1Anchrom Enterprises, Mumbai, Maharashtra, India.
2Guru Nanak Institue of Research and Development, G.N. Khalsa College of Arts, Science and Commerce, Mumbai, Maharashtra, India.
3Ismail Yusuf College of Arts, Commerce and Science, Ismail College Campus, Mumbai, Maharashtra, India.
4School of Environment and Sustainable Development, Central University of Gujarat, Gujarat, India.
Corresponding author email: rama.yadav.5464@gmail.com
Article Publishing History
Received: 21/09/2021
Accepted After Revision: 24/02/2022
In this present study, a simple, rapid, cheap, sensitive and reproducible HPTLC-MS Method has been developed for the identification of two important bioactive compounds, Sanguinarine and Dihydrosanguinarine in Argemone Mexicana Linn seeds. The work further discussed and developed a sensitive HPTLC – MS method to analyse the adulteration and/or contamination of argemone oil in the edible mustard oil by spiking Sanguinarine and Dihydrosanguinarine as biomarkers. The n-hexane: diethyl ether (1:1 v/v) solvent system has been used as an extraction medium to extract the Sanguinarine and Dihydrosanguinarine from the seeds followed by HPLC-MS detection.
The CAMAG HPTLC system modules consisted with ATS-4, ADC-2, visualizer-2, TLC scanner-4; Derivatizer and TLC-MS interface-2 have been used for sample application, HPTLC plate development, plate photo documentation, scanning of plate, spraying of derivatization reagent and elution of biomarkers directly from HPTLC plate respectively. The n-hexane: acetone (23:7, v/v) and dragendorff’s reagent has been used as mobile phase and derivatization reagent respectively. The UV- spectra and MS data confirmed the detection of selected biomarkers in the samples/spiked samples. Thus, the work highlights the use of HPTLC-MS to develop simple and routine method for the sensitive detection of Sanguinarine and Dihydrosanguinarine in Argemone Mexicana seeds and able to become bases to these biomarkers’ evaluations in other samples such as various edible oils, agro-products, drugs and biomedical products etc.
Adulteration, Argemone Oil, Edible Mustard oil, HPTLC, Sanguinarine.
Yadav R, Wavha S, Charegaonkar A, Bajwa R. K, Tekale P, Dawane V. High Performance Thin-Layer Chromatography-Mass Spectrometry Evaluation of Sanguinarine and Dihydrosanguinarine from the Argemone mexicana Seeds in Edible Mustard Oil. Biosc.Biotech.Res.Comm. 2022;15(1).
Yadav R, Wavha S, Charegaonkar A, Bajwa R. K, Tekale P, Dawane V. High Performance Thin-Layer Chromatography-Mass Spectrometry Evaluation of Sanguinarine and
Dihydrosanguinarine from the Argemone mexicana Seeds in Edible Mustard Oil. Biosc.Biotech.Res.Comm. 2022;15(1). Available from: <a href=”https://bit.ly/3CijwzA“>https://bit.ly/3CijwzA</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Oils is a product widely used for various preparation of food products such as snacks, sweets, and homemade foods etc., thus Oils have a major role in various edible articles (Mishra and Manchanda 2012; Cárdenas et al. 2021). Food products prepared in good quality standards have been associated with several health benefits while if prepared by contaminated oil may lead to health issues like food poisoning and other long-lasting diseases (Kaur et al. 2019; Almoselhy 2021). Many times, the oils become contaminated or adulterated with other oils and lead to change the quality of pure oil (Li et al. 2021). Several reports have been published about the adulteration of costly edible oils with cheap edible and/or non-edible oils to gain huge profit margin that leads to human poisoning/toxicity or major health issues related to adulterated oils (Salah and Nofal 2021; Xia et al. 2021).
Thus, the proper quality testing of edible oils is the major requirement of today’s world. In this regards the adulteration of mustard oil with argemone oil is a serious issue and critical analytical problem (Gomber et al. 1994; Thatte and Dahanukar 1999; Singh et al. 2000; Ghosh et al. 2005; Xia et al. 2021). Argemone oil contamination in mustard oil is one of the important issues, which are faced by oil processing industries (Das and Khanna 1997; Babu et al. 2007). The source of this contamination and adulteration is the seeds from the matured Argemone mexicana plant (Bui 1974; Babu et al. 2007).
Argemone mexicana Linn (Mexican poppy, Papaveracea) is an annual weed which grows at the side of agricultural fields and is found mostly in Mexico and Florida of South America as well as in Asian, African and Caribbean countries (Namkeleja et al. 2014). The plant grows under warm temperatures and found commonly on roadsides of Indian fields. Being a medicinal plant, it is reported to have various medicinal properties like antimicrobial, antimalarial, wound healing and cytotoxic effects etc. (Osho and Adetunji 2010; Dash and Murthy 2011; Priya and Rao 2012; Khan and Bhadauria 2019; Xia et al. 2021).
The plant shows the presence of fatty acids, alkaloids, phenolics and other phytoconstituents (Apu et al. 2012). There are two types of alkaloids present namely Sanguinarine and Dihydrosanguinarine that are proven toxic to humans, by simple process of reduction and oxidation both the alkaloids are interconvertible to each other (Sarkar 1948; Vrba et al. 2009; Absolínová et al. 2009). The seeds of Argemone mexicana has similar physical appearance as mustard oil seeds, thus after the maturation of the plant, the light weight black colour seeds of Argemone mexicana gets contaminated with mustard seeds and during the oil processing, the oil forms argemone seeds have been mixed with oil of mustard seeds.
A clinical condition named as Epidemic dropsy is caused due to consumption of mustard oil adulterated with argemone oil; the alkaloid responsible for this is sanguinarine and dihydrosanguinarine from the seeds of Argemone mexicana Linn (Sanyal 1950; Sharma et al. 1999; Xia et al. 2021). Various diseases related to the sanguinarine and dihydrosanguinarine such as glaucoma, epidemic dropsy and sometimes leads total blindness were reported in FSSAI oils and global Fat manuals (Mahajan et al. 2014; Srivastava 2015; Xia et al. 2021).
The earlier analytical methods for identification of sanguinarine in argemone oil have been neither reliable nor reproducible because many of the methods contain manual processes and less proper software control associated with instrumental methods. Paper chromatographic earlier reports and/or previous HPLC methods have been facing the lack of LOD detection as well as clean-up alongwith need of high grade of solvents respectively (Hakim et al. 1961; Li et al. 2021). Thus, the present study is an attempt to provide a HPTLC-MS method for this evaluation along with the attributes of method simplicity, quickness, cost effectiveness and solvent saving approach. The developed methods are significantly useful in the evaluation of these two bioactive alkaloids in the Argemone mexicana Linn plant and able to target the adulteration issues associated with the respective oils.
MATERIAL AND METHODS
For chemicals and reagents, all the solvent used for analysis was of analytical grade, solvent like methanol, n-hexane, acetone of Merck (Brand). For the derivatization process Dragendorff’s reagent is prepared by using, Bismuth nitrate, Potassium iodide. HPTLC Glass Silica Gel 60 F254, 20×10 cm (Merck Catalogue No. 1.05642.0007). If no. of samples and standards to be applied is less than 10, then use a 10 x 10 cm sized plate. Argemone mexicana plant seeds used were directly collected from the fields of Saphale, Palghar (19.577778°N 72.819167°E), edible Mustard oil and seeds is procured from the local market of Mumbai, Maharashtra, India. For standards and sample solutions in the present study, standard sanguinarine and dihydrosanguinarine was not used, instead of standards, argemone seeds were used to isolate sanguinarine and dihydrosanguinarine and to identify them in mustard oils. The two benzylisoquinoline alkaloids isolated and identified from Argemone mexicana seeds were used as a standard and their identity and purity was confirmed by MS (Mass Spectrometer).
Working standard stock solution of Argemone mexicana seed was prepared by taking 1gram of argemone seeds in 15 ml tarson tube, the seeds were crushed with glass rod and extracted with 10 ml of 1:1 n-hexane: Diethyl ether. The solution was shaken vigorously for 2-3 minutes; Sonicated for 15 minutes, centrifuged at 1500rpm for 5 minutes, after the centrifugation the clear supernatant is used for analysis. This supernatant can be used as working standard. Working standard stock solution was kept in standard volumetric flask rapped with paraffin tape and stored at 8°C throughout the analysis. The working standard was applied in an aliquot of 0.5 µl, 1.0 µl, 2.0 µl, 3.0 µl and 4.0 µl on TLC plate with CAMAG Automatic TLC applicator (ATS 4).
For spiking studies, the spiking of the working standard was done on pure edible mustard oil samples. 2.0 µl of working standard was spiked in each track of pure mustard oil. Spiking on mustard oil was done on plate with CAMAG ATS 4 (Automatic TLC sampler) by over-spotting the working standard on the Mustard oil sample tracks.
For derivatization reagent to detect Alkaloids, derivatization was performed with Dragendorff’s reagent. Dragondorff’s reagent: Solution 1: Weigh 0.85 g of basic bismuth nitrate in a glass bottle and add 40 ml of water and 10 ml glacial acetic acid. Solution 2: Weigh 8gm of potassium Iodide in a glass bottle and dissolve in 30ml of water. Just before dipping, 20ml of each solution was diluted with 80 ml Acetic acid and 200 ml Water. During chromatography for separation and isolation of the two important alkaloids, CAMAG HPTLC modules from (Muttenz, Switzerland) was used. The HPTLC modules consist of Automatic TLC Applicator (ATS 4) equipped with a 25-µl syringe, CAMAG ADC-2, TLC Scanner 4, operated by CAMAG server client software vision CATS 2. HPTLC Silica gel 60 F254 (glass plate), was from Merck.
For sample preparation, two types of mustard oil samples were analysed, mustard oil directly from the market and other is oil extracted from mustard seeds. From Seeds:1 g of Mustard seeds was taken in a 15 ml Tarson tube, the seeds were crushed with a glass rod, add 10 ml of 1:1 n-Hexane: Diethyl ether. The mixture was shaken vigorously for 2-3minutes, then sonicated for 15 mins. After this,it was centrifuged at 1500rpm for 5 min, and taken for the supernatant to continue analysis. From Oil: 2 ml of mustard oil was taken in 15 ml Tarson tube. Then 10 ml of 1:1 n-Hexane: Diethyl ether was added. The mixture was shaken vigorously for 2-3 min and then sonicated for 15 mins. Finally, the solution was used for analysis.
For the chromatographic conditions at the time of application of the working standard and samples, the HPTLC plates were heated at 105 °C for 5 minutes on CAMAG plate heater III, for activation of plate. For The application of standard and sample CAMAG Automatic TLC Sampler (ATS 4) is used. The working standard solution and sample solution was applied in an aliquot of 0.5 µl, 1.0 µl, 2.0 µl, 3.0 µl and 4.0 µl. The spiking of mustard oils samples was done by over spotting the working standard on edible oil bands on plate. Application was done with dosage speed of methanol (150 nl/s) in the form of 8mm bands. The separation was done in a CAMAG ADC-2 automatic developing chamber. The ADC-2 was pre-saturated for 20 minutes with 20 ml of Mobile Phase [n-hexane: acetone (23:7, v/v)] and relative humidity was controlled at 33 % by using MgCl2. 6H2O (saturated salt solution). The plates were developed up to solvent front of about 70mm from bottom edge. The developed plate was dried automatically by ADC-2. The developed plate was photo documented by CAMAG Visualizer 2 under R White, R 254 nm and R 366 nm.
For scanning, CAMAG scanner 4 scanned the developed plate. The plates were scanned at multiple wavelengths (254 nm, 366nm and 480 nm) by using single wavelength and multiple wavelengths in scanner type. Optimization was done for light sensitivity The scanning was done under measurement mode (Absorbance/Fluorescence) mode by using a D2 Lamp for 254 nm Hg lamp for 366 nm and W lamp for 480 nm. The filter used was K 400 and the slit dimensionselected was 6.0 mm x 0.45 mm micro. The Scanning was performed with the scanning speed of 20 mm/s and data resolution of 25µm/step. The scanning of the tracks was done from 4.9mm from bottom of the plate and up to 73.1mm.
For the spectrum analysis, the plates were scanned under scanner type (Spectrum), optimization was done for resolution and the measurement mode selected to absorbance. Scanning was done from 190 to 700 nm to get the UV visible spectra of the two alkaloids at Rf: – 0.20 mm and 0.42 mm. After the spectrum analysis, the well separated bands at Rf:-0.20 mm of sanguinarine and Rf:-0.42 mm of dihydrosanguinarine was marked with pencil under visible light. The marked bands were eluted with the help of TLC-MS interface 2 and analysed by using Acuity QDA 3 mass spectrometer (waters, USA) in chemical ionization mode for TIC /MS Scan/Positive (+) scan.
RESULTS AND DISCUSSION
An HPTLC-MS Method has been developed for simple and rapid isolation of two toxic alkaloids namely sanguinarine and dihydrosanguinarine from argemone seeds. The method can be used to detect the contamination or adulteration of argemone oil in mustard oil.
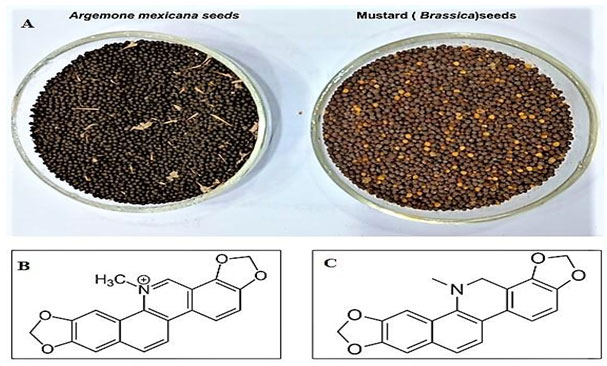
Figure 1: The seeds of Argemone mexicana and Brassica (A) and the structure of two biomarkers; Sanguinarine (B) and Dihydrosanguinarine (C) respectively.
The Mobile phase used was n-hexane: Acetone in the ratio of 23:7 v/v. The mobile phase provides very good separation between sanguinarine and dihydrosanguinarine with reduced matrices from argemone oil and mustard oil extracts. This method was very specific to these two QBA’s Sanguinarine and Dihydrosanguinarine; alkaloids other than these two do not interfere and are not detected by this mobile phase. As per FSSAI regulations it is claimed on every pouch or container of mustard oil ‘‘Free from Argemone oil’’ therefore the mustard oil should be free from argemone oil. The Method is very simple and fast, as it requires simple steps and less time for the extraction process. The process consists of crushing the seeds, sonicating it for 15 minutes and centrifuge for 5 minutes and collection of the clear supernatant for the analysis. For this simple process, the time required is 30 minutes. Procedures reported in previous research papers include tedious procedures for sample preparation.
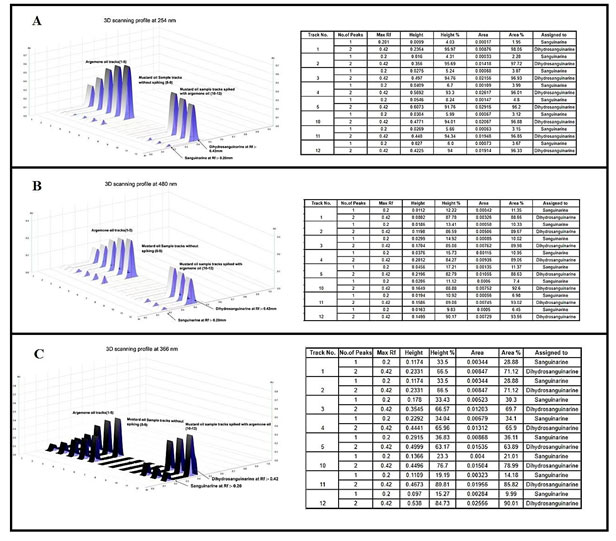
The two alkaloids sanguinarine and dihydrosanguinarine at Rf: 0.20 mm and 0.40 mm was active in short and long UV and also in the visible light. The best response of the two alkaloids was in R 366 nm image, which is shown in the Fig-2 (B). The developed plate was scanned at various wavelength such as R 254 nm, R 366 nm and 480 nm and the corresponding 3D Densitogram and the peak data is shown in Fig 3 as (A), (B) and (C) and the alkaloids also show their presence under white (Orange colours bands) when they were treated with dragondorffs reagent. After the photo documentation the UV spectra for both bands at Rf: 0.20 mm and Rf:0.42 mm was recorded. The developed plate was further used for HPTLC-MS analysis, the bands at Rf:0.20 mm and Rf: 0.42 mm were marked with pencil and the elution of both the analytes were done by TLC-MS interface 2, mass dat is shown in the Fig 4 as (A) and (B) (Almoselhy 2021).
Figure 2: Developed plate under various electromagnetic radiation zones. Where, A – Developed plate image in R 254nm, shows the separation of Dihydrosanguinarine (Rf: 0.42) and Sanguinarine (Rf: 0.20) from argemone oil, argemone oil spiked to edible mustard oil. B – Developed plate image in R 366nm, shows the separation of Dihydrosanguinarine (Rf: 0.42) and Sanguinarine (Rf: 0.20) from argemone oil, argemone oil spiked to edible mustard oil. In R366 nm, both the alkaloids Sanguinarine and Dihydrosanguinarine can be easily detected with intense yellow colour fluorescence bands, the upper band of higher intensity is of Dihdrosanguinarine and lower band is Sanguinarine. C -. Developed plate image in R White, tracks 1-5; TLC separation of (i)Sanguinarine (Rf:0.20) and (ii) Dihydrosanguinarine (Rf:0.42) from argemone oil. Track 6- 9 Unspiked mustard oil sample. Tracks 10-12 spiked mustard oil sample. D – Derivatized plate image in R White after derivatization with dragondorff’s reagent. Both the alkaloids give reddish orange colour bands after derivatizing it with dragondorff’s reagent. Sanguinarine at (Rf: 0.20) and Dihydrosanguinarine (Rf:0.42).
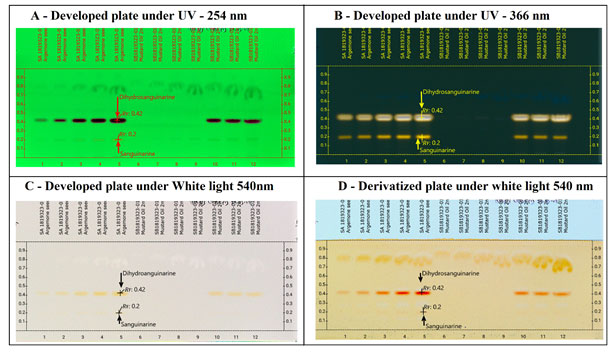
After the extraction of oils from Argemone mexicana seeds, the identification of sanguinarine and dihydrosanguinarine from argemone oil has been done by checking the maximum Rf value, spectrum data, λ max values followed by the m/z ratios of both alkaloids by TLC-MS interface 2. After confirming the two alkaloids with the above steps, argemone oil was spiked in mustard oil sample by over spotting with CAMAG ATS 4 applicator. When the developed method was used for the identification of the two alkaloids in the spiked sample, both the alkaloids were identified in the spiked samples without any interference from mustard oil. Thus, this method was suitable for checking the contamination or adulteration of mustard oil by argemone oil. The developed method gives reproducible results (Cárdenas et al. 2021).
Figure 3: 3D – Densitogram profiles and their respective data table images including no. of peaks, Rf max, Height and area of the peaks and their relative percentage profiles. Here, tracks 1-5; TLC separation of (i) Sanguinarine (Rf:0.20) and (ii) DihydroSanguinarine (Rf:0.42) from argemone oil. Track 6- 9 Unspiked mustard oil sample. Tracks 10-12 spiked mustard oil sample. Where, A – Densitogram profile at UV – 254 nm, B – Densitogram profile at 430 nm, C – Densitogram profile at UV – 366 nm.
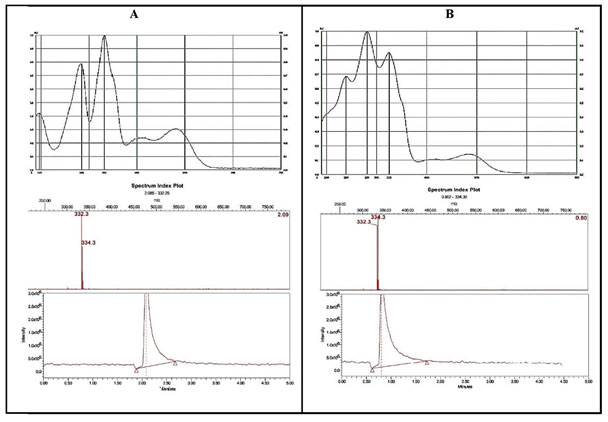
The identification of Sanguinarine in mustard oil samples was further confirmed by comparing the in-situ UV spectra of Sanguinarine from argemone oil and spectra of Sanguinarine in spiked mustard oil. The Mass spectral analysis of bands corresponding to Sanguinarine (Rf: 0.20) and Dihydrosanguinarine (Rf: 0.42) after exposure to UV-radiation was done with the help of TLC-MS interface 2. The bands were marked with pencil under UV 254nm light and the analytes are eluted from the plate to Mass Spectrophotometer. The base peak (m/z 332 and m/z 334) shown for Sanguinarine and Dihydrosanguinarine (Cárdenas et al. 2021).
Figure 4: UV- spectra and correspondent MS- spectra of Sanguinarine (A) and Dihydrosanguinarine (B) respectively. A – In situ UV spectra of Sanguinarine in argemone oil showing the λ max at 194 nm, 281nm and 332 nm along with the peak at 332 m/z in mass spectra. B – In situ UV spectra of Dihydrosanguinarine in argemone oil after UV irradiation (366 nm, for 15 min). Showing the λ max at 240 nm, 281 nm and 325 nm along with the peak at 334 m/z in mass spectra.
CONCLUSION
The findings of the present study suggest that the HPTLC-MS method has been developed to isolate and identify the alkaloids namely Sanguinarine and Dihydrosanguinarine from Argemone mexicana seeds. By using this method, Sanguinarine and Dihydrosanguinarine, which is well separated without any interference from oil matrices, can be used as specific markers to detect the adulteration or contamination of mustard oil with argemone seed oil. This method found to be cheap, very simple to use and less time consuming, when compared with earlier reported methods that are having complex sample preparation and reproducibility issues. This method found to be useful to screen multiple numbers of mustard oil samples simultaneously thus significantly potent in industries in the screening of samples on large scale and industries dealing with mustard oil related products as well as applicable in forensic labs for argemone oil poisoning case investigations.
ACKNOWLEDGEMENTS
The necessary technical help, infrastructure and HPTLC facilities were provide by the Anchrom Enterprises, Mumbai, Maharashtra, India and Ismail Yusuf college of Arts, Commerce and Science, Mumbai, Maharashtra, India.
Conflict of Interests: Authors declare no conflict of interests to disclose.
Data Availability Statement: The database generated and /or analysed during the current study are not publicly available due to privacy, but are available from the corresponding author on reasonable request.
REFERENCES
Absolínová, H., Jančář, L., Jančářová, I. et al. (2009). Acid-base behaviour of sanguinarine and dihydrosanguinarine. Central European Journal of Chemistry, 7: 876-883.
Almoselhy, R. I. (2021). A Review on Health risks from processing contaminants in edible oils and foodstuffs. International Journal of Advanced Technology & Science Research.
Apu, A. S., Al-Baizyd, A. H., Ara, F. et al. (2012). Phytochemical analysis and bioactivities of Argemone mexicana Linn. leaves. Pharmacology online, 3: 16-23.
Babu, C. K., Khanna, S. K., and Das, M. (2007). Adulteration of mustard cooking oil with argemone oil: do Indian food regulatory policies and antioxidant therapy both need revisitation?. Antioxidants and Redox Signaling, 9: 515-525.
Bui, T. I. (1974). Chemical study of the seeds of the poppy Argemone mexicana L. cultivated in the USSR and growing in Vietnam. Farmatsiia, 23: 36.
Cárdenas, J., Orjuela, A., Sánchez, D. L. et al. (2021). Pre-treatment of used cooking oils for the production of green chemicals: A review. Journal of Cleaner Production, 289: 125129.
Das, M., and Khanna, S. K. (1997). Clinicoepidemiological, toxicological, and safety evaluation studies on argemone oil. Critical reviews in toxicology, 27: 273-297.
Dash, G. K., and Murthy, P. N. (2011). Evaluation of Argemone mexicana Linn. Leaves for wound healing activity. J Nat Prod Plant Resour, 1: 46-56.
Ghosh, P., Reddy, M. K., and Sashidhar, R. B. (2005). Quantitative evaluation of sanguinarine as an index of argemone oil adulteration in edible mustard oil by high performance thin layer chromatography. Food chemistry, 91: 757-764.
Gomber, S., Daral, T. S., Sharma, P. P. et al. (1994). Epidemic dropsy in Trans Yamuna areas of Delhi and UP. Indian pediatrics, 31: 671-671.
Hakim, S. A., Mijović, V., and Walker, J. (1961). Experimental transmission of sanguinarine in milk: detection of a metabolic product. Nature, 189: 201-204.
Kaur, R., Sharma, A. K., Rani, R. et al. (2019). Medicinal qualities of mustard oil and its role in human health against chronic diseases: a review. Asian Journal of Dairy and Food Research, 38: 98-104.
Khan, A. M., and Bhadauria, S. (2019). Analysis of medicinally important phytocompounds from Argemone mexicana. Journal of King Saud University-Science, 31: 1020-1026.
Li, C., Li, C., Yu, H. et al. (2021). Chemical food contaminants during food processing: sources and control. Critical reviews in food science and nutrition, 61: 1545-1555.
Mahajan, R., Garg, S., and Sharma, P. B. (2014). Global food safety: determinants are Codex standards and WTO’s SPS food safety regulations. Journal of Advances in Management Research.
Mishra, S., and Manchanda, S. C. (2012). Cooking oils for heart health. J Pre-Cardio, 1: 123-131.
Namkeleja, H. S., Tarimo, M. T., and Ndakidemi, P. A. (2014). Allelopathic effects of Argemone mexicana to growth of native plant species. American journal of plant sciences, 2014.
Osho, A., and Adetunji, T. (2010). Antimicrobial activity of the essential oil of Argemone mexicana Linn. Journal of Medicinal Plants Research, 4: 019-022.
Priya, C. L., and Rao, K. V. B. (2012). Ethanobotanical and current ethanopharmacological aspects of Argemone mexicana Linn: an overview. International Journal of Pharmaceutical Sciences and Research, 3: 2143.
Salah, W. A., and Nofal, M. (2021). Review of some adulteration detection techniques of edible oils. Journal of the Science of Food and Agriculture, 101: 811-819.
Sanyal, P. K. (1950). Argemone and mustard seeds. The Indian medical gazette, 85: 498.
Sarkar, S. N. (1948). Isolation from argemone oil of dihydrosanguinarine and sanguinarine: toxicity of sanguinarine. Nature, 162: 265-266.
Shanbhag, V. V., Jha, S. S., Kekre, M. S. et al. (1968). Epidemic dropsy in Bombay city and suburbs. Indian journal of medical sciences, 22: 226-36.
Sharma, B. D., Malhotra, S., Bhatia, V. et al. (1999). Epidemic dropsy in India. Postgraduate medical journal, 75: 657-661.
Srivastava, S. (2015). Food adulteration affecting the nutrition and health of human beings. Journal of Biological Sciences and Medicine, 1: 65-70.
Tandon, R. K., Singh, D. S., Arora, R. R. et al. (1975). Epidemic dropsy in New Delhi. The American journal of clinical nutrition, 28: 883-887.
Thatte, U., and Dahanukar, S. (1999). The Mexican poppy poisons the Indian mustard facts and figures. The Journal of the Association of Physicians of India, 47: 332-335.
Vrba, J., Doležel, P., Vičar, J. et al. (2009). Cytotoxic activity of sanguinarine and dihydrosanguinarine in human promyelocytic leukemia HL-60 cells. Toxicology in vitro, 23: 580-588.
Xia, Q., Du, Z., Lin, D. et al. (2021). Review on contaminants in edible oil and analytical technologies. Oil Crop Science.


