Department of Zoology, Bankura Sammilani College, Bankura, West Bengal, India.
Corresponding author email: surajitnajumder.sm@gmail.com
Article Publishing History
Received: 17/03/2021
Accepted After Revision: 18/06/2021
The Bankura town of district Bankura, West Bengal, India is full of lentic water bodies like pond, reservoir and water tank etc. In these lentic water bodies, some are perennial. Most of the rest waterbodies are seasonal. Out of these, a large number of waterbodies are partly seasonal; that means, these waterbodies can retain the water to some extent during winter to summer, but the water level become low due to various causes. We referred this type of waterbodies as shallow-water pond. And the ultimate rest type of waterbodies is totally seasonal and get dried during winter to summer season. Our point of interest is to compare between the perennial and shallow-water ponds of Bankura town. The fish yield from the shallow-water ponds of Bankura town is quiet low. On the contrary, in the perennial ponds where the water depth is minimum 3-4 metre, do not face this problem at all.
Till today it is said that, in Bankura town the pisciculture is less efficient due to various natural and anthropogenic causes. But the actual cause was unknown till date. After studying various physico-chemical and hydrobiological parameters following standard methods of APHA we have observed a remarkable change in these shallow-water ponds especially during that season. A rapid and exceptionally dense accumulation of algae (Euglena sp.) was observed floating over these shallow-water ponds. This floating algal bloom is hampering the pisciculture of Bankura town a lot by oxygen depletion. The correlation between dissolved oxygen and pH was not showing significant result in shallow-water ponds but, showed totally significant result in case of perennial ponds. The main aim of the study is to make the town dwellers as well as the pond owners of Bankura town aware about the cause of low yield in these shallow-water ponds, so that they can overcome the problem.
Algal Bloom, Euglena, Shallow-Water Pond, Perennial Pond, Bankura Town.
Majumder S. Excessive Growth of Euglena Sp. and its Effects on Shallow-Water Ponds. Biosc.Biotech.Res.Comm. 2021;14(2).
Majumder S. Excessive Growth of Euglena Sp. and its Effects on Shallow-Water Ponds. Biosc.Biotech.Res.Comm. 2021;14(2). Available from: <ahref=”https://bit.ly/3w1nDfp“>https://bit.ly/3w1nDfp</a>
Copyright © Majumder This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
About 71 percent of the Earth’s surface is covered with water and the oceans hold about 96.5 percent of it. The rest 3.5 percent exists in the air as water vapour; in rivers and lakes as liquid; in icecaps, glaciers as solid and in the ground as soil moisture. Out of this 3.5 percent only 2% we found as fresh water in the form of surface and subsurface water bodies and are usable for both human consumption and aquaculture. Water is essential for the functioning of every single cell, tissue, organ and organ system in all animals as well as human being also. It is observed, during the last several decades the water quality of the Indian water bodies has been deteriorating, due to continuous discharge of industrial, agricultural and domestic sewage (Majumder and Dutta, 2014; López-Felices et al., 2020; Wato and Amare, 2020). Both lotic and lentic inland waterbodies of freshwater ecosystem are being subjected to constant environmental stress (Goswami et al., 2017; Borics et al., 2021).
Ponds are useful in many ways, as it is the most common source of open freshwater. The main nutritional cycles of an aquatic ecosystem are constituted by phytoplankton as these are the only primary food for many organisms, such as fish, crustaceans and shellfish (Goswami et al., 2017; Borics et al., 2021). These planktons can also act as indicators of trophic status of water bodies. Freshwater zooplankton is also an important component of an aquatic ecosystem and plays a crucial role for maintaining the chain of the ecosystem as they maintain the link between the producer and primary consumer. So, the distribution of both phyto as well as zooplanktons and the level of their abundance are useful for calculating the potentiality of a water body from fishery point of view (Majumder, 2020).
It is known to all that, Bankura district is one of the backward districts of West Bengal. Still Bankura has ranked first in pisciculture (particularly in spawn production), as the whole Bankura district contains large number of waterbodies than the other districts of West Bengal (according to the Office of the Additional Director of Fisheries, Bankura, West Bengal, India; www.bankura.org.in/site/Fisheries.htm). But in case of Bankura town some ponds are perennial and most of the rest waterbodies are seasonal. Out of these seasonal ponds of Bankuta town, a large number of waterbodies are partly seasonal; that means, these waterbodies can retain the water to some extent during winter to summer.
And the water level become low due to various causes. We referred this type of waterbodies as shallow-water pond. And the ultimate rest type of waterbodies is totally seasonal that get dried during winter to summer season. We compared the values of physico-chemical and hydrobiological parameters of perennial and shallow-water ponds of Bankura town to know the actual cause of low yield of fish production. This study has been done to make the town dwellers as well as the pond owners of Bankura town aware about the fact of low yield in these shallow-water ponds (Majumder et al., 2019b). For the rapid growth and development of different organs of fish, zooplanktons provide the necessary amount of protein; and the zooplanktons themselves get their nutrients from phytoplanktons of the water body (Islam and Bhuiyan, 2007; Anton-Pardo and Adamek 2015; Radhakrishnan et al., 2019). The Rotifers, one of the well-known zooplanktons that play a significant role in the food chain of pond ecosystem and became a pollution indicator or water quality monitor (Kamble and Meshram, 2005; Majumder et al., 2019b).
A eutrophic condition was observed due to excessive growth of Euglena sanguinea, a microalga that inhabits freshwater habitats throughout the world. In his species large amounts of the red xanthophyll astaxanthin (Ast) have been reported to occur (Frassanito et al., 2008; Wołowski, 2011; Guiry, 2017). By 2018 onwards report of dense growth of planktonic algae of one or few species came to know imparting a distinct colour to the water body, that is referred to as algal blooms. This universal phenomenon was termed as flowering of the water (Guiry, 2017; Majumder et al., 2019b).
Bloom formation was attributed to the algal genera belonging to the classes Bacillariophyceae, Chlorophyceae, Cyanophyceae, Dinophyceae, and Euglenophyceae. These water bodies were studied for different physico-chemical parameters and the plankton analyses were also conducted to study the dominance of Euglena in water bodies. Very high concentrations of organic matter and phosphates were the chief features of the pond’s waters having Euglena blooms (Date et al., 2018). In 2019 onwards harmful algal bloom (HAB) namely red tide were reported to cause change in water colour of sea which has a serious impact on environment along the coast and aquatic ecosystem (Zohdi and Abbaspour, 2019; Gokul et al., 2020).
Review article of 2020 disclose the methods of algal control (Fentie, 2020). But what happens to those pond (perennial and shallow-water) ecosystems, most importantly the changes taken place in physico-chemical and hydro-biological parameters are still unknown. And is there any relation between algal growth of pond and pisciculture is also unknown. This work may give some light to know the relation between algal growth and fish yield.
MATERIAL AND METHODS
Study area: Figure 1 shows both Type I (perennial) and Type II (shallow-water) ponds of Bankura town of district Bankura, West Bengal, India. The total fieldwork was carried out consecutively for six months from January to June, 2020 at two different sites (Type I and Type II ponds) of Bankura town (Figure: 2 to 4). Figure 2 shows both these two sampling sites are located at two different sided of Dwarkeswar river at Bankura. Actually, the Type I ponds are well managed for aquaculture and these ponds are perennial (water retains throughout the year up to 4 to 5 metre depth) in nature. On the other hand, the Type II ponds are not well managed for aquaculture purpose and retain shallow water depth up to one metre or so.
These Type II ponds are located throughout the Bankura town. Figure 3 shows pond Daser Bandh (located at 23°12’54”N and 87°2’12”E), Rajgram, Bankura [beside Bateswar Temple] and this is a Type I (pond with yellow marking in Figure 2 and 3) pond. Figure 4 shows pond Natun Bandh (located at 23°13’35”N and 87°3’4”E), Kankata, Bankura and this is a Type II (pond with red marking) pond. For the analysis of physico-chemical and hydrobiological parameters the water samples were collected in the morning between 6 to 7 AM from each of two collection sites.
The hydrogen ion concentration (pH), dissolved oxygen, free and dissolved CO2 were determined following standard methods of APHA-AWHA-WPCF (2005). The values were compared with standard values of BIS (Bureau of Indian Standards, 2003; Khanna and Bhutiani, 2008). A Celsius thermometer (scale ranging from 0°C to 100°C) was used to measure air and surface water temperature. For measuring intensity of light, the Digital Lux meter (HTC, Model No. LX-101A, Range 0 to 2,00,000) was used. pH of water was measured directly using a digital electrode pH meter (Systronics, Model No. SYS-335).
The planktons were collected with a modified nylon bolting silk plankton net (No. 25 mesh size 50μ) with a round metallic frame of 0.625 sq.m. area was used for collection of planktons. Collected samples were transferred to the labelled vials which contain 5% formalin solution. The plankton was observed and documented using Magnus Trinocular Microscope (Model MLX TR) attached with Nikon Coolpix Camera. During the laboratory experiment, most chemicals used (Merck, India) for analysis of the physico-chemical parameters were with highest purity available or of analytical AR grade. Statistical analysis was done by MS Excel, 2007.
RESULTS AND DISCUSSION
The pH values were recorded here ranging from 6.14 to 7.33 (Table 1) at Type I and Type II ponds respectively; both the values are marginally acidic to neutral. The water temperature of Type I and II pond water showed negative correlation with water pH (r=-0.961, p<0.01 and r=-0.705, p<0.05 respectively) (Table 2 and 3). The water temperature of both the ponds water shows negative correlation with dissolved O2 (r=-0.929, p<0.01 and r=-0.756, p<0.01 respectively).
But the water temperature of both the pond waters showed positive correlation with free CO2 of water (r=0.912, p<0.01 and r=0.789, p<0.01). The pH values of both pond waters also showed positive correlation with dissolved O2 (r=0.803, p<0.01 and r=0.819, p<0.01 respectively). These findings are comparable with several workers in their studies on different water bodies (Rai and Gary, 1980; Shardendu and Ambashth, 1988; Sinha, 1995; Zang et al., 2011; Liu et al., 2020).
Table 1. The physico-chemical and hydro-biological parameters of two different types of productive ponds of Bankura district of WB, India from January to June, 2020 are being summarized here. The values of different physico-chemical parameters are Mean ± S.E. where N=12.
| Sampling sites
Parameters observed |
Type I Pond Daser bandh of Rajgram, Bankura (High productive) | Type II Pond Natun bandh, Kankata, Bankura (Less productive) | BSI
standard |
| Average water depth (metre) | 0.75 – 1.15 | 3.45 – 4.34 | — |
| Latitude | 23°12’54”N | 23°13’35”N | — |
| Longitude | 87°2’12”E | 87°3’4”E | — |
| Air Temp (0C) | 29 ± 5.2 | 29 ± 3.4 | — |
| Water Temp (0C) | 25 ± 3.6 | 22 ± 2.5 | < 400C |
| Light intensity (Lux) | (349-943)x100 | (768-1111)x100 | — |
| pH | 7.33 ± 0.71 | 6.14 ± 0.54 | 6.5 – 8.2 |
| Dissolved O2 (mg/L) | 5.00 ± 0.25 | 2.64 ± 0.79 | Upto 6.0 |
| Free CO2 (mg/L) | 355 ± 26.5 | 372 ± 28.1 | — |
| Dissolved CO2 (mg/L) | 330 ± 34.6 | 405 ± 38.6 | — |
| Qualitative analysis of plankton | Planktons observed in moderate to high amount | Huge number of plankton (mainly Euglena sanguinea) observed | — |
Table 2. Correlation matrix among the physico-chemical parameters of Type I pond water during January to June, 2020.
| Parameters | pH | Dissolved O2 (mg/L) | Free CO2 (mg/L) |
| Water Temp (0C) | -0.961**
(p value 0.00001) |
-0.929**
(p value 0.000013) |
0.912**
(p value 0.000036) |
| pH | – | 0.803**
(p value 0.001661) |
-0.746**
(p value 0.005335) |
| Dissolved O2 (mg/L) | – | -0.817**
(p value 0.001178) |
**= Correlation is significant at p<0.01 level, *= Correlation is significant at p<0.05 level, N=12
Table 3. Correlation matrix among the physico-chemical parameters of Type II Pond water during January to June, 2020.
| Parameters | pH | Dissolved O2 (mg/L) | Free CO2 (mg/L) |
| Water Temp (0C) | -0.705*
(p value 0.010447) |
-0.756**
(p value 0.004445) |
0.789**
(p value 0.002283) |
| pH | – | 0.819**
(p value 0.001119) |
0.647*
(p value 0.022962) |
| Dissolved O2 (mg/L) | – | -0.619*
(p value 0.031863) |
**= Correlation is significant at p<0.01 level, *= Correlation is significant at p<0.05 level, N=12
It is also known that the dissolved oxygen (DO) plays a crucial role in sustaining flora and fauna in aquatic ecosystem. Among the two ponds, the pond of Daser bandh of Rajgram, Bankura showed the highest (5.00 ± 0.25 mg/L) level of DO in comparison to the other pond water (2.64 ± 0.79 mg/L) (Table 1). A moderate number of phyto as well as zooplankton were observed in Type I ponds but in Type II or the less productive ponds possess comparatively huge amount of Euglena sp (Figure 5, 7, 9 and 10). As the timing of water collection was between 6 to 7 AM; the level of DO was lower due to low intensity of sunlight.
The value of light intensity was measured (from 349 to 943)x100 Lux in Type I ponds and (from 768 to 1111)x100 Lux in Type II ponds. The pond surroundings were full of trees in case of Type I ponds, where as in Type II ponds the pond surroundings were devoid of plants. Furthermore, the algal bloom of the phytoplankton showed a blanketing effect on the Type II Pond, thereby preventing the penetration of sunlight into the pond water. It greatly affected the growth of beneficial algae by hampering photosynthesis. As a result, DO level was depleted in Type II ponds (Majumder et al., 2019a and Liu et al., 2020).
Dissolved and free CO2 in water plays an important role in maintaining the aquatic life. Main sources of carbon dioxide are respiration of aquatic organisms and also mixing of atmospheric CO2 with the pond water. Due to the high affinity of CO2 towards water, they can react to form carbonic acids and carbonates which alters the pH of water. The pH values of Type I and II pond water showed both negative and positive correlation with free CO2 value of pond water (r=-0.746, p<0.01 and r=0.647, p<0.05 respectively) (Table 2 and 3). In this study no such remarkable change was observed (Table 1) in free and dissolved CO2 values. But the DO value of Type I and II pond water showed negative correlation with free CO2 of water (r=-0.817, p<0.01 and r=-0.619, p<0.05 respectively) (Liu et al., 2020).
Figure 1: Two different types of ponds are observed at Bankura town

Figure 2: Two sampling sites (marked red and yellow oval) at the two different sided of Dwarkeswar river of Bankura town. Type I (Yellow) and Type II (Red)
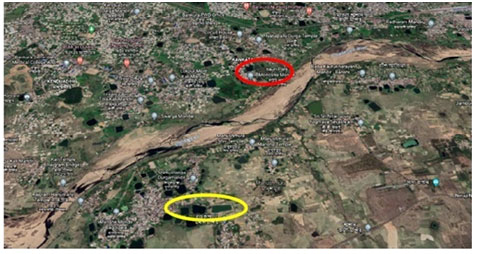
Figure 3: Daser Bandh (23012’54”N and 8702’12”E), Rajagram, Bankura [beside Bateswar Temple]
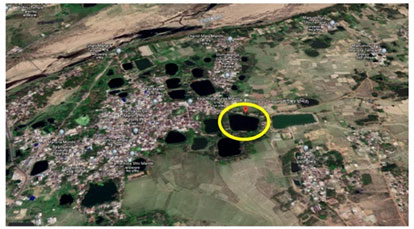
Figure 4: Natun Bandh (23013’35”N and 8703’4”E), Kankata, Bankura Type I (pond with yellow marking)Type II (pond with red marking)

Planktons observed: The study of plankton is necessary in fisheries and aquaculture research as it provides food for fish in freshwater lakes and plays a major role in fish production. Zooplanktons are heterotrophic planktonic organisms floating in water. They are delicate aquatic organisms and some of them are very weak swimmers. They contribute significantly to biological productivity of freshwater ecosystem. They serve as good indicators of change in water quality, because it is strongly affected by the environmental conditions and it quickly responds to change in environmental quality (Guy, 1992; Majumder et al., 2019b; Majumder, 2020). The freshwater zooplanktons constitute mainly Cladocera, Copepods, Rotifers, Ostracods, Protozoans and larva.
Figure 5: Four different ponds of Type I, their zooplanktons and huge fish yields

Among zooplanktons, the cladocera are the dominant group. This group is represented by Daphnia sp., Moina sp., Ceriodaphnia sp. and Bosmina sp. As per Murugan et. al., (1998) this group feeds on smaller zooplankton, bacterio-plankton and algae and they are highly responsive against pollutants. Cladocera are important food source for fry, fingerlings and adult of many economically important fish species. Cladocera were observed in both the two types of ponds but upto 13% in Type I ponds and only 2% in Type II ponds (Figure 9 and 10). Growth of Cladocera also been observed upto 18-25 % in Indian ponds (Majumder 2020; Sakhare and Chalak 2020).
Figure 6: Eight different ponds of Bankura town of Type II along with algal bloom on the water surface

Copepoda comprises of the third most abundant group of zooplankton & this group is represented by Cyclops sp. and Diaptomus sp. Depending on the feeding habits there are three orders of copepods. Copepods of the order cyclopoida are the most important food items in freshwater aquaculture and their Nauplius is especially valuable for feeding fry (Wojciech et al., 2004). Cyclopoid copepods are commonly carnivorous who live on other zooplankton and fish larvae, though they also feed on algae, bacteria and detritus also. Copepoda were observed in both the two types of ponds but upto 51% in Type I and only 4% in Type II ponds (Figure 9 and 10). Increased level in number of Copepoda also been observed in ponds of Bankura (Majumder et al., 2019b; Majumder 2020).
Figure 7: Planktons mainly found (Euglena sanguinea) in Type II ponds
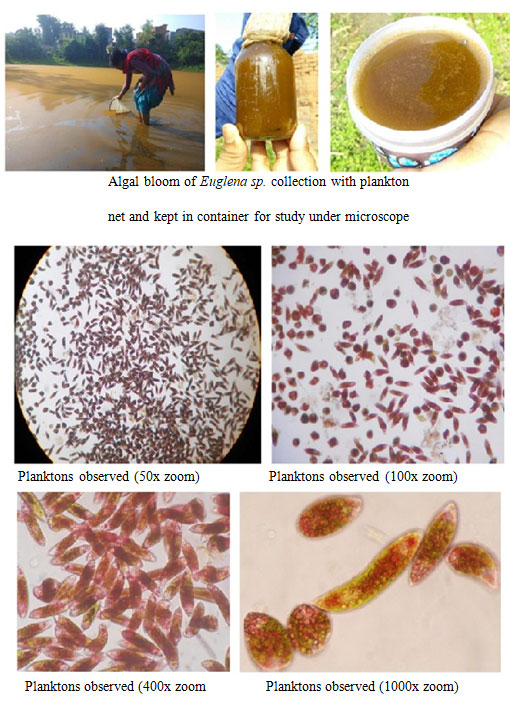
Figure 8: Due to excessive growth of Euglena sanguinea fish yield decreases a lot in Type II ponds of Bankura town
Rotifers are the most important soft-bodied invertebrates having a very short life cycle among the zooplanktons. These are globally recognized as pollution indicator organisms in the aquatic environment (Kamble and Meshram, 2005). Rotifers occasionally become plenty when sufficient food is available and it can obtain population density of over 5000 individual/L. Quantitative exploration during the period of study showed that the family Branchionidae exhibit maximum diversity of species (Joshi et al., 2015).
Rotifers were observed in both the two types of ponds but upto 21% in Type I and only 7% in Type II ponds (Figure 9 and 10). The decreased number of Rotifers from perennial to shallow-water pond indicates the fact of low yield in pisciculture (Sakhare and Chalak 2020; Majumder 2020). The most dominant rotifer is Brachionus sp. It is illustrated by 4 species; among them Brachionus bidentata was found to be the predominant species. Brachionidae an important family of monogonont Rotifera and of the Rotifer fauna of India has received relatively more attention of the Indian workers relying on limnetic collections (Sharma and Sharma, 2014, Majumder 2020).
Figure 9: Planktons observed in Type I ponds
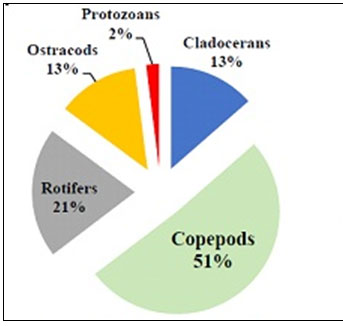
Figure 10: Planktons observed in Type II ponds
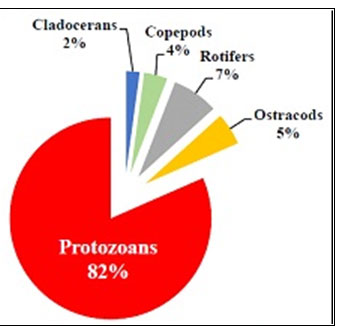
Ostracods are small, poorly-segmented Crustacean in which the body parts are enclosed within a calcareous bivalve carapace. Ostracoda comprises of the least abundant group of zooplankton and this group is represented by Cypris sp. and Heterocypris sp. Ostracods are mainly bottom dwellers of lakes and live on detritus and dead phytoplankton. These organisms are food of fish and benthic macro-invertebrates (Chakrapani et. al., 1996). Ostracods were observed in both the two types of ponds but upto 13% in Type I and only 5% in Type II ponds (Figure 9 and 10). The decreased number of Ostracods from Type I to II pond indicates the fact of low yield in pisciculture (Majumder 2020). As ostracods occur in almost all aquatic habitats, the extreme antiquity of the group and the preservation of their valves in a wide variety of depositional environments make them an important tool in both palaeoecological and bio stratigraphic analysis (Armstrong and Brasier, 1980).
Some of the most common protozoa that can be found are Euglena sp., Chlamydomonas sp., Phacus sp. etc. Protozoa mainly Euglena sp. showed very dense population (82%) in Type II ponds whereas only 2% observed in Type I ponds. Euglena sanguinea is generally cylindrical with a blunt pointed end. This alga is found in fresh water environments all over the planet. Like many other members of the algae (Protists subgroup) it seems to be capable of both sexual and asexual reproduction. It produces an interesting compound toxic to many fish species, and can also overgrow under certain conditions also leading to fish death (Grung and Liaaen-Jensen, 1993; Gerber and Hader, 1994; Paul et al, 2010). Understanding this microorganism is very important to fishing and fish-farming industries because under certain conditions these algae can have a detrimental impact on its surroundings (Zimba et al., 2004; Zohdi, 2019). They are capable of altering their shape, especially during motility: for example, they can extend their length up to ten times their width (Majumder, 2020).
They have a retractable flagellum, that remains mostly within the cell body, even when it is fully extended. They have also been noted to have granular eye spots of many varieties. They have been shown to reproduce asexually by mitosis. The photosynthetic algae are the autotrophs containing chlorophyll and an accessory pigment namely astaxanthin (a carotenoid). The accessory pigment prevents the cell’s chloroplast from being overwhelmed under excessively bright conditions. The cells will appear red when utilizing this accessory pigment and green when these pigments are retracted into vesicles. This alga produces a compound known as euglenophycin that exhibits ichthyotoxic, herbicidal and anticancer activity at low ppm to ppb dosages (Paul et al., 2010). Actually, ichthyotoxic means toxic to fish. Euglena sanguinea a protist that is found in freshwater environments all over the world (Majumder, 2020).
Some researchers suggested that the toxin functions as a neurotoxin based upon the behavioural changes. Juvenile catfish exposed to cultures of the algal isolates died within 2h of exposure (Zimba et al., 2004). Some euglenophyte species have been reported to be red or to have the ability to become red with Euglena sanguinea. Euglena sanguinea is a microalga that inhabits eutrophic lentic freshwater habitats throughout the world (Wołowski, 2011; Guiry, 2017). It was originally described from a blood-coloured water sample from Silesia, although it was noted that cells were at first green and then red (Laza-Martínez et al., 2018). Present study revealed that Euglena sp. grows enormously in shallow-water ponds during winter to summer season. These algae produce a compound known as euglenophycin. The compound euglenophycin exhibits ichthyotoxic compound; means toxic to fish. Euglena sanguinea a protist that is found in freshwater environments all over the world (Laza-Martínez et al., 2018).
CONCLUSION
Present study revealed that Euglena grows enormously in shallow-water (not in perennial) ponds during winter to summer season which creates oxygen depletion leading to mass mortality of fish. Actually, Euglena cannot be mechanically or physically controlled, except by replacing the pond water which is not a practical option for most pond owners. Still, some of them are doing so along with aeration at night for several days. This may help to control the oxygen depletion. Rather the fish farmers can take necessary steps to reduce the algal growth in the pond by controlling the mixing of phosphate rich nutrients and effluents of agricultural land nearby the pond.
ACKNOWLEDGEMENTS
The author is thankful to the Principal, Bankura Sammilani College for providing space to do the laboratory-based works and to the owners of the above-mentioned ponds of Bankura districts for their cordial help during the sampling and also thankful to the Eastern Regional Office (ERO) of University Grants Commission (UGC), Kolkata for providing the instrumental and chemical support to do this work (Grant No. F. PSW-005/13-14(ERO) ID No. WB1-009; S. No. 219564).
Conflict of Interests:The author declares no conflict of interest while finalizing the manuscript.
REFERENCES
Anton-Pardo M and Adamek Z (2015) The role of zooplankton as food in carp pond farming: a review. J. Appl. Ichthyol. Vol 31 No Suppl. 2 pp 7–14.
APHA, AWHA and WPCF (2005) Standard methods for the examination of water & wastewater. 21st edn, American Public Health Association, American Water Works Association and Water Pollution Control Federation, Washington DC.
Armstrong HA and Brasier MD (1980) Microfossils 2nd edn, Blackwell Publishing, USA.
Borics G, Abonyi A, Salmaso N and Ptacnik R (2021) Freshwater phytoplankton diversity: models, drivers and implications for ecosystem properties. Hydrobiologia Vol 848 pp 53–75.
Bureau of Indian Standards, (2003) In: Indian Standard Drinking Water-Specification (First Revision), Edition 2.1 (1993-01), IS 10500: 1991, (c) BIS (2003), Manak Bhavan, 9, Bahadur Shah Zafar Marg, New Delhi, 110002.
Chakrapani BK, Krishna MB and Srinivasa TS (1996) A Report on the water quality, plankton and bird populations of the lakes in and around Bangalore and Maddur, Karnataka, India. Department of Ecology and Environment, Government of Karnataka.
Date DW, Rayate PS, Chaugule BB (2018) Ecological and physico-chemical observations on three blooms of Euglena in Pune, (M.S.) area, International Journal of Researches in Biosciences, Agriculture and Technology Vol VI No Special Issue 2 pp 45-48.
Fentie D (2020) Methods of Algal Control: A Review Paper. Int. J of Engineering Science and Computing Vol 10 No 9 pp 27284-27288.
Frassanito R, Cantonati M, Flaim G, Mancini I and Guella G (2008) A new method for the identification and the structural characterization of carotenoid esters in freshwater microorganisms by liquid chromatography/ electrospray ionization tandem mass spectrometry. Rapid Communications in Mass Spectrometry Vol 22 pp 3531–3539.
Gerber S and Hader DP (1994) Effect of enhanced UV-B irradiation on the red-coloured freshwater flagellate Euglena sanguinea. FEMS Microbiology Ecology Vol 13 pp 177–184.
Gokul EA, Raitsos DE2, Gittings JA and Hoteit I (2020) Developing an Atlas of Harmful Algal Blooms in the Red Sea: Linkages to Local Aquaculture. Remote Sens. Vol 12 pp 3695-3709; doi:10.3390/rs12223695.
Goswami SN, Trivedi RK, Saha S, Mandal A and Jana S (2017) A study on plankton diversity of three urban ponds in Kolkata of West Bengal State, India IJABR Vol 7 No 4 pp 687-691.
Grung M and Liaaen-Jensen S (1993) Carotenoids in a natural bloom of Euglena sanguinea. Biochemical Systematics and Ecology Vol 21 pp 757–763.
Guiry MD and Guiry GM (2017) Algaebase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org.
Guy D (1992) In: The ecology of the fish pond ecosystem with special reference to Africa. Pergamon Press, New York 220–230.
Islam SN and Bhuiyan AS (2007) Monthly vertical occurrence of some copepods in a pond in Rajshahi City Research Journal of Fisheries and Hydrobiology Vol 2 No 1 pp18-20.
Joshi A, Kalgutkar A and Joshi N (2015) Quantitative analysis of diversity during seasonal variations of Sanjay Gandhi National Park (SGNP) by Quadrate Method Int. J. of Life Sciences Vol 3 No 1 pp 76-80.
Kamble BB and Meshram CB (2005) A preliminary study on zooplankton diversity of Khatijapur tank near Achalpur, Dist. Amaravati, M.S. J. Aqua. Biol. Vol 20 No 2 pp 45-47.
Khanna DR and Bhutiani R (2008) In: Laboratory Manual of Water and Wastewater Analysis, Astral International.
Laza-Martínez A, Fernández-Marín B and García-Plazaola JI (2018) Rapid colour changes in Euglena sanguinea (Euglenophyceae) caused by internal lipid globule migration. European Journal of Phycology Vol 54 No 1 pp 90-101. https://doi.org/10.1080/09670262.2018.1513571
Liu G, He W and Cai S (2020) Seasonal Variation of Dissolved Oxygen in the Southeast of the Pearl River Estuary. Water Vol 12 pp 2475-2493. doi:10.3390/w12092475
López-Felices B, Aznar-Sánchez JA, Velasco-Muñoz JF and Piquer-Rodríguez M (2020) Review Contribution of Irrigation Ponds to the Sustainability of Agriculture. A Review of Worldwide Research, Sustainability Vol 12 pp 1-18.
Majumder S (2020) Plankton diversity changes with slight variation in physico-chemical parameters of some lotic and lentic water bodies of Bankura district of WB, India. Uttar Pradesh Journal of Zoology Vol 41 No 4 pp 9-22.
Majumder S and Dutta TK (2014) Studies on seasonal variations in physico-chemical parameters in Bankura segment of the Dwarakeshwar River (W.B.) India, Int. J. Adv. Res. Vol 2 No 3 pp 877-881
Majumder S, Bhowmik B, Dey D, Banerjee S and Ghosh S (2019a) A Comparative Study Among Three Different Types of Productive Ponds of Bankura District of WB, India. Environment and Ecology Vol 37 No 1 pp 120-126.
Majumder S, Patra M, Banerjee S, Konar S and Saha D (2019b) A Study of Plankton Diversity of Some Ponds of Bankura Town, West Bengal, India. Environment and Ecology Vol 37 No 4 pp 1115-1123.
Murugan N, Murugavel P and Kodarkar MS (1998) Cladocera: The biology, classification, identification and ecology. Indian Association of Aquatic Biologists (IAAB), Hyderabad.
Paul VZ, Peter DM, Kevin B, Hannah EL and Richard ET (2010) Identification of euglenophycin – A toxin found in certain euglenoids. Toxicon. Vol 55 No 1 pp 100-104. http://dx.doi.org/10.1016/j.toxicon.2009.07.004
Radhakrishnan DK, Ali IA, Schmidt BV, John EM, Sivanpillai S and Vasunambesan ST (2019) Improvement of nutritional quality of live feed for aquaculture: An overview. Aquaculture Research Vol 51 No 1 pp 1-17.
Rai H and Gary H (1980) Classification of Central Amazon Lake (South America) on the basis of their microbiological and physico-chemical characteristics. Trop. Ecol. 1-23.
Sakhare VB and Chalak AD (2020) Seasonal variation of cladocera in a perennial pond, Ambajogai (Maharashtra), India. Journal of Fisheries Vol 8 No 2 pp 1-3.
Shardendu and Ambasht RS (1988) Limnological studies of a rural pond and urban tropical aquatic ecosystem: oxygen forms and ionic strength Tropical Ecology Vol 29 No 2 pp 98-109.
Sharma BK and Sharma S (2014) The diversity of Indian Brachionidae (Rotifera: Eurotatoria: Monogononta) and their distribution, Opusc. Zool. Budapest Vol 45 No 2 pp 165-180.
Sinha SK (1995) Potability of some rural pond’s water at Muzzafarpur (Bihar). A note on water quality index. Poll. Res. Vol 14 No 1 pp 135-140.
Wojciech P, Goodwin AE, Eiras JC and Nowak BF (2004) Importance of copepoda in freshwater aquaculture. Zoological Studies Vol 43 No 2 pp 193-205.
Wato T and Amare M (2020) The Agricultural Water Pollution and Its Minimization Strategies– A Review. Journal of Resources Development and Management Vol 64 pp 10-22.
Wołowski K (2011) Phylum Euglenophyta (Euglenoids) In: The Freshwater Algal Flora of the British Isles: An Identification Guide to Freshwater and Terrestrial Algae. 2nd ed. Pp 181–239.
Zang C, Huang S, Wu M, and Du S (2011) Comparison of Relationships Between pH, Dissolved Oxygen and Chlorophyll a for Aquaculture and Non-aquaculture Waters. Water Air and Soil Pollution Vol 219 No 1 pp 157-174.
Zimba PV, Rowan M and Triemer R (2004) Identification of euglenoid algae that produce ichthyotoxin(s). Journal of Fish Diseases Vol 27 pp 115-117.
Zohdi E and Abbaspour M (2019) Harmful algal blooms (red tide): a review of causes, impacts and approaches to monitoring and prediction. Int. J. Environ. Sci. Technol Vol 16 No 3 pp 1789-1806. https://doi.org/10.1007/s13762-018-2108-x


