Pharmacology Department, PDEA’s S.G.R.S. College of Pharmacy, Saswad, MS India
Corresponding author email: pnj1511@gmail.com
Article Publishing History
Received: 09/04/2020
Accepted After Revision: 27/05/2020
The allopathic system of medicine includes two conventional lines of treatment for rheumatoid arthritis, which come along with certain side effects. Hence, turning to safe, effective and time-tested Ayurvedic herbal drug formulation would be a preferable option. With this view transdermal films incorporating herbal drug components such as boswellic acid (Boswellia serrata) and Aloe gel (Aloe barbadensis) was envisioned. Transdermal patches of herbal extracts were prepared by solvent casting method. The patches were optimized on the basis of physicochemical evaluation such as thickness, folding endurance, physical appearance, uniformity of weight, moisture content, moisture uptake, pH and in vitro drug release, Dissolution studies. The graphs obtained for the average absorbance release with respect to time through transdermal film indicate drug release occurred at a constant rate. The skin irritation study done on wister rats skin showed that the formulation does not produce irritation to the skin. Overall, present study assures a novel approach in execution of transdermal delivery technology in the field of herbals.
Boswellic Acid, Aloe Gel, Herbal Extract, Tdds, In–Vitro Drug Release Study
Shelke P. S, Jagtap P. N. Formulation and In–Vitro Evaluation of Transdermal Patches of Anti–Arthritic Ayurvedic Medicinal Plants. Biosc.Biotech.Res.Comm. 2020;13(2).
Shelke P. S, Jagtap P. N. Formulation and In–Vitro Evaluation of Transdermal Patches of Anti–Arthritic Ayurvedic Medicinal Plants. Biosc.Biotech.Res.Comm. 2020;13(2). Available from: https://bit.ly/3fgfSub
Copyright © Shelke and Jagtap This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
The transdermal patch is a medicated adhesive patch that is placed on the skin to deliver a specific dose of medication through the skin and into the bloodstream (Tanwar et al., 2016). Transdermal drug delivery system (TDDS) has been an increased interest in the drug administration via the skin for both local therapeutic effects on diseased skin (topical delivery) as well as for systemic delivery of drugs. The skin as a site of drug delivery has a number of significant advantages over many other routes of drug administration, including the ability to avoid problems of gastric irritation, pH and emptying rate effects, avoid hepatic first-pass metabolism thereby increasing the bioavailability of drug, reduce the risk of systemic side effects by minimizing plasma concentrations compared to oral therapy (Dulan et al., 2016; Lakhani et al., 2015; Kharia et al., 2019).
The purpose of this research work was to Formulation and evaluation of transdermal drug delivery system of ayurvedic drug incorporating boswellic acid & aloe gel using various polymers such as PVP and Ethyl cellulose by solvent casting technique for improvement of bioavailability of drug and reducing toxic effects.Boswellic acid, a constituent of Boswellia serrata (Family- Burseraceae) showed anti-inflammatory and anti-rheumatic activities along with anti-pyretic effect with no ulcerogenic effect and well tolerated in as high a dose as 2 gm/kg (p.o) in mice (Umar et al.,2014). It improves blood supply to joints and restores integrity of vessels obliterated by spasm of internal damage (Chaodhary et al.,2015). B. serrata (Sallai guggul) shows its superiority over conventional drugs as it is a plant product being used since ages and is absolutely free from any toxic and side effects (Bhardwaj,2016).
Aloe barbadensis is a member of the lily family, and is a succulent plant that contains a lot of liquid in its think, spiny leaves. Aloe vera has found to be helpful in curing arthritis symptoms mainly inflammation and pain without side effects. Its anti-oxidant properties regulate expression of cyclooxygenase-2 (inhibition of this enzyme helps relieve pain and inflammation. The study reported significant inhibition of inflammation in arthritic models on using Aloe vera extracts (Khemkaran et al.,2011,Tiwari et al.,2018; Kar and Bera, 2018 (Sethi,2020).
MATERIAL AND METHODS
Herbal extract powders of Boswellia serrata and Aloe barbadensis are obtained as an gift sample from Sane Guruji Hospital and Ayurvedic Medical Store, Hadapsar, Pune.Ethyl cellulose, Polyvinylpyrrolidone (PVP), Propylene glycol were purchased from research lab centre, Pune. The laboratory chemicals other than mentioned above used in the study were of analytical reagents grade, and several types of equipment employed in the formulation of herbal patches were magnetic stirrer, Petri dish, ultrasonic cleaner, vernier caliper, electronic balance, pH meter, hot water bath, ultraviolet-visible spectrophotometer, tray dryer and hot air oven & dissolution apparatus etc.
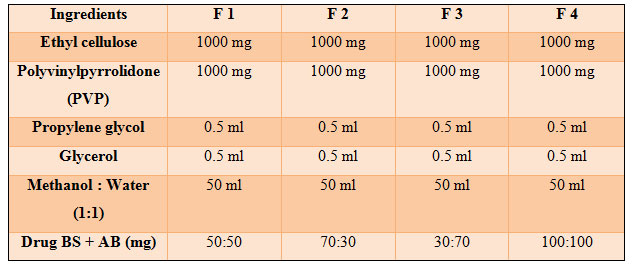
Ethyl cellulose, PVP was used as the skeletal type polymer material of preparation. Propylene glycol as penetration enhancer and plasticizer. PVP (1g) and ethyl cellulose (1g) were weighed in requisite ratios and mixed in 50ml solvent containing distilled water : methanol (1:1 ratio) . Stirred the mixture over a hot water bath until dissolved. After the mixture was cooled down to 25oc, the drug AB + BS 500mg in the ratio of 50:50, 70:30, 30:70 & 100:100 was added. After that, propylene glycol (0.5ml), glycerol (0.5ml) were added. The mixture was then poured into glass Petri dish and dried at room temperature for 24 hrs. The petri dish was left undisturbed at room temperature for one day. The patch was obtained intact by slowly lifting from the petri dish and transdermal patches were cut into length of 2 x 4 cm. The patch was collected and stored in desiccator until further use (Chauhan et al., 2018). The drugs (boswellic acid and Aloe gel) were selected on the basis that they produce synergistic action in suppressing inflammation and are time tested and proven safe.
Table 1. Formulation Design
The thickness of each patch was measured by using Screw gauge or Vernier caliper at five different positions of the patch and the average was calculated (Santosh et al.,2009). Patches sizes of 2 x 4 cm was cut. The weights of five patches were taken and the weight variation was calculated (Kumar et al.,2013).
A patch of 2 x 4cm was cut evenly and repeatedly folded at the same place till it brakes. The numbers of times the film was folded at the same place without breaking give the value of the folding endurance (Prabhakar et al.,2011; Jadhav et al.,2009).The pH of the film was found by using pH paper. The prepared films were weighed individually and kept in a desiccators containing fuse calcium chloride at room temperature for 24h. After 24h, the films were reweighed and determined the percentage moisture content from the mentioned formula (Murthy et al.,2001; Saxena et al.,2006).% Moisture content = ( Initial weight – Final weight) / Initial weight x 100
The weighed films were kept in desiccators at room temperature for 24h containing saturated solution of potassium chloride in order to maintain 84% RH. After 24h, the films were reweighed and determined the percentage moisture uptake from the below mentioned formula (Mali et al.,2015; Darwhekar et al.,2011). % Moisture uptake = ( Final weight – Initial weight ) / Initial weight x 100
A specified area of patch was dissolved in a phosphate buffer solution. The content was stirred to dissolve the film. The content was transferred to a volumetric flask. The absorbance of the solution was measured at wavelength 253 nm and determines the drug content (Prajapati et al.,2011).For estimating the sensitivity of the formulation toward the skin, the formulated patches were applied to the hairless skin of the wistar rats for the duration of 24 hours and the skin inflammation effects were reported for all individual rats ( totally 4 rats used ) (Mohamad and Tabassum, 2012).
By using USP type 1 dissolution test apparatus, Drug release from the prepared TD patch was studied using 8 station dissolution rate test apparatus (Labindia, DS 8000) employing a paddle stirrer at 50 rpm and at 37±1°C. Phosphate buffer of pH 7.4 (500 ml) was used as dissolution fluid. Samples of 5 ml of each were withdrawn at different time intervals over a period of 6 h. Each sample withdrawn was replaced with an equal amount of fresh dissolution medium. Samples were suitably diluted and assayed at 253 nm for herbal transdermal patch using UV-visible spectrophotometer. The drug release was plotted on the graph as Time (min) on X- axis and Absorbance on Y – axis (Bhavani et al.,2016).
Figure 1: Formulation of Transdermal Patches Using Solvent Casting Method.
Figure 2: BS + AB Herbal Transdermal Patches
RESULTS AND DISCUSSION
The transdermal patches of Boswellia serrata and Aloe barbadensis were formulated successfully by solvent casting technique with an aim to improve the bioavailability of herbal drug in combination. The prepared film was found to be uniform, flexible, smooth and transparent as shown in Table 1 and figure 1and 2. The formulations were subjected to physical examination; films appeared to be slightly translucent suggesting that the drug was not completely solubilized rather dispersed/suspended in the matrix (Shinde et al.,2008).
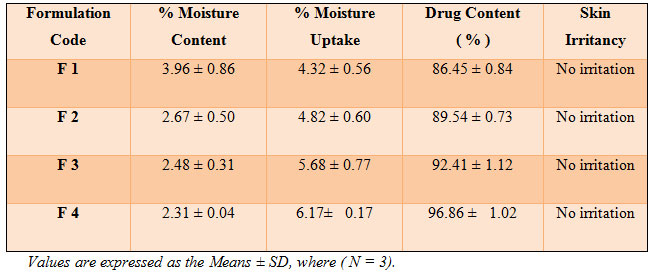
The results of thickness studies for different formulations are shown in Table 2. The above results obtained show that thickness of all the prepared formulations was found to be uniform (0.433 – 0.438 mm). It was found that the weights were uniform with respect to all prepared formulations and showed low standard deviation values. F4 formulation weight was found to be slightly increased to 0.2156 gms due to higher drug concentration present.
The results of folding endurance studies for different formulations are shown in Table 2. The above data show that the prepared formulations were found to be uniform with respect to folding endurance (12-17). The folding endurance number gives the mechanical property of the patches; high folding endurance number indicate that the patches have high mechanical property (Shirisha et al.,2017).
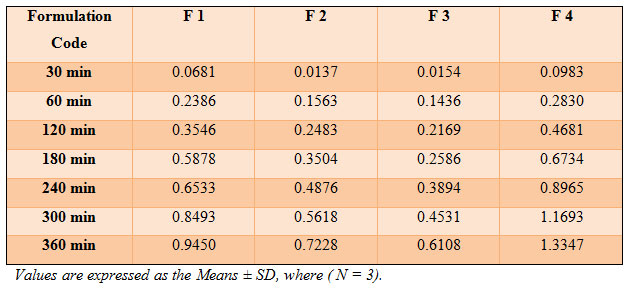
The results are shown in (Table 2 ). All the patches have around neutral pH 6. So it will not cause any kind of irritation to the skin.The moisture content in prepared formulation F1, F2, F3 and F4 was found to be increased in the order as follows: F1 > F2 > F3 > F4 ( Table 3). The small moisture content in the formulations help them to remain stable and not being a completely dried and brittle film. Again, low moisture absorption protects the patches from microbial contamination (Jatav et al.,2013).
Moisture content and moisture uptake studies provide information regarding stability of the formulation (Layek et al.,2010). The percentage moisture uptake was found to be increased with increasing drug concentration Ratio in all the four formulations.i.e. F1 < F2 < F3 < F4 as shown in ( Table 3).The percent drug content in BS + AB patches in various formulation ratio code was found in between 86.45% to 96.86 % in Table 3.
The skin irritation study done on wister rats skin showed that the formulation does not produce irritation to the skin (Table 3). After 24 hours, no inflammation of the skin was observed in all the formulations. Hence all the prepared formulations passed the skin irritation test and supposed to be safe for human use also. The patches were found to be free of any skin irritation. Based on the above observations, it can be reasonably concluded that plasticizers have a significant influence on the mechanical properties of the transdermal patches (Bharkatiya et al.,2010).
In vitro studies of Patches F1, F2, F3 and F4 were carried out by using USP type 1 dissolution test apparatus ( Table 4). As compared with other formulations, F4 shows the highest drug release profile, whereas lowest drug release was expressed by the patch F3 at the end of 6 hours ( Figure 3). From the results, it is clear that the best release profile was obtained with formulation F1 and F4 (containing 50:50 and 100:100 drug concentration). This may be due to the equal dug combination present in the formulations. The drug release was obtained in the following pattern: F4 > F1 > F2 > F3. The release of drugs from transdermal patch was found to be linear for the first 4 hours and attained a steady level upto 6 hours (Figure 3).
Release studies also indicate that by using two different polymeric combinations such as ethyl cellulose: PVP (1:1) and propylene glycol as a plastisizer were considered as the best formulations for the sustain drug release through the Transdermal Drug Delivery System ( TDDS). The incorporation of PVP was helpful for rapid dissolution of the surface hydrophilic drug which results in the formation of pores and thus leads to the decrease of mean diffusional path length of the drug molecules to permeate into dissolution medium and higher permeation rates (Prabhakara et al.,2010). The stability studies indicated that all the patches maintained good physicochemical properties and drug content after storing the patches in different storage conditions. Compatibility studies indicated that there was no interaction between the drug and polymers (Bhatnagar et al.,2010). While choosing polymer ratio, it was found that ethyl cellulose – PVP polymers are better suited than eudragit – PVP polymers for the development of transdermal patches (Kathe et al.,2017).
Table 2. Evaluation of Herbal Transdermal Patch of BS + AB
Table 3. Evaluation of Herbal Transdermal Patch of BS + AB
Table 4. In–Vitro Drug Release Study of Prepared Transdermal Patch of BS + AB
Figure 3: Drug Release Profiles of BS + AB Transdermal Patches
CONCLUSIONS
From the present study, it can be concluded that transdermal drug delivery system for Boswellia serrata + Aloe barbadensis with ethyl cellulose and PVP meet the ideal requirement for transdermal devices which can be a good way to bypass the extensive hepatic first – pass metabolism and increase bioavailability. BS + AB transdermal patches were provide sustained transdermal delivery for prolonged periods, hence suitable in the therapy of Rheumatoid Arthritis (RA). Through the present experimentation, it has been found that the drugs of ayurvedic origin can be utilized in a better form with enhanced efficacy for incorporation in modern dosage form. This work is one of the first few attempts to utilize Ayurvedic drugs through TDDS. The present work can further be proceeding with in – vivo study on healthy Wister rats to evaluate anti – arthritic activity.
ACKNOWLEDGEMENT
The authors wish to thank the Management and Faculty of Department of Pharmacology, College of Pharmacy (Saswad), for providing all necessary facilities and encouragement.
REFERENCES
Bhardwaj A, Harinath Dwivedi, Koshi Mamman and SA Saraf (2016). Solubility enhancement of Boswellia serrata Roxb. ex colebr extract through a self dispersible lipidic formulation approach. Indian Journal of Natural Products and Resources. 7(1):9-18.
Bharkatiya M, Nema R, Bhatnagar M, (2010). Designing and Characterization of Drug Free Patches for Transdermal Application. Int J of Pharma Sci and Drug Res. 2(1): 35-39.
Bhatnagar M, Bharkatiya M, Kumar R.N, (2010). Development and characterization of transdermal patches of Metapropol tartarate. Asian J of Pharma and Clinical Research. 3(2):130-134.
Bhavani B, Suhasini M.S, Niranjan P, Ramadevi M, Anusha N, (2016). Design, evaluation and optimization of fluconazole transdermal patch by 22 factorial method. Der Pharmacia Lettre. 8 (5): 280-287.
Chauhan L, Vashisht S, (2018). Formulation and evaluation of novel herbal antidiabetic transdermal patch. Innov Pharm Pharmacother. 6(4): 61-64.
Choudhary M, Kumar V, Singh S, (2015). Medicinal plants with potential anti-arthritic activity. J Intercul Ethnopharmacol. 4(2):147-179.
Darwhekar G, Jain D.K, Patidar P.K, (2011). Formulation and evaluation of transdermal drug delivery system of Clopidogrel bisulfate. Asian Journal of Pharmacy and Life Science. 1(3): 26-29.
Dulan A, Sheridan D, Laucher M.A, (2016). Using transdermal drug patches for older adults. Nursing. 46(11):69-71.
Hu Y, Xu J, Hu Q, (2003). Evaluation of Antioxidant Potential of Aloe vera (Aloe barbadensis Miller) Extracts. J Agr Food Chem. 51: 7788-7791.
Jadhav R.T, Kasture P.V, Gattani S.G, Surana S.J, (2009). Formulation and evaluation of transdermal films of diclofenac sodium. Int.J. Pharmtech Res. 1(4): 1507-1511.
Jatav V.S, Saggu J.S, Sharma A.K, Sharma A, Jat R.K, (2013). Design, development and permeation studies of nebivolol hydrochloride from novel matrix type transdermal patches. Adv Biomed Res. 2(1): 62-63.
Kar S.K , Bera T.K, (2018). Phytochemical constituents of Aloe vera and their multifunctional properties: A comprehensive review. Int J Pharm Sci Res. 9(4):1416-1423.
Kathe K, Kathpalia H, (2017). Film forming systems for topical and transdermal drug delivery. AJPS. 6: 487-497.
Kharia A, Gilhotra R, Singhai A.K, (2019). Formualtion and evalaution of transdermal patch for treatment of inflammation. Int J Pharm Sci & Res. 10(5): 2375-2384.
Khemkaran A, Jain S.K, (2011). Aloe-emodin novel anticancer herbal drug. International journal of phytomedicine. 3: 27-31.
Kumar S, Behury B, Sachinkumar P, (2013). Formulation and evaluation of transdermal patch of Stavudine. J Pharm Sci. 12(1): 63-69.
Lakhani P, Bahl R and Bafna P, (2015). Transdermal Patches: Physiochemical and in-vitro Evaluation Methods. Int J Pharm Sci Res. 6(5):1826-1836.
Layek B, Mukherjee B, (2010). Tamoxifen citrate encapsulated sustained release liposomes: Preparation and evaluation of physicochemical properties. Sci Pharm. 78: 507-515.
Mali A.D, Bathe R, Patil M, (2015). An updated review on transdermal drug delivery systems. International Journal of Advances in Scientific Research. 1(6): 244-254.
Mohamad M, Tabassum N, Ali J, Jan R, (2012). Preparation and evaluation of transdermal patch of aceclofenac. J Pharm Res. 5(1) : 331-332.
Murthy S.N, Rani S, Hiremath R, (2001). Formulation and evaluation of controlled release transdermal patches of theophylline-salbutamol sulphate. Indian Journal of Pharmaceutical Education and Research. 27(2): 1057-1062.
Prabhakar P, Shah S, Gundad, (2011). Formulation development and investigation of Domperidone transdermal patches. Int J of Pharm Investig. 1(4): 240-246.
Prabhakara P, Koland M, Vijaynarayana K, Harish N.M, et al., (2010). Preparation and evaluation of Transdermal patches of Papaverine hydrochloride. Int. J. Res. Pharm. Sci. 3(1): 259-266.
Prajapati S.T, Patel C.G, Patel C.N, (2011). Formulation and Evaluation of transdermal Patch of repaglinide. ISRN Pharma. 2: 65-78.
Santosh G, Dhaval P, Mantesh K, Ajay S, Vital V, (2009). Formulation and evaluation of matrix type transdermal patches of Glibenclamide. Int J of Pharmaceutical Sciences and Drug Research. 1(1): 46-50.
Saxena M, Mutalik S, Reddy M.S, (2006). Formulation and evaluation of transdermal patches of metoclopramide hydrochloride. Indian drugs. 43(9): 740-745.
Sethi P, (2020). Micromorphological and Phytochemical Studies of Aloe barbadensis Mill Root. International Journal of Clinical and Developmental Anatomy. 6(1):14-16.
Shinde A, Garala K, More H, (2008). Development and characterization of transdermal therapeutics system of tramadol hydrochloride. Asian J Pharm.1(2):265-267.
Shirisha S, Joshi G, Sahoo S.K, Rao Y.M, (2017). Preparation and Evaluation of Matrix Type Transdermal Patches of Domperidone Maleate: in vitro and ex vivo Characterization. Indian J of Pharmaceutical Education and Research. 51(4):517-524.
Tanwar H, Sachdeva R, (2016). Transdermal Drug Delivery System: A Review. Int J Pharm Sci Res. 7(6): 2274-2290.
Tiwari M, Upadhayay M, (2018). The medicinal plant components and applications (Aloe vera). Journal of Medicinal Plants Studies. 6(3):89-95.
Umar S, Umar K, Sarwar A.H, et al, (2014). Boswellia serrata extract attenuates inflammatory mediators and oxidative stress in collagen induced arthritis. Phytomedicine. 21(6):847-856.