be attributed to the reduction in chlorophyll content of the leaf and also a decline in protein. This change might have already affected the photosynthetic activity of the plant and hence the reduction in carbohydrate content (Macfie and Taylor, 1992).
Impaired carbon flow through the glycolytic pathway may sometimes due to disturbed carbohydrate metabolism which decreases the rate of sucrose formation as reported (Clemens, 2006). As a result of protein degradation, the availability of free amino acids is significantly high in hyperaccumulators. The free amino acid content is increased with increasing concentration of the arsenic chloride and nickel chloride. It may be due to the destruction of protein or increase in the biosynthesis of amino acids from the nitrate source, which was not utilized in the protein synthesis (Schmoger et al., 2000). The degradation of protein may lead to an increase in free amino acid content. It is an adaptive mechanism employed by the plant cell to overcome post-stress metabolism (Singh and Vijayakumar, 1974). Our work emphasized that to science-based decisions with standards and limitations set by regulatory bodies and a clearer understanding and explanation of observation on wastewater treatment systems.
Summary
The present study revealed the impact of various concentrations of arsenic on the biochemical characteristics, of Abelmoschus esculentus, L. Photosynthetic pigments such as chlorophyll and carotenoid also showed a similar significant declining trend. However, the anthocyanin accumulates in the leaves with an increase in the concentration of metal indicating its antioxidant property and protective function against heavy metal pollutants. The total soluble sugar and the protein content in the leaves were found to decrease with the increase in the concentration of heavy metals treatment. Reduction in protein level can be directly correlated to the observed increase in the accumulation of free amino acids. Proline accumulation was more in the stressed plants than in the control.
CONCLUSION
Extensive progress has been made in characterizing soil chemistry management needed for phytoremediation, and physiology of plants which hyperaccumulate and hypertolerate metals, there is still the need for more nutrient-related research monitoring to achieve unpolluted wastewater discharge. It is increasingly clear that hypertolerance is fundamental to hyperaccumulation, and high rates of uptake and translocation are observed in hyperaccumulator plants. Search for superior hyperaccumulator plants and agronomic technology to improve the annual rate of phytoextraction and to allow recycling of water toxic metals accumulated in plant biomass is very likely to support commercial environmental remediation which society can afford. In addition, opportunities should be identified for research and development to improve the efficiency of phytoremediation. This will help to ensure science-based decisions with standards and limitations set by regulatory bodies and a clearer understanding and explanation of observation on wastewater treatment systems.
ACKNOWLEDGEMENTS
I would like to thank the head of the department botany as well as my supervisor department of biochemistry for the immense support. I also like to pay sincere gratitude to my co-worker and supporting staff of the department who supported during paper write-up.
Conflict of Interest Statement: The author has no conflict of interest.
REFERENCES
Chakraborti, D., Singh, S. K., Rahman, M. M., Dutta, R. N., Mukherjee, S. C., Pati, S., and Kar, P. B. (2018). Groundwater Arsenic Contamination in the Ganga River Basin: A Future Health Danger. International journal of environmental research and public health, 15(2), 180. https://doi.org/10.3390/ijerph15020180
Chung, J., Li, X., and Rittmann, B. E. (2006). Bio-reduction of arsenate using a hydrogen-based membrane biofilm reactor. Chemosphere, 65(1), 24–34. https://doi.org/10.1016/j.chemosphere.2006.03.018
Clemens, S. (2006). Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie. 88: 1707 – 1719.
Dowton, W.J.S. (1977). Photosynthesis in salt stressed in grape wines. Aust. J.Plant Physiol. 4: 183 – 192.
Goodwin, F.W. and Mercer, F.T. (2005). Introduction to plant biochemistry, 2nd Edition, Pergamon Press. New York.
Handiseni, M., Sibiya, J., Ogunlela, V. and Koomen, I. (2010). Evaluation of non-chemical methods of soil sterilization in Paprika (Capsicum annum L.) Seedlings production in the small holder farming sector of Zimbabwe. Agricultura Tropica ET Subtropica. 43 (2): 97 – 108.
Inskeep, W.P., McDermott, T.R. and Fendorf, S. (2002). Arsenic (V)/(III) cycling in soil and batural waters: chemical and microbiological processes. In: Environmental Chemistry of Arsenic. Eds. Frankenberger, W.T., Jr., Marcel Dekker, New York, PP. 183 – 215.
Jayaraman, J. (1981). Laboratory manual in Biochemistry, Willey-Eastern Company Limited, Madras pp. 1– 65.
Macfie, S.M. and Taylor, G.J. (1992). The effects of excess manganese on photosynthetic rate and concentration of chlorophyll in Triticum aestivum grown in solution culture, Physiol. Plantarum. 85 467 –472.
Melo EEC, Costa ETS, Guilherme LRG, Faquin V, and Nascimento CWA (2009) Accumulation of arsenic and nutrients by castor bean plants grown on an As-enriched nutrient solution. J Hazard Mater 168:479–483
Prasad, D.P.H. and Prasad A.R.K. (1987). Effects of lead and mercury on chlorophyll synthesis in mungbean seedlings. Phytochem. 26: 881 –883.
Rahman, M. A., Hasegawa, H., Ueda, K., Maki, T., and Rahman, M. M. (2008). Influence of EDTA and chemical species on arsenic accumulation in Spirodela polyrhiza L. (duckweed). Ecotoxicology and environmental safety, 70(2), 311–318. https://doi.org/10.1016/j.ecoenv.2007.07.009
Schmoger, M.E., Oven, M. and Grill, E. (2000). Detoxification of arsenic by phytochelatins in plants. Plant Physiol. 122: 793 – 801.
Singh, D.S. and Vijayakumar, K.P. (1974). Carry out the effects of Salinity on yield and quality of wheat seed. Seed. Res. 21: 13 – 18.
Swaminathan, K., Arjunan, J. and Gurusamy, R. (1998). Effect of glucose factory effluents on the seed germination and seedling development of groundnut (Arachis hypogea,L.). Envirion. Biol. 2: 187 – 189.
Tlustos, P., Goessler, W., Szakova, J. and Balik, J. (2002). Arsenic compounds in leaves and roots of radish grown in soil treated by arsenite, arsenate and dimethylarsinic acid. Appl. Organometal. Chem. 16: 216 – 220.
Wan, Y., Huang, Q., Camara, A. Y., Wang, Q., and Li, H. (2019). Water management impacts on the solubility of Cd, Pb, As, and Cr and their uptake by rice in two contaminated paddy soils. Chemosphere, 228, 360–369. https://doi.org/10.1016/j.chemosphere.2019.04.133