1Department of Microbiology, Sri Ramakrishna College of Arts and Science for Women,
Coimbatore, Tamil Nadu, India
2Department of Microbiology, Sri Ramakrishna College of Arts and Science for Women,
Coimbatore, Tamil Nadu, India
3Department of Microbiology, Tiruppur Kumaran College for Women,
Tirupur, Tamil Nadu, India
Corresponding author email: pavaimicro@gmail.com
Article Publishing History
Received:
Accepted After Revision:
The Pharmacological properties of medicinal plants aid in the treatment of various diseases are emerging area of research in the present scenario due to the presence of secondary metabolites. The present study aims to explore the green synthesis of silver nanoparticles from leaf extracts and the evaluation of bioactive compounds and its antibacterial activity. The present research focused on the analysis of the phytochemical composition, antibacterial activity, and green synthesis of silver nanoparticles from the leaf extracts of Cassia fistula, Adhatoda vasica, and Aegle marmelos. Following aqueous extraction, phytochemical screening and antibacterial activity were carried out.
Green synthesised silver nanoparticles were characterized by using UV absorption, FTIR, and X-Ray Diffraction methods, and its antibacterial activity was evaluated. The hot aqueous combinatorial extract was quantitatively analyzed for the presence of tannins (3.03 mg), flavonoids (2.4 mg), alkaloids (10.4 mg), phenols (10.2 mg), and carbohydrates (7.8 mg). The green synthesis of silver nanoparticles was indicated with a UV-visible absorption peak at 460 nm. Functional groups identified by FTIR include amines, nitro compounds, alcohols, phenols, and alkenes. The highest size of synthesized silver nanoparticles from the combinatorial leaf extract was 23.63 nm (200) using XRD. Antibacterial activity of green synthesised silver nanoparticles demonstrated 13 mm zone of inhibition for Staphylococcus sp. and a zone of 15 mm for Escherichia coli. This present investigation paves a path for the establishment of green synthesised combinatorial leaf extracts that exhibit greater antibacterial efficacy against various pathogens. Hence, novel drug formulations may be adapted in the future.
Adhatoda Vasica, Antibacterial Activity, Cassia Fistula, Green Synthesis, Phytochemical Analysis, Silver Nanoparticles.
Pavai I. S, Selvi B.T, Karthika G.Green Synthesis of Silver Nanoparticles with Combinatorial Leaf Extracts: Phytochemical Screening and Antibacterial Activity. Biosc.Biotech.Res.Comm. 2025;18(1).
Pavai I. S,, Selvi B.T, Karthika G. Green Synthesis of Silver Nanoparticles with Combinatorial Leaf Extracts: Phytochemical Screening and Antibacterial Activity. Biosc.Biotech.Res.Comm. 2024;18(1). Available from: <a href=”https://shorturl.at/cqL2N“>https://shorturl.at/cqL2N</a>
INTRODUCTION
The primary source of natural products that are widely and successfully used in medicine is plants. Disease incidence is typically lower in populations that use a lot of natural herbal items. Recently, there has been a lot of attraction towards natural-based herbs as antimicrobial agents due to their eco-friendly and health hazardless in nature. The traditional Indian systems of Ayurveda and Siddha medicines support the importance of medicinal plants to treat diseases Ameer et al., (2021). Herbal medicines have been utilized in most countries since ancient times. Still medicinal plants in Asia are extensively employed as a therapy for infectious disease in rural and background areas, Omeje et al., (2023).
Herbal medications are commonly used in healthcare due to their inexpensive cost and abundance of antibacterial qualities (Enechi et al 2022). Many plants and herbal medicine-derived natural products could be used as an alternative therapeutic potential for RTI since they have antibacterial effects, Armutcu et al.,(2021). The size of nanocarriers was similar to biological molecules like viruses and proteins, which allows them to interact with cell surfaces and the cell wall Lombardo et al. (2019). Silver nanoparticles were used in a broad range of applications like drug delivery, food industries, anti-microbial and anti-cancer studies on the green synthesis of silver nanoparticles Zharkova et al. (2021). Therefore, plant extract–mediated green synthesis of nanoparticles is considered eco-friendly, cost-effective, and safe, and is a viable alternative for microbiological applications Garibo et al. (2020).
MATERIAL AND METHODS
The Following plants were used in the present study.
Cassia fistula: A deciduous tree with lovely yellow blossoms and grey bark is Cassia fistula. In Ayurvedic and Unani medicine, it is one of the most often utilized plants. According to some reports, this plant can help with skin conditions, liver issues, tuberculous glands, and diabetes. The fruit pod measures 20–27 mm in diameter and 40–70 cm in length. The distal extremities of the fruit pod are rounded and somewhat bent. Danish et al.(2011). This medium-sized, ornamental plant grows quickly and sheds its leaves after the growth season. Hanif et al.,(2007). Numerous quantities of primary and secondary metabolites are present in Cassia fistula. The pharmacological and biological effects of this substance depend on these metabolites. Anthraquinones, polyphenols, polysaccharides, flavonoids, tannins, glycosides, and amino acids are examples of primary and secondary metabolites.
Adhatoda vasica: For over two millennia, Adhotoda vasica has been utilized in India’s traditional medical system. It is also a well-known medication in Unani and Ayurvedic medicine. The antibacterial, anti-spasmodic, anti-arthritic, antiseptic, expectorant, anti-tuberculosis, and anti-cancer qualities of Adhatoda vasica are well-known.
Aegle marmelos: There are various uses for Aegle marmelos as a medicinal plant. Every portion of Aegle marmelos, including the leaves, fruits, pulp, flowers, stem bark, and root bark, had therapeutic value; however, the leaf had the strongest pharmacological activity. Leaves were employed as a mild laxative or to relieve asthmatic mucous membrane inflammation. Leaf decoction can be used as an expectorant, to help get rid of fever, or to help clear the bronchial passages of mucus discharge. Aegle marmelos leaves can treat severe conjunctival inflammation, including acute bronchitis, and they can also treat inflammation in other parts of the body.
The leaves of Cassia fistula, Adhatoda vasica, and Aegle marmelos were gathered from the Tirupur district in Tamil Nadu, India. Plants were identified and authenticated by Botanical Survey of India vide certificate no BSI/SRC/5/23/2024/Tech/892. After the collection plants being cleaned with water, the recently harvested leaves were promptly drenched with ethanol and allowed to dry at room temperature in the shade. In a mixer, the dried leaves were ground into a powder. For later use, the powdered plant leaf material was kept in sterile glass containers.
Extraction Of Plant Materials: All the dried leaf powders were combined into various concentrations such as 1:1:1, 0.5:0.5:1, 0.5:1:0.5, 1:0.5:0.5 and 0.5:0.5:0.5. The manufacture of the extract was done using these blended concentration granules. The combination was steeped for 24 hours after 10 grams of each concentration powder were suspended in 100 ml of hot and cold distilled water. The clear solution was stored in a water bath at 80°C for two hours after the residues were filtered through Whatman No. 1 filter paper. For later usage, the dehydrated crude extracts were kept at 4°C (Chessbrough (2000).
Collection of Clinical Pathogens: The Government Hospital in Erode was where the clinical pathogens were collected. For the following investigation, isolates were kept on nutrient agar at 4°C. The conventional Gram-staining, biochemical, and selective plate methods were used to confirm the clinical pathogens. After streaking the stains on selective media, they were incubated for 24 hours at 37°C. Escherichia coli streaked on an EMB agar plate and Staphylococcus sp. on an MSA plate.
Antibacterial Activity of Combinatorial Leaf Extract: The usual agar well diffusion method was utilized to test the plant extract’s antibacterial effectiveness against clinical pathogen microorganisms. After swabbing the cultures onto the nutrition agar plates, wells of 6 mm in diameter were created on the nutrient agar using gel pierce, and 100 µl of plant extract was added to the wells created using the gel borer. following a 24-hour incubation period at 37°C. Syeda and Riazunnisa (2020).
Qualitative Phytochemical Analysis: Using routine protocols, a preliminary screening for phytochemicals was conducted. Audu, Mohammed and Kaita,(2007). To analyze the phytoconstituents found in plants, tests for alkaloids, flavonoids, phenols, tannins, saponins, carbohydrates, glycosides, and proteins were conducted.
Quantitative Phytochemical Analysis: Alkaloids, flavonoids, carbohydrates, tannins, and phenols were all quantitatively analyzed using conventional techniques. Anandhu et al.,(2021).
Hemolytic Activity of Combinatorial Leaf Extract: With a little modification of the Yang, Sun and Fang , (2005) approach, the cytotoxicity of plant extract to normal, healthy cells was examined using in vitro hemolytic activity. The plant extracts were streaked over a blood agar plate, then incubated for 48 hours at 37°C. Extracts that did not produce hemolysis were chosen for additional processing after incubation.
Green Synthesis of Silver Nanoparticles Using Combinatorial Leaf Extract: After being soaked for the whole night, 15g/l of plant powder was extracted in a water bath at 60–70 °C for 20–30 minutes and then chilled. Next, two ml of 0.1 M AgNO3 solution were added to eight ml of extract. It was heated in a water bath for 40 minutes at a temperature of 60°C. After 30 minutes, the reaction mix’s color changed, but it was still incubated for 24 hours at room temperature to improve synthesis. Following incubation, the mixture was centrifuged for 20 minutes at 5000 rpm to confirm the formation of nanoparticles by UV-visible spectroscopy. For additional characterization research, the resulting pellets were gathered, suspended three times in distilled water, then centrifuged to eliminate any remaining unbound biomass. The nanoparticles of silver were kept in storage.
Characterization of Silver Nanoparticles Using Combinatorial Leaf Extract Uv Absorption Spectrophotometric Analysis of Green Synthesized Silver Nanoparticles: The UV absorption spectrophotometer was used to analyze the produced silver nanoparticles. About 1 mg of dried nanoparticles and 9 ml of ethanol were combined to prepare the sample, which had an absorption wavelength in the 350–600 nm. Iman et al.(2023).
Fourier Transform Infrared Spectrophotometric Analysis of Green Synthesized Silver Nanoparticles: The functional compounds that comprise green produced silver nanoparticles are identified by FTIR analysis. A sample for examination was made by mixing 0.05 mg of nanoparticles with KCl. After that, the sample was put into an FTIR device, and the spectrum was captured. With a resolution of 4 cm-1, spectra were captured between 400 and 4000 cm-1. Wilson and Venkateshwari (2022).
Structure Elucidation of Green Synthesized Silver Nanoparticles: The crystalline size and structure of green produced silver nanoparticles were ascertained by X-ray diffraction examination. The Shimadzu MODEL XRD-6000 equipment, which can be operated at 40 Kv of voltage with Cu KαX-radiation (λ=0.15418), was coated with about 200 mg of green produced silver nanoparticles. The size of the particles was measured using the Debye-Scherrer equation [D = kλ/ßCos˟]. Akintelu, Bo and Folorunso, (2020).
Antibacterial Activity of Green Synthesized Silver Nanoparticles: Using Muller Hinton Agar media at 37°C for 24 hours, the antibacterial activity of combinatorial silver nanoparticles at a concentration of 500 mg/ml was assessed using the agar well diffusion method. The diameter of the zone of inhibition was used to measure the development of microorganisms. Nair, Kalariya and Chanda, (2005).
RESULTS AND DISCUSSION
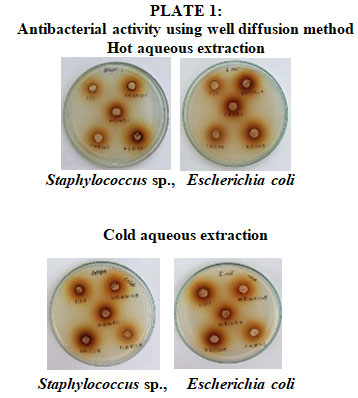
Antibacterial Activity of Combinatorial Leaf Extract: The bactericidal activity of combination aqueous (hot and cold) extracts of Adhatoda vasica, Aegle marmelos, and Cassia fistula was evaluated at five distinct concentrations. The antibacterial activity was evaluated using the agar well diffusion method shown in plate no 1. The average zone of inhibition for each isolate was used to illustrate the results in both hot and cold aqueous extracts. The zone in Seemaisamy et al., (2019) was similar to the aqueous extract of C. fistula, A. vasica and A. marmelos of Staphylococcus sp., and Escherichia coli.
Qualitative Phytochemical Analysis: Aegle marmelos, Adhatoda vasica, and Cassia fistula were the three different plants that underwent qualitative phytochemical study. The majority of the phytoconstituents in the leaves were alkaloids, flavonoids, protein, phenol, and carbs. Cassia fistula aqueous extract was subjected to a qualitative phytochemical examination. Alkaloids, flavonoids, protein, phenol, and carbs were found in a plant. Similar results were observed in Ali, (2014). Protein, carbohydrates, alkaloids, flavonoids, and saponins are all present in the Adhatoda vasica aqueous extract. Comparable outcomes were documented in Patel and Patel, (2023). Aegle marmelos‘s aqueous extract contains protein, carbs, tannin, alkaloids, and flavonoids. Results for Ratnampally and Venkateshwar (2017) were comparable. Table 1 displays the results of the qualitative phytochemical investigation.
Table 1. Qualitative phytochemical analysis
|
PHYTO CHEMICALS |
TEST |
RESULT | ||
| C.
fistula |
A.
vasica |
A.
marmelos |
||
| Alkaloid
|
Wangner’stest | + | + | + |
| lavonoid | Alkaline reagent test | + | + | + |
| Saponin
|
Foam test | – | + | – |
| Tannin
|
Ferric chloride test | – | – | + |
| Protein
|
Ninhydrin test | + | + | + |
| Phenol
|
Ferric chloride test | + | + | + |
| Steroids | Liebermann Burchard
Reaction Test |
– | – | – |
| Glycosides
|
Kellerkilliani test | – | – | – |
| Carbohydrates
|
Fehling’s test | + | + | + |
(+) indicates positive result and (-) indicates negative result
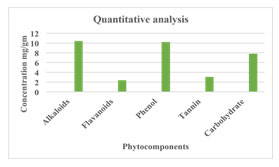
Quantitative Phytochemical Analysis of Combinatorial Leaf Extract: The quality of the phytochemical components contained in the leaf extracts from Cassia fistula, Adhatoda vasica, and Aegle marmelos was assessed. The phytochemical components were examined using the hot aqueous extract at a 1:1:1 concentration is tannins (3.036), phenol (10.2), alkaloids (10.4 mg/gm), flavonoids (2.4 mg/gm), and carbohydrates (7.8%). It was found that the flavonoid content was lower, and the alkaloid concentration was higher than other phytochemical components. Priya et al.,(2010) offered comparable explanations. The findings of a quantitative phytochemical analysis are displayed in Figure 1.
Figure 1: Quantitative phytochemical analysis
Hemolytic Activity of Combinatorial Leaf Extract: To assess their cytotoxic effect, combinatorial leaf extracts were examined for hemolytic activity in blood agar plates. The findings indicate that the hemolytic activity of plant extracts was demonstrated by the concentrations of hot (0.5:1:0.5, 1:0.5:0.5, 0.5:0.5:1) and cold (1:1:1, 1:0.5: 0.5, 0.5:0.5:1, 0.5:1:0.5, and 0.5:0.5:0.5). wherein the plant extracts’ non-hemolytic activity was demonstrated by the hot (1:1:1 and 0.5:0.5:1). Plant extracts with non-hemolytic activity were used for other purposes Kalita et al. ( 2011), and the hemolytic activity of a plant extract in water was examined. Mathur et al., (2011) talked about the plant’s aqueous extract’s non-hemolytic action, Table 2 presents the results.
Table 2. Hemolytic activity of combinatorial leaf extract
| DIFFERENT RATIOS OF
AQUEOUS (HOT & COLD) EXTRACTS |
| Hot (C.f:A.v:A.m-1:1:1) |
| Hot (C.f:A.v:A.m- 0.5:0.5:1) |
| Hot (C.f:A.v:A.m-1:0.5:0.5) |
| Hot (C.f:A.v:A.m- 0.5:1:0.5) |
| Hot (C.f:A.v:A.m- 0.5:0.5:0.5) |
| Cold (C.f:A.v:A.m- 1:1:1) |
| Cold (C.f:A.v:A.m- 0.5:0.5:1) |
| Cold (C.f:A.v:A.m- 1:0.5:0.5) |
| Cold (C.f:A.v:A.m- 0.5:1:0.5) |
| Cold (C.f:A.v:A.m-
0.5:0.5:0.5) |
C.f – Cassia fistula; A.v- Adhatoda vasica; A.m- Aegle marmelos
Green Synthesis of Silver Nanoparticles Using Combinatorial Leaf Extract: Silver nanoparticles were created using the green synthesis technique. For the synthesis of silver nanoparticles, a nonhemolytic ratio of hot 0.5:0.5:1 was used from the earlier investigation. The color shift from yellow to brown signifies the formation of silver nanoparticles. With the use of UV-visible spectroscopy, it was further verified. The color shift from yellow to brown indicated the presence of silver nanoparticles. There was a correlation between the outcomes with Pang et al.,(2020).
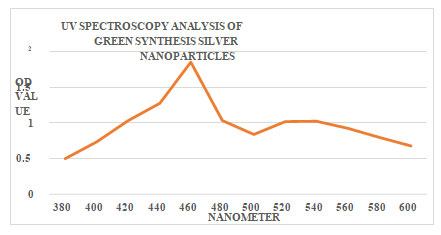
Characterization of Silver Nanoparticles Using Combinatorial Leaf Extract Uv Absorption Spectrophotometric Analysis of Green Synthesis of Silver Nanoparticles: A UV absorption spectrophotometer was used to absorb the green produced silver nanoparticles. The peaks demonstrate that the creation of silver nanoparticles was indicated by the combinatorial leaf extract. The observed silver nanoparticles were in the 380–600 nm range. The synthesis of nanoparticles at 460 nm was indicated by the largest peak, which also showed the formation of nanoparticles at that wavelength. The descending peaks were anticipated. In Lakkim et al.(2020, the same findings were discussed. The outcomes of UV absorption spectroscopy are displayed in Figure 2.
Figure 2: UV Spectroscopy analysis of silver nanoparticles
Fourier Transform Infra Red Spectrophotometric Analysis Of Green Synthesized Silver Nanoparticles: The functional group of the nanoparticles determines their stability and activity. In order to determine the various functional groups present in green produced silver nanoparticles, FTIR analysis was carried out. Peaks were used to represent the functional groups. The several functional groups, including alcohols, phenols, alkenes, carboxylic acids, nitro compounds, aromatics, aliphatic amines, aldehyde, carbon dioxide, and alkyl halides, are indicated by the green produced silver nanoparticles. The formation of silver nanoparticles was announced by the following functional groups. Although it serves as a capping and reducing agent throughout the silver nanoparticle process, the leaf extract was primarily covered with the nanoparticles. It was determined by the investigation that the leaf extract was used to create the silver nanoparticles. The combinatorial leaf extract was used to create silver nanoparticles, according to the results of the FTIR study. Kale and Dandge (2020) stated that green produced silver nanoparticles had the same functional groups. The FTIR analysis is displayed in Figure 3 and Table 3.
Figure 3: Ftir analysis of silver nanoparticles
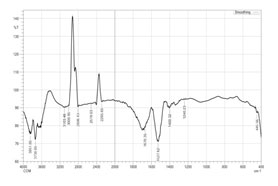
Table 3. Ftir analysis of silver nanoparticles
| Frequency cm-1 | Bond | Functional group |
| 3851.85 | O-H stretch, free hydroxyl | Alcohols, Phenols |
| 3738.05 | O-H stretch, free hydroxyl | Alcohols, Phenols |
| 3103.46 | =C-H stretch
|
Alkenes |
| 3008.95 | =C-H stretch
|
Alkenes |
| 2806.43 | H-C=O:C-H
stretch
|
Aldehyde |
| 2519.03 | O-H stretch
|
Carboxylic acid |
| 2285.65 | O=C=O
stretch
|
Carbon dioxide |
| 1670.35 | -C=C- stretch
|
Alkenes |
| 1527.62 | N-O asymmetric stretch
|
Nitro compounds |
| 1400.32 | C-C stretch
|
Aromatics |
| 1240.23 | C-N stretch
|
Aliphatic amines |
| 445.56 | C-Br stretch | Alkyl halides |
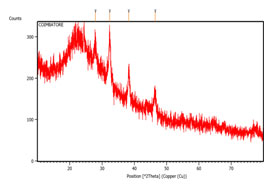
X Ray Diffraction Of Combinatorial Leaf Extract: XRD analysis was used to examine the green produced silver nanoparticles amorphous and crystalline structures. The primary peaks and associated planes were identified by comparing the XRD data with the JCPDS file 040783. The peaks that were found were 38.269 for (111) and 46.442 for (200). In the (111) plane, the green produced silver nanoparticles’ face-centered cubic (fcc) shape was clearly visible. According to Wilson and Venkateshwari (2022), the planes were a sign that silver nanoparticles were forming. Figure 4 illustrates the existence of XRD analysis structural elucidation.
The size of the green produced silver nanoparticles was determined using the Debye-Scherrer equation, D = kλ/ßCos̟. Green produced silver nanoparticles ranged in size from 15 to 30 nm, with the greatest being 181.36 nm and the smallest being 159.76 nm. According to these findings, the current investigation showed rapid estimates, with the smallest size created at 15.71 nm, which corresponds to (111), and the highest size of synthesized silver nanoparticles from the combinatorial leaf extract being 23.63 nm (200).
Figure 4: Xrd analysis of silver nanoparticles
Antibacterial Activity Of Green Synthesized Silver Nanoparticles: Combinatorial leaf extract’s silver nanoparticles antibacterial properties were investigated. Green produced silver nanoparticles demonstrated a 13 mm zone of inhibition for Staphylococcus sp. and a maximal zone of 15 mm for Escherichia coli. The findings make it abundantly evident that silver nanoparticles have strong antibacterial action through zone formation. Zones obtained by Khadka et al. (2020) were nearly identical. Table 4 and Plate 2 displayed the findings.
Plate 2: Antibacterial activity of green synthesized silver nanoparticles
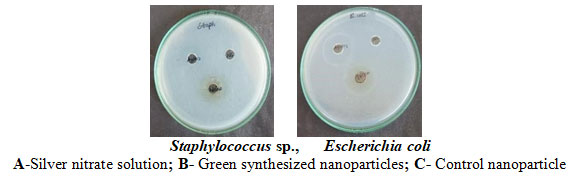
Table 4: Antibacterial activity of silver nanoparticles
| ORGANISMS | ZONE OF
INHIBITION(mm) |
||
| SILVER
NANOPARTICLE |
|||
| AgNPs
|
AgNO3 | NC | |
| Staphylococcus sp.,
|
13 mm | R | R |
| Escherichia coli | 15 mm
|
10 mm | R |
R- Resistant; AgNPs- Silver nanoparticles; AgNO3- Silver nitrate solution; NC- Negative Control
CONCLUSION
The hot and cold leaf extracts show the existence of different phytochemical components, according to the research mentioned above. In a hot 1:1:1 aqueous extract, the combinatorial leaf extract of all three plants included 10.4 mg of alkaloids, 10.2 mg of phenol, 3.03 mg of tannin, 2.4 mg of flavonoids, and 7.8 mg of carbohydrates. Combinatorial leaf extracts are used to create green-manufactured silver nanoparticles, which display a wide variety of phytochemical components. When compared to plant-based antibacterial agents, silver nanoparticles showed increased antibacterial activity. When compared to other commercial antimicrobial medications, the green synthesis approach has several advantages, including being easy to use, quick, inexpensive, and most significantly, environmentally benign. Green-manufactured silver nanoparticles successfully inhibit the microorganisms.
REFERENCES
Mathur A , A.M., Reena Purohit, R.P., Deepika Mathur, D.M., Prasad, G.B.K.S. and Dua, V.K., 2011. Pharmacological investigation of methanol extract of Syzigum cuminii seeds and Crateva nurvula bark on the basis of antimicrobial, antioxidant and anti-inflammatory properties. Der Chemica Sinica 2(1) ,pp.174-181
Akintelu, S.A., Bo, Y. and Folorunso, A.S., 2020. A review on synthesis, optimization, mechanism, characterization, and antibacterial application of silver nanoparticles synthesized from plants. Journal of Chemistry, 2020(1), p.3189043.
Ali, M.A., 2014. Cassia fistula Linn: a review of phytochemical and pharmacological studies. Int J Pharm Sci Res, 5(6), pp.2125-2130.
Ameer, M.R., Khalid, Z.M., Shinwari, M.I. and Ali, H., 2021. Correlation among Antidiabetic Potential, Biochemical Parameters and Gc-Ms Analysis of The Crude Extracts of Justicia adhatoda L. Pak. J. Bot, 53(6), pp.2111-2125.
Anandhu, K.S., Jose, M., Kuriakose, S. and Jayalakshmi, P.M., 2021. Phytochemical analysis and In vitro Antidiabetic activity of aqueous extract of Lagerstroemia speciosa and Aegle marmelos. Research Journal of Pharmacy and Technology, 14(9), pp.4697-4701.
Armutcu, F. and Kucukbayrak, A., 2021. Herbal remedies for respiratory tract infections. In Infectious Diseases (pp. 46-71). Bentham Science Publishers.
Audu, S.A., Mohammed, I. and Kaita, H.A., 2007. Phytochemical screening of the leaves of Lophira lanceolata (Ochanaceae). Life Science Journal, 4(4), pp.75-79.
Chessbrough, M., 2000. Medical Laboratory Manual for Tropical Countries, Linacre House.
Danish, M., Singh, P., Mishra, G., Srivastava, S., Jha, K.K. and Khosa, R.L., 2011. Cassia fistula Linn.(Amulthus)-An important medicinal plant: A review of its traditional uses, phytochemistry and pharmacological properties. J Nat Prod Plant Resour, 1(1), pp.101-118.
Enechi, O.C., Okeke, E.S., Isiogugu, O.N., Umeh, B.U., Eze, C.G., Emencheta, S.C., Ezeorba, T.P., Izuchukwu, C., Agbo, N.C., Ugwu, L. and Iloh, C.V., 2022. Evaluation of the Anti-Inflammatory and Antioxidant Properties of Flavonoid-Rich Seed Extract of Buchholzia coriacea Engler (Capparaceae). Tropical Journal of Natural Product Research, 6(10).
Garibo, D., Borbón-Nuñez, H.A., de León, J.N.D., García Mendoza, E., Estrada, I., Toledano-Magaña, Y., Tiznado, H., Ovalle-Marroquin, M., Soto-Ramos, A.G., Blanco, A. and Rodríguez, J.A., 2020. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Scientific reports, 10(1), p.12805.
Hanif, M.A., Nadeem, R., Bhatti, H.N., Ahmad, N.R. and Ansari, T.M., 2007. Ni (II) biosorption by Cassia fistula (Golden Shower) biomass. Journal of Hazardous Materials, 139(2), pp.345-355
Iman, M., Moosavian, S.A., Zamani, P. and Jaafari, M.R., 2023. Preparation of AS1411 aptamer-modified PEGylated liposomal doxorubicin and evaluation of its anti-cancer effects in vitro and in vivo. Journal of Drug Delivery Science and Technology, 81, p.104255
Kale, S.S., Kale, S. and Dandge, P., 2020. Structural identification of antibacterial components isolated from Cassia fistula. International Journal of Herbal Medicine, 8(5), pp.134-142.
Kalita, S., Kumar, G., Karthik, L. and Rao, K.V.B., 2011. Phytochemical composition and in vitro hemolytic activity of Lantana camara L.(Verbenaceae) leaves. Pharmacologyonline, 1, pp.59-67.
Khadka, D., Regmi, R., Shrestha, M. and Banjara, M.R., 2020. Green Synthesis of Silver Nanoparticles using Medicinal Plants Berberis asiatica and Cassia fistula and Evaluation of Antioxidant and Anti-bacterial Activities. Nepal Journal of Science and Technology, 19(2), pp.25-32.
Lakkim, V., Reddy, M.C., Pallavali, R.R., Reddy, K.R., Reddy, C.V., Inamuddin, Bilgrami, A.L. and Lomada, D., 2020. Green synthesis of silver nanoparticles and evaluation of their antibacterial activity against multidrug-resistant bacteria and wound healing efficacy using a murine model. Antibiotics, 9(12), p.902.
Lombardo, D., Kiselev, M.A. and Caccamo, M.T., 2019. Smart nanoparticles for drug delivery application: development of versatile nanocarrier platforms in biotechnology and nanomedicine. Journal of nanomaterials, 2019(1), p.3702518.
Nair, R., Kalariya, T. and Chanda, S., 2005. Antibacterial activity of some selected Indian medicinal flora. Turkish Journal of biology, 29(1), pp.41-47.
Nussbaumer, S., Bonnabry, P., Veuthey, J.L. and Fleury-Souverain, S., 2011. Analysis of anticancer drugs: a review. Talanta, 85(5), pp.2265-2289.
Omeje, K.O., Ezema, B.O., Onaebi, C.N., Onoyima, S.C., Ezeorba, T.P. and Eze, S.O., 2023. HPLC fingerprint of flavonoids, enzyme inhibition and antioxidant activity of Newbouldia laevis stem-bark: an in vitro and in silico study. Future Journal of Pharmaceutical Sciences, 9(1), p.36.
Pang, Z., Li, H., Liu, J., Yu, G. and He, S., 2020. Nano-silver preparation using leaf extracts of Youngia japonica and its in hibitory effects on growth of bacteria from cut lily stems. Northern Horticulture, 14, pp.103-109.
Patel, M. and Patel, P., 2023. Extraction, Characterization and Antibacterial activity of phytoconstituents from the leaves of Adhatoda vasica. Bull. Env. Pharmacol. Life Sci, 12, pp.147-154.
Priya, C.L., Kumar, G., Karthik, L. and Rao, K.V.B., 2010. Antioxidant activity of Achyranthes aspera Linn stem extracts. Pharmacologyonline, 2(2), pp.228-237.
Ratnampally, S.K. and Venkateshwar, C., 2017. Quantitative analysis of phytochemicals in the bark extracts of medicinally important plant Cassia fistula Linn. Int J Curr Microbiol Appl Sci ISSN, 6(4), pp.2319-7706.
Seemaisamy, R., Faruck, L.H., Gattu, S., Neelamegam, R., Bakshi, H.A., Rashan, L., Al-Buloshi, M., Hasson, S.S. and Nagarajan, K., 2019. Anti Microbial and Anti Cancer activity of Aegle marmelos and Gas Chromatography Coupled Spectrometry Analysis of their Chemical Constituents. International Journal of Pharmaceutical Sciences and Research, 10(1), pp.373-380.
Syeda, A.M. and Riazunnisa, K., 2020. Data on GC-MS analysis, in vitro anti-oxidant and anti-microbial activity of the Catharanthus roseus and Moringa oleifera leaf extracts. Data in brief, 29, p.105258.
Wilson, P. and Venkateshwari, S., Green Synthesis and Characterization of Silver Nanoparticles from Thymus vulgaris Leaf Extract.International journal for research in Applied science & Engineering Technology,10(7), pp.4035-4042
Yang, Z.G., Sun, H.X. and Fang, W.H., 2005. Haemolytic activities and adjuvant effect of Astragalus membranaceus saponins (AMS) on the immune responses to ovalbumin in mice. Vaccine, 23(44), pp.5196-5203.
Zharkova, M.S., Golubeva, O.Y., Orlov, D.S., Vladimirova, E.V., Dmitriev, A.V., Tossi, A. and Shamova, O.V., 2021. Silver nanoparticles functionalized with antimicrobial polypeptides: Benefits and possible pitfalls of a novel anti-infective tool. Frontiers in microbiology, 12, p.750556.


