1Department of Biotechnology, P.S. Science & H.D. Patel Arts College, Kadi, Gujarat, H.N.G. University, Patan India
2Institute of Science, Nirma University, Ahmadabad, Gujarat, India
Corresponding authour email: jignesh.pathak56@gmail.com
Article Publishing History
Received: 14/12/2020
Accepted After Revision: 23/03/2021
Custard apple has many alkaloids, such as aporphine, roemerine, norcorydine, corydine, norisocorydine, glaucine and anonaine in different parts of the plant. The roots are used to treat acute dysentery, depression and spinal marrow diseases, while leaves have been used in cases of prolapse of the anus, sores and swelling. Ripe fruit is sweet and good tonic for human health and it is enriching the blood, increases muscular strength and lessens vomiting. Its seeds are used as abortifacients. Seed oil is used in paint and soap industry. This investigation involves preliminary screening, detection, and separation of secondary metabolites from the seed extract of Annona squamosa Linn. Oil was extracted from seeds of Annona squamosa Linn. by Soxhlet extraction method; methanol was used as a solvent for extraction.
Absorbance and functional group detection were studied using FTIR, seed oil supernatant was used to detect the functional groups of secondary metabolites those were presented in sample. A potent Cyanidin-3-O-(2-O-beta-xylopyranosyl-6-O-acetyl)-beta-galactopyranoside, 4-[4-(2aminoethyl)-2,6Diiodophenoxy]2-iodophenol, Amikacin. The study was done using atmospheric pressure chemical ionization (APCI) Liquid Chromatography mass Spectroscopy technique for identification of Phytochemical. Various phytochemicals were detected using this technique. The analysis by APCI-LC-MS reveals presence of several compounds like cyclopeptides and acetogenins. Cyclosqamosin A, Cyclosqamosin B, Cyclosqamosin H, Acetogenins (polyketides); Annonacin, Squamocin, Annonin VI, which were detected by peaks of m/z ratio between 605 to 640 positive ions shift and Tenacissoside F (steroid) at 667 m/z negative ion shift. Identified compounds were compared with reference of earlier investigations, it was clear that this compound played a major role as anti-diabetic, anticancer, anti-inflammatory and have insecticidal property. However, further Research is required to study phytochemicals.
FTIR, APCI-LC-MS, Tenacissoside F, Cyanidin-3-O-Beta-Galactopyranoside, Squamocin,
Pathak J, Patel P. K, Suthar R, Shah K. R . Identification of Phytochemicals from seed extract of Custard Apple (Annona squamosa L.). Biosc.Biotech.Res.Comm. 2021;14(1).
Pathak J, Patel P.K, Suthar R, Shah K.R . Identification of Phytochemicals from seed extract of Custard Apple (Annona squamosa L.). Biosc.Biotech.Res.Comm. 2021;14(1). Available from: <a href=”https://bit.ly/3bnwal1“>https://bit.ly/3bnwal1</a>
Copyright © Pathak et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Custard apple is tropical fruit which belongs to genus Annona and family Annonaceae and are collectively known as annonaceous fruits. There are over 120 species of genus Annona and are commonly found in India as a fruit consuming plant. This plant is commonly known as custard apple in English and Sitafal in Gujarati. Custard apple contains alkaloids, such as aporphine, roemerine, norcorydine, corydine, norisocorydine, glaucine and anonaine in various parts of the plant (Kowalska and Puett, 1990; Pinto et al., 2005; Hiwale, 2015). The Custard apple roots are used in the treatment of acute dysentery, spinal marrow diseases and some cases of depression. The leaves of Custard apple are used in cases of prolapse of the anus, sores and swelling. Custard apple contains alkaloids, such as aporphine, roemerine, norcorydine, corydine, norisocorydine, glaucine and anonaine in various parts of the plant (Kowalska and Puett, 1990; Chao-Ming et al., 1997; Pinto et al., 2005).
Seeds of Custard apple contains acetogenins namely; squamocins B to N, coumarinoligans, annotemoyin-1, annotemoyin-2, squamocin and cholesteryl, glucopyranoside (Zahid et al., 2018). Seeds of Custard apple are toxic, but they are used to treat head lice as they have insecticidal properties (its preparation causes eye irritation and can cause blindness). Seeds of Annona also have insecticidal properties. Farmers use pesticides to protect their crops from pest infestation, using chemical pesticides is no longer preferable. Seeds of custard apple can be used as biopesticide which can be used as suitable alternatives for pest control. Oil content is high in seeds which can be used to make soap or, if treated to remove the toxic alkaloids, it can be used as a cooking oil (Das et al., 2016; Vetal and Pardeshi, 2019). Earlier studies have been done to study phytochemicals obtained from seeds, leaves. Investigations done in past suggests that the alkaloids from Annona species have rarely been explored for their medicinal applications (Nugraha et al., 2019).
Many volatile components have been isolated from A. squamosa such as bullatacin, 12,15-cis-squamostatin-A, α-Pinene, β-caryophyllene, camphene, β-pinene, myrcene, anonaine, spathulenol, germacrene Duvariamicin-III, annonacin, squamocin, liriodenine, and molvizarin (Alkazman, Harnett and Hanrahan, 2020). This investigation involves preliminary screening, detection, and separation of secondary metabolites from the seed extract of Annona squamosa Linn. By comparing with reference of earlier studies, it was clear that seed extract contains compound that played a major role as anti-diabetic, anticancer, anti-inflammatory and have insecticidal property (Mangal et al., 2015; Ribeiro et al., 2018). The present investigation involves identification of such compounds that can be used for medicinal purposes.
MATERIAL AND METHODS
Seeds of Annona squamosa were collected from fruit of Annona squamosa L. seeds were tested and determined viability, viable seeds were selected for identification of phytochemicals as previous study (Patel et al., 2019). Seeds were crushed using mixer grinder and powdered seeds were filled in a paper cup for further oil extraction. Finely crushed seed powder packed in paper cup was taken for oil extraction using Soxhlet extraction method, methanol was used as a solvent for extraction. After few cycles the crude oil was obtained and stored in dark bottles for further phytochemical analysis.
FTIR spectral analysis was conceded from the Annona squamosa seed oil supernatant to detect the functional groups of secondary metabolites those were presented in sample. It was performed on BRUKER-FITR instrument in Department of chemistry, P. S. Science & H. D. Patel Arts College, Kadi. We used atmospheric pressure chemical ionization (APCI) Liquid Chromatography mass Spectroscopy technique for identification of Phytochemicals. According to APCI-LC-MS technique we have obtained major peaks, APCI (Positive) m/z at 623, 605, 587, 639 and APCI (Negative), we have obtained major peaks of m/z at 667, 683. The oil was used to check antibacterial activity against Bacillus subtills & Staphylococcus aureas, (Chavan, 2006).
RESULTS AND DISCUSSION
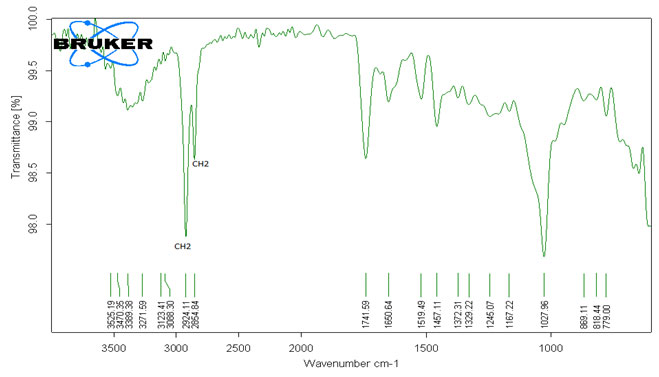
The Upper layer of Methanolic extract FTIR spectra had 20 peaks. The Peaks at 3625.19 cm-1, 3470.35 cm-1, 3389.38 cm-1, 3271.59 cm-1, 3123.41 cm-1, 3088.30 cm-1, 2924.11 cm-1, 2854.84 cm-1, 1741.59 cm-1 indicates the presence of O-H stretch, O-H free hydroxyl, Aromatic O-H free, Aromatic O-H (H-bonded), Dimer OH, #CH stretch, =CH stretch, CH stretch, C=O stretch doublet, Aromatic H stretch and Functional group free hydroxy Alcohol, Phenols, Carboxylic acids, Alkanes, Ketones and Aromatics. The Peaks formed at 1650.64 cm-1, 1519.49 cm-1, 1457.11 cm-1, 1372.31 cm-1, 1329.22 cm-1, 1245.07 cm-1, 1167.22 cm-1, 1027.96 cm-1, 869.11 cm-1, 818.44 cm-1, 779.00 cm-1 specify the presence of C=C stretch, C=O stretch (H-Bond), C=N, =NOH, N-O asymmetric stretch, N=O, Aromatic C-C stretch, -CH3, -CH2, S=O, R-F (C-F stretch), S=O (sulfone), N-O Symmetric stretch.
P-H bending (phosphine), P=O (phosphonate), P=O (phosphoramide), Si-CH3, N-O (aromatic), C-O stretch, R-F (C-F stretch), C=S (thiocarbonyl), P-H (phosphine), P=O (phosphine oxide), P=O (phosphate), C-O stretch, P-OR esters, Si-OR, C-H out of Plan, S-OR, RNH2, RNH, R2C=CHR (=CH out of plan), R-Cldemonstrated for the presence of Alkenes, Amides, Quinone or conjugated ketone, Imine, Oxime, Nitro compounds, Nitroso compounds, Aromatics, Sulphate ester, Alkyl halides, Sulfone, Ethers, Phosphine, Phosphonate, Phosphoramide, Trimethylsilyl, Amine Oxide, Carboxylic acids, Esters, Thiocarbonyl, Phosphine oxide, Phosphate, Phosphite Esters, Organosilicon, Amines respectively (Table-I) (Harnett and Hanrahan, 2020).
Table 1. Analysis of functional group present in first separated layer (Upper leyer) from crude oil of Annona squamosa L. by FTIR peaks values.
| Sr. No | Peak Value | Bond | Functional group |
| 1 | 3625.19 | O-H free hydroxyl, Aromatic O-H free | Alcohols, Phenols |
| 2 | 3470.35 | O-H stretch, Aromatic O-H H-bonded | Alcohols, Phenols |
| 3 | 3389.38 | Dimer OH, Aromatic O-H H-bonded | Carboxylic acids, Phenols |
| 4 | 3271.59 | #C-H stretch, Dimer OH, Aromatic O-H H-bonded | Alkenes, Carboxylic acids, Phenols |
| 5 | 3123.41 | =C-H stretch, Dimer OH | Alkenes, Carboxylic acids, |
| 6 | 3088.30 | =C-H stretch, Dimer OH, Aromatic-H stretch | Alkenes, Carboxylic acids, Aromatics |
| 7 | 2924.11 | C-H stretch, Dimer OH | Alkanes, Carboxylic acids |
| 8 | 2854.84 | C-H stretch, -CH2, Dimer OH, | Alkanes, Carboxylic acids |
| 9 | 1741.59 | C=O stretch doublet, C=O stretch | Ketones, Amides |
| 10 | 1650.64 | C=C stretch, C=O stretch (H-Bond), C=N, =NOH | Alkenes, Amides, Quinone or conjugated ketone, Imine, Oxime |
| 11 | 1519.49 | N-O asymmetric stretch, N=O | Nitro compounds, Nitroso compounds |
| 12 | 1457.11 | Aromatic C-C stretch, -CH3, -CH2 | Aromatics, Alkanes |
| 13 | 1372.31 | -CH2 and -CH3, S=O | Alkanes, Sulphate ester |
| 14 | 1329.22 | R-F (C-F stretch), S=O (sulfone), N-O Symmetric stretch | Alkyl halides, Sulfone, Nitro Compound |
| 15 | 1245.07 | R-F (C-F stretch), C-O stretch, P-H bending (phosphine), P=O (phosphonate), P=O (phosphoramide), Si-CH3, N-O (aromatic)
|
Alkyl halides, Ethers, Phosphine, Phosphonate, Phosphoramide, Trimethylsilyl, Amine Oxide, Carboxylic acids, Esters |
| 16 | 1167.22 | R-F (C-F stretch), C=S (thiocarbonyl), P-H (phosphine), P=O (phosphine oxide), P=O (phosphate), C-O stretch | Alkyl halides, Thiocarbonyl, Phosphine, Phosphine oxide, Phosphate, carboxylic acid, esters |
| 17 | 1027.96 | R-F, P-H phosphine, P-OR esters, Si-OR, C-O Stretch | Alkyl halides, Phosphine, Phosphite Esters, Organosilicon, Carboxylic acids, Esters |
| 18 | 869.11 | C-H out of Plan, S-OR, RNH2, RNH | Aromatics, esters, Amines |
| 19 | 818.44 | R2C=CHR (=CH out of plan), R-Cl, C-H out of Plan, S-OR, RNH2, RNH | Alkenes, Alkyl halides, Aromatics, esters, Amines |
| 20 | 779.00 | R-Cl, C-H out of Plan, S-OR, RNH2, RNH | Alkyl halides, Aromatics, esters, Amines |
Figure 1: FTIR Spectral pattern of Methanolic extracts of seeds (Upper Layer) from Annona squamosa L.
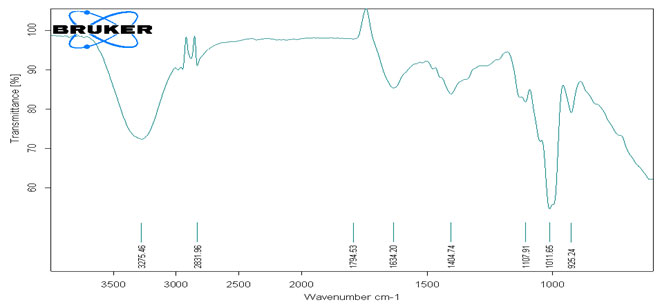
The Second separated (Lower layer) layer of Methanolic extract FTIR spectra had 8 peaks. The Peaks at 3275.46 cm-1, 2831.96 cm-1, 1794.53 cm-1, 1634.20 cm-1, 1404.74 cm-1, 1107.91 cm-1, 1011.65 cm-1, 925.24 cm-1 indicated the presence of Alkynes, Carboxylic acids, Phenols, Acid halide, Aryl carbonate, Five-membered ring Anhydride, Amides, Imine, Sulfate esters, Aromatics, Alkyl halides, esters, ethers, Thiocarbonyl, Phosphine, Phosphine oxide, Phosphate, Organosilicon, Alkyl halides, Phosphine, Phosphite Esters respectively (Table-II) (Harnett and Hanrahan, 2020).
Table 2. Analysis of functional group present in Second separated (lower level) layer from crude oil of Annona squamosa L. by FTIR peaks values.
| Sr. NO | Peak Value | Bonds | Functional Group |
| 1 | 3275.46 | #C-H stretch, Dimer OH, Aromatic O-H H-bonded | Alkynes, Carboxylic acids, Phenols |
| 2 | 2831.96 | Dimer OH | Carboxylic acids |
| 3 | 1794.53 | Unknown | Acid halide, Aryl carbonate, Five-membered ring
Anhydride |
| 4 | 1634.20 | NH out of plan, C=N | Amides, Imine |
| 5 | 1404.74 | C-O stretch, S=O (Sulfate ester), Aromatic C-C stretch | Carboxylic acids, Sulfate esters, Aromatics |
| 6 | 1107.91 | R-F (C-F stretch), C-O stretch, C=S (thiocarbonyl), P-H bending (phosphine), P=O (phosphine oxide), P=O (phosphate), Si-OR, | Alkyl halides, carboxylic acid, esters, ethers, Thiocarbonyl, Phosphine, Phosphine oxide, Phosphate, Organosilicon |
| 7 | 1011.65 | R-F (C-F stretch), P-H bending (phosphine), P-OR (esters), Si-OR, C-O stretch | Alkyl halides, Phosphine, Phosphite Esters, Organosilicon, Carboxylic acids, Esters |
| 8 | 925.24 | P-OR (esters), RCOOH O-H bend | Phosphite Esters, Carboxylic acids |
Figure 2: FTIR Spectral pattern of Methanolic extracts of seeds (Lower Layer) from Annona squamosa L.
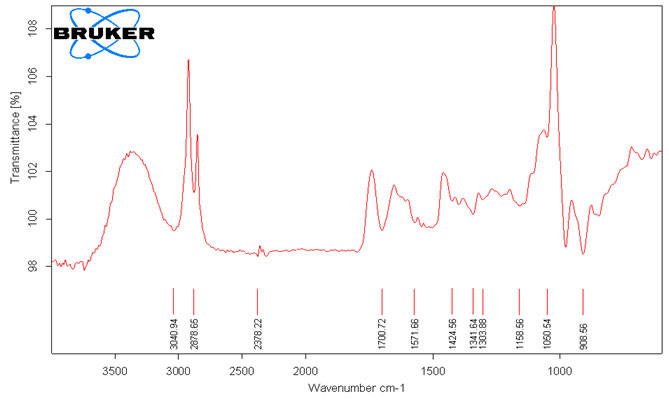
The third separated (Crystal) layer of Methanolic extract FTIR spectra had 11 peaks. The Peaks at 3040.94 cm-1, 2878.65 cm-1, 2378.22 cm-1, 1700 cm-1, 1571.66 cm-1, 1424.56 cm-1, 1341.64 cm-1, 1303.88 cm-1, 1158.56 cm-1, 1050.54 cm-1, 908.56 cm-1 shows the presence of Alkenes, Carboxylic acids, Aromatics, Phosphine, Aldehydes, Amides, Imine, Nitroso Compounds, Sulfate esters, Alkyl halides, Sulfone, Sulfonic acid, Nitro Compounds, Esters, thiocarbonyl, Phosphine oxide, Phosphate, Phosphite Esters, Organosilicon (Harnett and Hanrahan, 2020).
Table 3. Analysis of functional group present in Crystal (lower level) from crude oil of Annona squamosa L. by FTIR peaks values.
| Sr. NO | Peak Value | Bonds | Functional Group |
| 1 | 3040.94 | =C-H stretch, Dimer OH, Aromatic H stretch | Alkenes, Carboxylic acids, Aromatics |
| 2 | 2878.65 | C-H stretch, Dimer OH | Alkanes, Carboxylic acids |
| 3 | 2378.22 | P-H (Phosphine) | Phosphine |
| 4 | 1700.72 | C=O stretch, C=O stretch, C=N | Aldehydes, Amides, Imine |
| 5 | 1571.66 | C=C stretch, C-O stretch, N=O | Alkenes, Carboxylic acids, Nitroso Compounds |
| 6 | 1424.56 | S=O (Sulfate ester), Aromatic C-C stretch | Sulfate esters, Aromatics |
| 7 | 1341.64 | R-F (C-F stretch), S=O (sulfone 1), S=O, N-O symmetric stretch, | Alkyl halides, Sulfone, Sulfonic acid, Nitro Compounds |
| 8 | 1303.88 | R-F (C-F stretch), S=O (sulfone 1), N-O symmetric stretch, C-O stretch | Alkyl halides, Sulfone, Nitro Compounds, Carboxylic acids, Esters |
| 9 | 1158.56 | R-F (C-F stretch), C=S (thiocarbonyl), S=O (sulfone 2), P-H bending (phosphine), P=O (phosphine oxide), P=O (phosphate), C-O stretch, | Alkyl halides, thiocarbonyl, Sulfone, Phosphine, Phosphine oxide, Phosphate, Carboxylic acids, Eesters |
| 10 | 1050.54 | R-F (C-F stretch), C-O stretch, C=S (thiocarbonyl), P-H bending (phosphine), P-OR (esters), Si-OR, | Alkyl halides, Alcohols, carboxylic acids, esters, ethers, Thiocarbonyl, Phosphine, PhosphiteEsters, Organosilicon, |
| 11 | 908.56 | =CH out of plan, P-OR (esters) | Alkenes, PhosphiteEsters |
Figure 3: FTIR Spectral pattern of Methanolic extracts of seeds (Crystal) from Annona squamosa L.
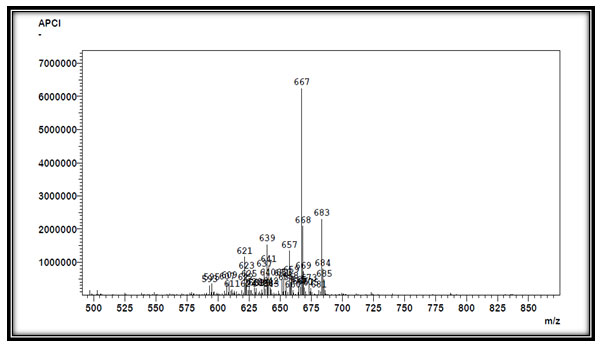
According to LC-APCI-MS technique we have obtained major peaks, APCI (Positive) m/z at 623, 605, 587, 639 (Fig 4) and APCI (Negative), we have obtained major peaks of m/z at 667, 683 (Fig 5). The analysis by APCI-LC-MS revealed presence of several compounds namely cyclopeptides and acetogenins (Zahid et al., 2013). Cyclopeptides like Cyclosqamosin A, Cyclosqamosin B, Cyclosqamosin H, Acetogenins (polyketides) like Annonacin, Squamocin, Annonin VI, which were detected by peaks of m/z ratio between 605 to 640 positive ions shift and Tenacissoside F (steroid) at 667 m/z negative ion shift. Investigations done in past suggests that the alkaloids from Annona species have rarely been explored for their medicinal applications. Many volatile components have been isolated earlier from A. squamosa such as Annonacin, Squamocin which suggest that these chemicals have medicinal properties (Nugraha et al., 2019; Alkazman, Harnett and Hanrahan, 2020). By comparing with reference of earlier studies, it was clear that this compound played a major role as anti-diabetic, anticancer, anti-inflammatory and have insecticidal property (Mangal et al., 2015; Ribeiro et al., 2018).
Figure 4: MS analysis of Annona squamosa L. seed oil. (positive ion shift)
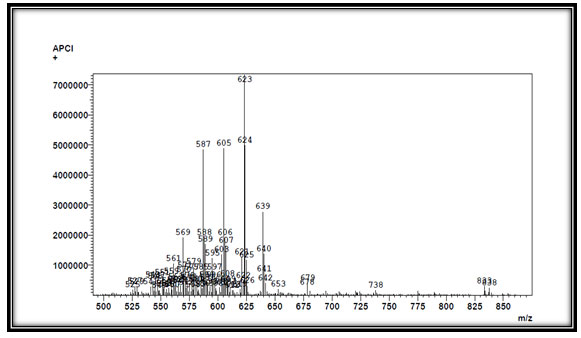
Figure 5: MS analysis of Annona squamosa L. seed oil. (Negative ion shift)
CONCLUSION
The present study explicates the therapeutic application of the seeds which is a rich source of antioxidants such as phenols and flavonoids. The analysis by APCI-LC-MS and FTIR reveals presence of several compounds; cyclopeptides and acetogenins. Cyclopeptides like Cyclosqamosin A, Cyclosqamosin B, Cyclosqamosin H and other groups of cyclopeptides. Acetogenins (polyketides), Annonacin, Squamocin, Annonin VI and Tenacissoside F. By comparing with reference of earlier studies, it was clear that this compound played a major role as anti-bacterial, anti-diabetic, anticancer, anti-inflammatory and have insecticidal property. Research and development would be an important area to focus on medicinal importance of plant. The isolated compounds can be used in future to make antidiabetic, anticancerous, anti-inflammatory medicines, further in future this compound can be used in anti-leaching cream, anti-dandruff shampoo, hair oil and seed oil can also be used as biopesticide.
ACKNOWLEDGEMENTS
This study has been supported by Department of biotechnology, Pramukh swami science & H.D Patel Arts College, Kadi. We are highly thankful to principal Dr. Ajay S. Gor, Lab Assistant Mayur Shah and Viral patel for constant technical support.
Conflict of interest: On behalf of all authors, the corresponding author states that there is no conflict of interest.
REFERENCES
Al Kazman, B.S., Harnett, J.E. and Hanrahan, J.R., (2020). The Phytochemical Constituents and Pharmacological Activities of Annona atemoya: A Systematic Review. Pharmaceuticals, 13(10), p.269.
Chao Ming, L., Ning Hua, T., Qing, M., Hui Lan, Z., Xiao Jiang, H., Yu, W., and Jun, Z. (1997). Cyclopeptide from the seeds of Annona squamosa. Phytochemistry, 45(3), 521-523.
Chavan, M. J., Shinde, D. B., and Nirmal, S. A. (2006). Major volatile constituents of Annona squamosa L. bark. Natural product research, 20(8), 754-757.
Das, S., Bhattacharya, A., Ghosh, B., and Maji, H. S. (2016). Analytical and Phytochemical Exploration of the Seeds of Annona squamosa. Journal of Analytical & Pharmaceutical Research, 3(4), 00065-00070.
De Fátima, A., Modolo, L. V., Conegero, L. S., Pilli, R. A., Ferreira, C. V., Kohn, L. K., and De Carvalho, J. E. (2006). Styryl lactones and their derivatives: biological activities, mechanisms of action and potential leads for drug design. Current medicinal chemistry, 13(28), 3371-3384.
Hiwale, S., (2015). Sustainable horticulture in semiarid dry lands (pp. 1-393). Springer India.
Koduru, S., Grierson, D. S., and Afolayan, A. J. (2006). Antimicrobial Activity of Solanum aculeastrum. Pharmaceutical biology, 44(4), 283-286.
Kowalska, M. T., and Puett, D. (1990). Potential biomedical applications for tropical fruit products. Tropical Garden Fruit World, 1(4), 126-127.
Mangal, M., Khan, M. I., and Agarwal, S. M. (2015). Acetogenins as Potential Anticancer Agents. Anti-cancer agents in medicinal chemistry, 16(2), 138–159.
Meurer Grimes, B., McBeth, D. L., Hallihan, B., and Delph, S. (1996). Antimicrobial activity in medicinal plants of the Scrophulariaceae and Acanthaceae. International journal of pharmacognosy, 34(4), 243-248.
Nugraha, A.S., Damayanti, Y.D., Wangchuk, P. and Keller, P.A., (2019). Anti-infective and anti-cancer properties of the Annona species: Their ethno medicinal uses, alkaloid diversity, and pharmacological activities. Molecules, 24(23), p.4419.
Pinto, A., Cordeiro, M., de Andrade, S., Ferreira, F., Filgueiras, H., Alves, R., and Kinpara, D. (2005). Annona species. International Centre for Underutilized Crops, University of Southampton, Southampton, UK.
Patel, P.K, Pathak, J., and Suthar, R., (2019). Effect of various physicochemical treatments on seed germination of Annona squamosa L. Asian J. Applied Sci., 12: 128-132.
Ribeiro, L. P., Zanardi, O. Z., Gonçalves, G., Ansante, T. F., Yamamoto, P. T., and Vendramim, J. D. (2018). Toxicity of an Annonin-Based Commercial Bioinsecticide Against Three Primary Pest Species of Stored Products. Neotropical entomology, 47(1), 145–151.
Shahidi, F. (2000). Antioxidant factors in plant foods and selected oilseeds. Biofactors, 13(1‐4), 179-185.
Sharma, A., Chand, T., Khardiya, M., Yadav, K. C., Mangal, R., and Sharma, A. K. (2013). Antidiabetic and antihyperlipidemic activity of Annona squamosa fruit peel in streptozotocin induced diabetic rats. International journal of toxicological and pharmacological research, 5(1), 15-21.
Vetal, D.S. and Pardeshi, A.B., (2019). Insecticidal potential of ethanol and hexane solvent seed extract of Annona squamosa against Spodoptera litura Fab. Journal of Pharmacognosy and Phytochemistry, 8(3), pp.842-845.
Zahid, M., Mujahid, M., Singh, P. K., Farooqui, S., Singh, K., Parveen, S., and Arif, M. (2018). Annona squamosa Linn. (custard apple): An aromatic medicinal plant fruit with immense nutraceutical and therapeutic potentials. International journal of pharmaceutical sciences and research, 9, 1745-1759.


