1Federal and Regional Centre for Aerospace and Surface Monitoring of the Objects and Natural Resources, Belgorod State National Research University, Belgorod, Russian Federation
2Institute of Earth Sciences, Belgorod State National Research University, Belgorod, Russian Federation
3Department of Biology, Belgorod State National Research University, Belgorod, Russian Federation
Corresponding author Email: liset@bsu.edu.ru
Article Publishing History
Received: 10/12/2018
Accepted After Revision: 21/02/2019
The differentiation of soil fertility vertically and its territorial distribution on the slopes have a genetic origin. The main aim of the study was to establish a link between the level of effective soil fertility and its biological activity in two dimensions. The degree interaction between the cellulose decomposition activity and the yield of barley on separate layers of the southern Chernozem was established. The erosion catena demonstrates that the cellulose decomposition intensity in non-eroded and moderately eroded soils was determined by the differences in magnitude and variation soil water reserves in individual soil layers. The results showed that the microbiological activity of the soil refl ecting the intensity of the transformation process of organic matter and the degree of nutrients availability in the yield is likely to act as the sensitive indicator of hydrothermal conditions. In addition, according to the research results, the effective fertility of individual soil layers both in the vertical dimension and in the topographic gradient was evident.
Cellulose-Decomposing Microorganisms, Soil Fertility, Catena, Soil Erosion
Lisetskii F, Vladimirov D, Cherniavskih V. Evaluation of Soil Biological Activity by a Vertical Profile and Erosion Catena. Biosc.Biotech.Res.Comm. 2019;12(1).
Lisetskii F, Vladimirov D, Cherniavskih V. Evaluation of Soil Biological Activity by a Vertical Profile and Erosion Catena. Biosc.Biotech.Res.Comm. 2019;12(1). Available from: https://bit.ly/2YSWYk5
Copyright © Lisetskii et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
Most abiotic factors and, particularly, air and soil humidity and temperature, concentration and migration of chemical elements and compounds in the soil and ultimately soil fertility tends to change regularly along hillslope gradient. Studies of vegetation patterns along a vertical gradient have shown that microtopography driven soil moisture has an important role in the formation of vegetation patterns (Deák et al., 2014) and soil-plant cover (Lisetskii et al., 2014a). Effective fertility is mostly materialized through microorganism activity; therefore, there is a direct link between soil fertility and its biological activity. This applies to natural changes in fertility and microbiota activity conditions both by vertical measurement in profi le and by topographic gradient. About 65% of the world’s land is subject to water erosion and 28% – to defl ation (Webb et al., 2006). In intensive agriculture in semi- arid regions the area of eroded soils increased, on the slopes with eroded soils, the humus content decreased (Prudnikova and Savin, 2015, Haddad et al., 2018; Upton et al., 2018).
Erosion on slope soils changes not only soil profi le morphology, the OM content (Lisetskii, 2008), the texture of soils at different degrees of erosion on slopes with free runoff became coarser by one gradation (Gabbasova et al., 2016), but also the complex of physico-chemical properties (Volungevicius et al., 2018) and content chemical elements useful for plants (Zelenskaya et al., 2018), which ultimately affects soil formation speed and soil loss tolerance value (Shtompel et al., 1998). During prolonged ploughing of chernozems as compared with a pasture or virgin, the number, size, and biomass of bacterial cells and microfungi biomass decrease (Polyanskaya et al., 2016). The aim of this study was to determine changes in the morphological, physical and chemical properties of soils caused by their agrogenic transformation.
The main soil fertility factor is the organic matter (OM) contained in it. The intensity of plant residues decomposition OM in the soil can be judged by its biological activity, which refl ects the related complex of biological processes. For the entire life of soil, the main source of energy is cellulose, which, for example, amounts to 57% in winter wheat straw. Cellulose mineralization synthesizes compounds, which are essential for plant growth,
humifi cation processes and soil microorganism vital activities. The cellulose-decomposing group of bacteria decomposing plant residues release oxidative enzymes into the environment, which causes humus synthesis to run more intensively using decay products of these residues (Zakharov, 1978). It is not always possible to assess the level of fertility of individual layers of soil profi le by number of nutrients available to the plants. Therefore, it is necessary to search for such indicators of soil fertility that correlate with yields. It was previously found (Tikhomirova, 1970; Zakharov, 1978; Yemtsev and Mishustin, 2005; Ulrich et al., 2008; Widhiya et al., 2016; Haddad et al., 2018; Upton et al., 2018), that the complex of soil conditions required for the vital activity of aerobic microorganisms, which decompose cellulose, coincide with the conditions of crop formation.
Cellulolytic soil activity can be used as an indicator of the vital functions of cellulose decomposing microorganisms, and conditions for formation of cultivated plant yield. It is known that cellulose constitutes the bulk of plant residues, and the role of cellulose-destroying microorganisms is signifi cant, since they are producers of biologically active substances, which replenish the pool of these compounds in the OM soil. For example, many types of cellulolytic bacteria (genus Cellulomonas, Cellvibrio, Bacillus, Myxococcus, Pseudomonas, Flavobacterium and others) synthesize amino
acids, growth agents, vitamins, pigments and oxidative enzymes which are involved in redox processes of plant residue humifi cation (Kozlov et al., 2017). When cellulose is decomposed, the cellulolytic microorganisms release a signifi cant amount of mucus involved in soil conditioning as well as pigments of various nature directly involved in humus synthesis (Zavarzin, 2014).
The need for environmental conditions varies in different cellulose-destroying microorganisms; therefore, it is common for different types of soils to have certain groups of cellulose-destroying microorganisms (Yemtsev and Mishustin, 2005). Semiarid regions with obvious shortage of sediments are characterized by formation of soil conditions in the presence of moisture redistribution along hillslope gradient, and they are signifi cantly changed when using irrigation. The aims of the study were to establish a link between the level of effective fertility based on irrigation soils profi le and cellulolytic activity and to identify differences in the rate of organic matter destruction in soils with varying erosion index on erosion catena.
Materials and Methods
Study area: The study area is a part of the southern steppe subzone located seaside and characterized by a weakly dissected surface. Here landscapes are formed in the conditions of hot summer, dry warm autumn and moderately warm wet winter. Average annual air temperature (at the nearest weather station (Odessa)) is10.2 °C. The average air temperature in summer varies from 19.4 °C (June) to 22.2 °C (July). The average annual
amount of precipitation (since 1894) was 386 mm. Evaporation value is 800 mm, humidity factor is 0.48 and i.e. the correction exceeds precipitation 1.5–2 times. The century-old precipitation trend is characterized by alternation of wet and dry periods (with a reduction to 310–320 mm per year). Heliothermal cycles with major periods of 11 and 10 years which are part of a lower-frequency natural periodicity (Ivanov and Lisetskii, 1995),
consist in the region covered by our study of 3–4 year phases of change in the amount of heat and moisture.
The studies were carried out on the following two test areas: irrigation land on a relatively fl at watershed (Pir) and non-irrigated agrolandscapes (boghara) with slopes characterized by active soil erosion (Pboh). The Pir test area is a Loess Plateau with a slight eastward slope.
Belyaevka region (Ukraine). Medium-steppe subzone. Lower Dniester irrigation system (Shkodogorsky array). It was built in 1964. The irrigation area exceeds 37 thou. ha. Groundwater occurs at a depth of 2–5 m. The source of irrigation is the water of the Dniester River. Dominating soils are Southern heavy loam solonetz Chernozem. Humus horizon thickness is 55 cm. Corg is 1.79%. It boils from HCl starting with 70 cm and demonstrates
vigorous boiling from 78 cm.
The Pboh test area is a gently undulating loess plain (slopes of steepness<2 occupy 39%) which belongs to a very dry and moderately hot agro-climatic zone. Share of eroded soils (Southern heavy loam Chernozem) is 28%, including moderately eroded soils, which occupy 29% of the total area of eroded soils. We examined the erosion catena on the slope of western exposition with average slope of 2° (experience 3).
Data used: Problem-solving was based on the research program, which included four experiments.
Experiments 1A and 1B included a fi eld experiment on assessment of biological activity with southern irrigation (1A) Chernozem layers (within the genetic horizons) and a vegetation experiment with the same layers to assess the level of effective fertility (1B). The results of these experiments complemented each other. Experiment 1A was carried out in the fi eld to determine differences in the rate of cellulose (%) decomposition in
individual layers of irrigated southern Chernozem. Oats and peas planting. Oat phase gives rise to booting (fi fth sheet, height 47 cm). A cellophane fi lm of 3×6 cm in size was put into a nylon mesh with mesh size of 0.25 mm and vertically attached to the soil (3-fold repeatability). Experiment 1B was conducted in a phytotron with separate soil layers. We cultivated Odessa-82, a recognized spring barley variety, by 14 plants per a vessel with capacity of 2520 cm3. Since individual soil layers had different moisture content (from 43 to 62%), the moisture in the soil was maintained using gravimetric method at from 25.9% to 37.4%.
The phytotron-maintained average temperature and relative humidity values (according to thermograph and hygrograph records) were 21–25 and 55–65% respectively between seeding and barley earing periods (90 days). We equalized the illumination conditions by periodic vessel rearrangement. For the vegetation experiment, we followed the previous recommendation that for options with transitional horizons (AB, BC) a suffi cient number of repetitions at a probability level of 0.95 should be considered as 4–6-fold repeatability, and for options with other horizons reliable results can be obtained when using 3–4 vessels. This made it possible to conduct more reasonable analysis of the mean options with due account for marginal error of difference of the means. Due to different option repeatability in the experiments, the non-equivalence of contrast of means was taken into account when assessing signifi – cance of particular differences.
Experiment 2 was carried out in the fi eld to assess differences in linen fabric decomposition for 2 periods of biotest exposure in layers 0–10 and 10–20 cm under fallow conditions. As for the experiment location, it was the western exposure ravine slope near water divide, where the southern unwashed Chernozem (depth of horizon A is 32 cm, humus horizon thickness is (A+AB) 50 cm) was present. Experiment 3 was conducted in the fi eld on the erosion catena. Objects under study: 1) Medium loamy non-eroded at the water divide Southern Chernozem, depth of horizon A is 32 cm, humus horizon thickness is (A+AB) is 53 cm; 2) Medium-eroded Chernozem 270 m down the slope. Soil moisture and bulk density were monitored at the depths of 10 and 20 cm for 459 days using 22 observation periods. The experiment was carried out for two calendar years in the same periods of exposure (August – October). The initial phase of the experiment ran under the stubble of winter wheat (minor cultivation), and since September processes took place in winter wheat rhizosphere, then after the wheat was plowed in May corn was sown and harvested for silage, and in September the plowing was done.
Methods
Activity of microfl ora destroying cellulose in individual layers of the ploughing horizon was determined by the rate of pure linen fabric decomposition since fl ax fi bers consist of cellulose for 80% and impurities in the form of minerals and lignin for 20%. Decomposing cellulose with simple communities of microorganisms (1–2 species) is signifi cantly more sensitive to the soil conditions than plant residues, and its usage provides more accurate results due to uniformity of chemical composition of biotests. The method of “applications” is objective enough to assess the intensity of microbiological activity in different layers of the soil profi le and to compare agricultural technologies, including the use of irrigation. The method of applications use is modifi ed (Mirchink, 1988), but it is based on the assessment of intensity of linen fabric decomposition by the difference in dry weight before and after exposure. Biotesting was carried out using squares of linen fabric size 1010 cm, which was covered with a nylon mesh with mesh size of 0.25 mm and laid horizontally in the soil at depths of 10 and 20 cm in order to preserve defragmented material after decomposition. In addition, we used chemically pure cellulose fi lm in the applications method. The bags removed with fi lms were washed from soil particles fi rst with tap water and then with distilled water. The loss of fi lm weight was determined using analytical scales, and then we calculated the average percent age (%) of decomposition for each examined layer and computed the average percentage for examined layers in each option of the experiment. We used the Euclidean distance calculation to make cumulative comparison of soil layers by parameters, such as humidity (%), bulk
density (t m-3) and soil moisture reserve (mm). Chemical analyses of soils included the following standard procedures: Corg content, by Tyurin’s method and CO2 by acidometry. The determination of cation exchange capacity (CEC) in calcareous soils was performed using EDTA–Na2.
Results and Discussion
Microbiological activity of irrigation soil Soil irrigation results in increased number of certain groups of microorganisms: algae, nitrifi ers, denitrifi – ers, but less actinomycetes, aerobic organisms, which decompose cellulose (Table 1).
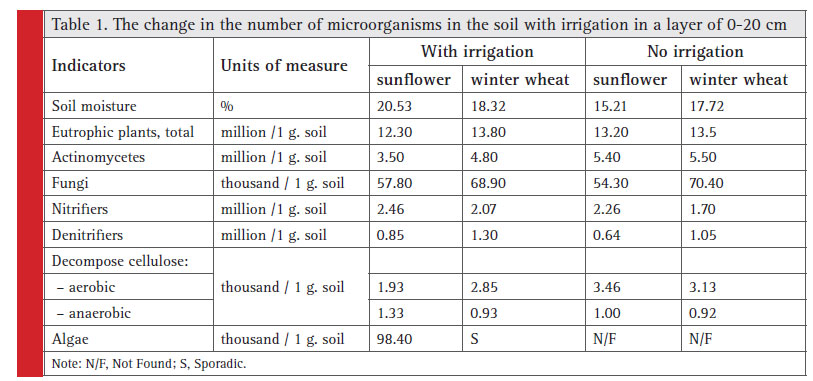 |
Table 1: The change in the number of microorganisms in the soil with irrigation in a layer of 0-20 cm |
An increase in erosion resistance of steppe soils during irrigation is explained largely due to growth by higher numbers of algae, fungi, aerobic cellulose-degrading microorganisms, and oligonitrophiles, in particular (Lisetskii et. al., 2018). Our results (Table 1) showed that irrigation had maximum effect on the number of groups, such as denitrifi ers, eutrophic plants, anaerobic bacteria and nitrifi ers. Under comparable conditions, sunfl ower and wheat have no advantages due to irrigation in terms of the number of microorganisms (with the exception of algae).
During three periods of vegetation it was natural in May–June for biological activity to show a decrease within meter-deep layer in certain layers of irrigation Chernozem, except for the upper part of carbonate horizon B1 (Table 2), which is associated with its chemical properties.
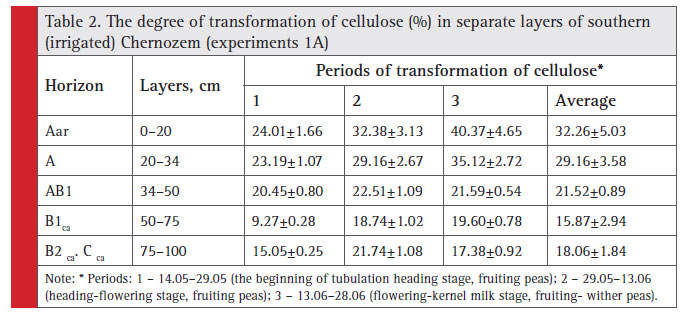 |
Table 2: The degree of transformation of cellulose (%) in separate layers of southern (irrigated) Chernozem (experiments 1A) |
Due to increased duration of irrigation reclamation in seven arid zones of Ukraine, it becomes relevant to study transformation tendencies in soil fertility profi le distribution under irrigation impact. In response to 35 years of irrigation the soil is reported to have humus profi le extension by 9 cm as compared to bogharic soil (Lisetskii, 1988), and increased humus reserve within one meter thick layer. The experiment revealed the existence of more clear horizon-oriented differences in effective fertility than in the non-irrigated Southern Chernozem. For example, the subsurface layer fertility decreased dramatically (F20-34 is 0.49). The results of dispersion analysis of barley grain yield obtained from individual layers of Southern Chernozem in experiment 1B showed that at the level F01 there were proven differences of effective fertility between all layers, except for layers to 50–64 and 64–75 cm that can be combined (Table 3). The relative (%) evaluation of effective fertility is determined by the ratio of grain yield for each tested layer (i) to ploughing layer yield (denoted by Fi).
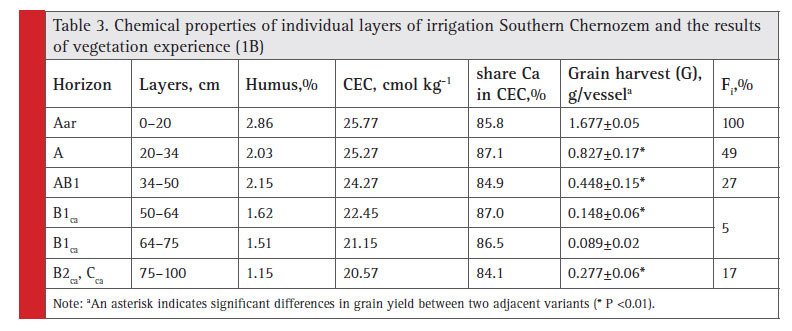 |
Table 3: Chemical properties of individual layers of irrigation Southern Chernozem and the results of vegetation experience (1B) |
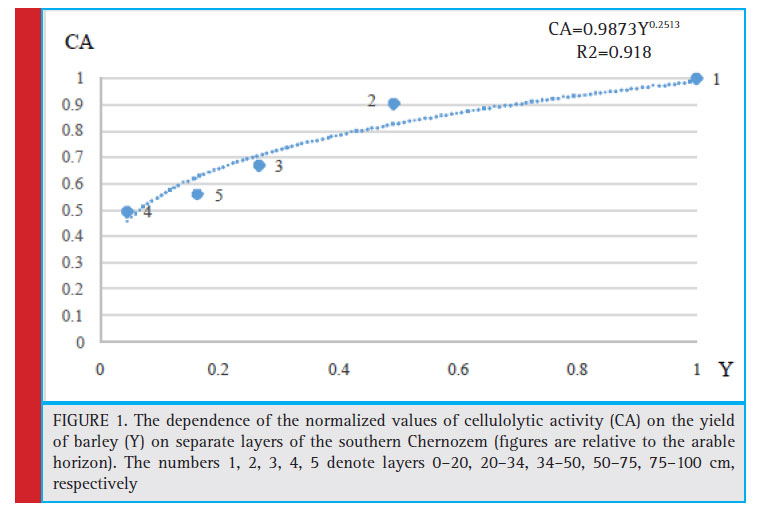
Features of effective fertility profi le distribution in irrigated soil are closely interrelated with microbiological activity. As shown by our two-year observations in the most important vegetation period (May-July), this is facilitated by absence of signifi cant horizon-oriented differences in the moisture content of a meter-thick layer of soil. On average, over three periods of microbiological tests exposure (May-June), the subsurface and underlying horizons of irrigated Chernozem showed decreased cellulolytic activity of soils in proportion to the reduced level of effective fertility (Figure 1). It is noteworthy that as for potential fertility (Table 3), the 75–100 cm layer is less fertile than layers to 50–64 and 64–75 cm, but it
appeared that this layer demonstrated higher cellulolytic activity and yield.
The results in Figure 1 show that the statistical dependence is virtually proportional: cellulolytic activity tends to grow by 18% with increased yield by each 20%. Therefore, microbiological soil activity can act as an indicator of effective fertility of individual layers of soil profi le. It shows the intensity of organic matter transformation process and the degree of possible materialization of available nutrients in the crop. However, the microbiota activity is ahead of those pedogenesis processes, which determine effective soil fertility.
Biotesting of in layer 0–20 cm fallow land
Fallow lands can signifi cantly restore the lost soil fertility even for a short period. The results of experiment 2 showed that in fallow condition the chemical parameters of virgin soil were virtually achieved, except for carbonate content, which obviously requires longer time (Table 4).
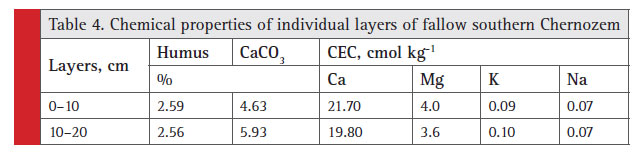 |
Table 4: Chemical properties of individual layers of fallow southern Chernozem |
In the 0 to 20 cm layer of the Chernozems the basal respiration rate and the content of the microbial biomass were minimal in the soil irrigated for 40 years without fertilizer and maximal in the fallow ones (120 yrs) (Prikhod’ko et al., 2013).Our results (Table 5) show that the intensity of microbiological processes can vary vertically under the infl uence of differences in moisture content even in 10 centimeter soil layers.
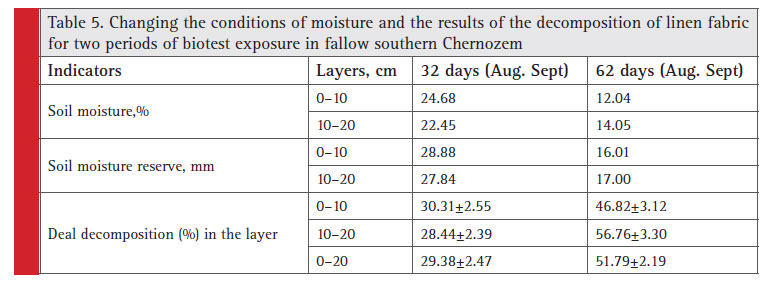 |
Table 5: Changing the conditions of moisture and the results of the decomposition of linen fabric for two periods of biotest exposure in fallow southern Chernozem |
The experiments demonstrated that the differentiation of the ploughing horizon into different in terms of effective fertility parts occurred after 2.5–3 months (Il’ina, 1980). And as for fallow land, as in our experiment, any change in hydrothermal conditions (decrease in average soil moisture from 23 to 14% and in-layer to 0–20 cm soil moisture reserve on 17 mm in the second month as compared to the fi rst month) is followed by multidirectional biological activity, when maintaining minor differences in humus and carbonates content and the composition of cation exchange (Table 5). In the fi rst month, the linen fabric decomposition proportion was slightly higher in the upper 10 cm layers as compared to the lower ones, but in two months, vice versa, cellulose destruction processes were more intense in 10–20 cm layer than in the upper 10 cm soil layers. This is due to the more soil moisture in the 10–20 cm layer as compared with the upper 10 cm during the second period of exposure (from 32 to 62 days).
Dynamics of moisture content and microbiota activity in erosion catena soils
Experiment 3 was conducted on the erosion catena in agrolandscape, which distinguishes this experiment from experiment 2. With the research territory, being signifi cantly eroded (about 60%), the percentage of moderately eroded soils is 13–14% (Lisetskii et al., 2014 b). Due to erosion, the slope soils lose OM and become less fertile (Table 6). The content of available cellulose in the form of surface residues on any moderately eroded
soil is 55–60% of its amount on any non-eroded soil (Lisetskii, 1992).
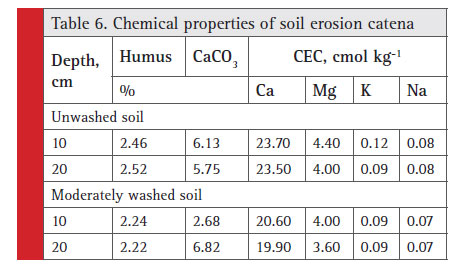 |
Table 6: Chemical properties of soil erosion catena |
The variability of indicators of microbocenosis conditions of steppe soils correlated with the dynamics of the climate (Kashirskaya et al., 2018). During the fi rst year of experiment the climatic conditions changed as follows: during the second period (30–60 days) the air temperature was 2.3°C higher than the long-term average annual normal temperature, and it corresponded to the normal value at the beginning and at the end of the experiment; the precipitation was lower than the normal level in the fi rst and second 30 days on 10 and 22.4 mm respectively, but in the third month precipitation indicator was by 30.8 mm more. The second year (starting from the eighth observation period) was very dry: if temperatures were close to the normal range, the moisture defi cit increased from 18.8 and 28.8 to 36 mm in each of three months (August – October, 16–21 timing of bservations). Naturally, this was mostly repeated in soil moisture dynamics (Figure 2). Moderately eroded soil had more severe moisture deficit.
On average, during the observation period (1 year and 3 months), the soil moisture reserve in the 0-20 cm layer was by 4 mm higher in unwashed soil as compared to the moderately washed soil. However, these soils (0–10 and 10–20 cm) have different layer-by-layer moisture characteristics. If the second layer of unwashed soil was more compacted than the upper one and had more moisture content and reserves, the lower layer of the moderately washed soil had lower moisture content, density and soil moisture reserve than the 0–10 cm layer. The Euclidean distance calculations make it possible for separate soil layer parameters to be considered as individual ones, and based on that to determine resulting differences of the investigated soil layers. The results obtained show that the unwashed and moderately washed soils have the greatest differences in terms of amount and variations of soil moisture reserves in individual soil layers. On average, during 22 observation periods (for 459 days) the soil moisture reserves were by 10% more in non-eroded Chernozem as compared to the moderately eroded one (Figure 2).
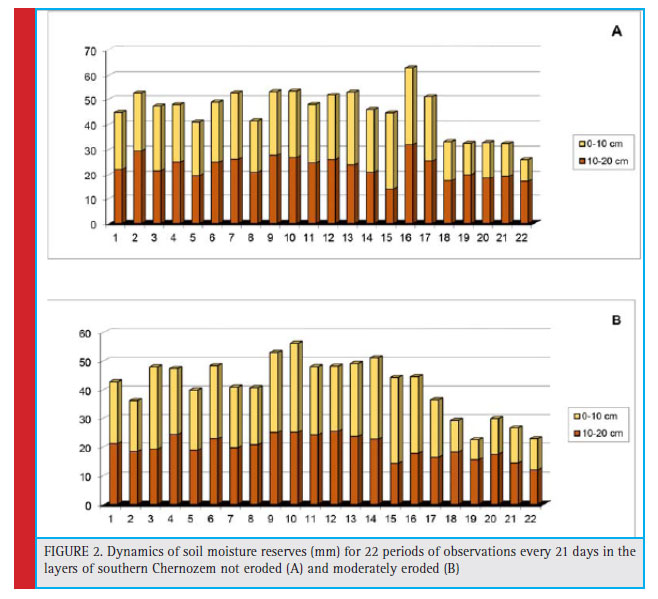 |
Figure 2: Dynamics of soil moisture reserves (mm) for 22 periods of observations every 21 days in the layers of southern Chernozem not eroded (A) and moderately eroded (B) |
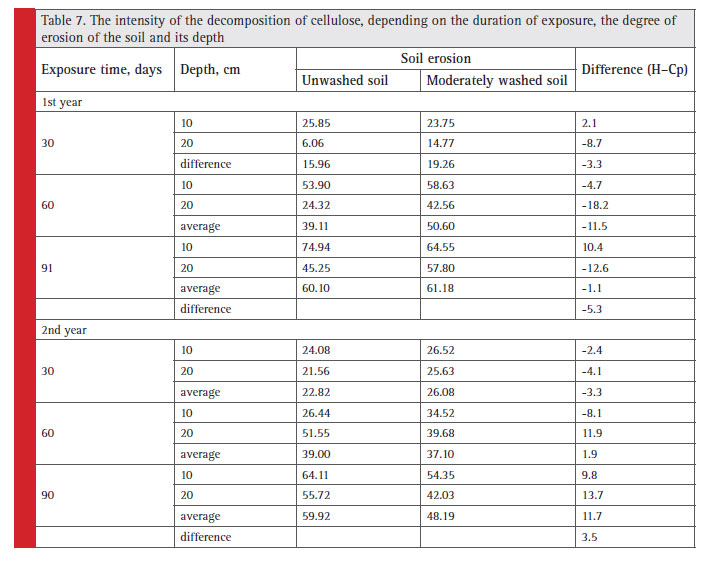 |
Table 7: The intensity of the decomposition of cellulose, depending on the duration of exposure, the degree of erosion of the soil and its depth |
The results of two-year experiment on cellulose (Table 7) decomposition showed that in the fi rst year the rate of cellulose decomposition was more often higher in the ploughing horizon of the moderately washed soil than in the unwashed one. Therefore, over 3 months of decomposition this difference reached 5%, although the total amount of cellulose destruction became almost the same in the ploughing horizons in both soils: 60–61%. However, decomposition that is more active occurred at a depth of 20 cm in moderately washed soil rather than at a depth of 10 cm, and in the unwashed soil — vice versa. During the second year of the experiment, we could observe similar situation as in the previous year only at the beginning of the decomposition process, then the decomposition rate was higher in unwashed soil, which ultimately resulted in higher transformation rate. In addition, after 3 months the degree of organic matter decomposition in the 0–20 cm layer of moderately washed soil was inferior to that of unwashed one by 11%.
For two years of experiments, during the biotest exposure periods the soil moisture reserve differences were greater between non-eroded and moderately eroded soils in the second year rather than in the fi rst one: 28 and 11% respectively. This refl ected in the differences in cellulose
decomposition rate (Table 7).
Comparison of data on rate of decomposition in the fallow land (Table 5) and in winter wheat sowing (Table 7) showed that over 60 days the biological activity of the unwashed soil (0–20 cm) was higher by 13% in the fallow land than in the agrocenosis. These features are confi rmed
by earlier studies as well. The rate of OM destruction is lower in winter wheat due to the less favorable moisture conditions for development and functioning of cellulosedestroying microorganisms (Val’kov and Kargaltsev, 1982).
Earlier (Mirchink, 1988) it was suggested that one can judge about “the total intensity of microbiological processes in general” by the degree of biotests decay. Indeed, our results have shown that in agricultural landscapes the degree of cellulose microorganism activity depends on differences in hydrothermal conditions as well as the entire set of soil fertility resources.
Conclusion
Distribution of soil fertility by vertical profi le and based on erosion catena is genetically related, since long-term erosion action involves lower soil layers into the ploughing horizon. However, anthropogenic pedogenesis changes the living conditions of microorganisms in the processed horizon. It turned out that the size and variation of soil moisture reserve in individual layers of non-eroded and moderately eroded soil on erosion catena were the most important parameters for microbiological activity among the hydrothermal parameters. Soil conditions, which are essential for the vital
activities of cellulose-decomposing microorganisms, are largely similar to the conditions that ensure crop formation. The study results have shown that microbiological soil activity, which refl ects the intensity of organic matter transformation process and the degree of possible materialization of available nutrients in the crop can act as a sensitive indicator of hydrothermal conditions and effective fertility of individual soil layers both in vertical
measurement and by topographic gradient.
We associate prospects for further studies with an increase in the number of soil objects on the erosion catena (from weakly to highly eroded) and evaluation of the transformation of microbiological activity due to differences in the hydrothermal regime, which are caused by the exposure of the slopes, especially for the northern and southern slopes.
Acknowledgements
The work was done in the framework of the implementation of the base part of the state assignment of the Ministry of Education and Science of the Russian Federation for the Belgorod State National Research University on 2017–2019 years (Project No. 5.4711.2017/6.7).
References
Deák, B., Valkó, O., Alexander, C., Mücke, W., Kania, A., Tamás, J. and Heilmeier H. (2014). Fine-scale vertical position as an indicator of vegetation in alkali grasslands – case study based on remotely sensed data. Flora, 209: 693–697. https://doi. org/10.1016/j.fl ora.2014.09.005
Gabbasova, I.M., Suleimanov, R.R., Komissarov, M.A., Garipov, T.T., Sidorova, L.V., Khaziev, F.K., Khabirov, I.K., Fruehauf, M. and Liebelt P. (2016). Temporal changes of eroded soils depending on their agricultural use in the southern Cis–Ural region. Eurasian Soil Sci., 49(10): 1204–1210. https://doi.org/10.1134/ S1064229316100070
Haddad, S.A., Lemanowicz, J. and Abd El-Azeim, M.M. (2018). Cellulose decomposition in clay and sandy soils contaminated with heavy metals. Int. J Env Sci and Technology: 1–16. https://doi.org/10.1007/s13762-018-1918-1
Il’ina, L.V. (1980). Differentiation of the arable layer of gray forest soil and the ways of its regulation. Proceedings of the Gorky Agricultural Institute, 142: 16–23. (in Russian) Ivanov, I.V. and Lisetskii, F.N. (1995). Manycentury periodicity of solar-activity and soil formation. Biofi zika, 40(4): 905–910.
Kashirskaya, N.N., Khomutova, T.E., Kuznetsova, T.V., Shishlina, N.I. and Borisov, A.V. (2018). Dynamics of Chemical and Microbiological Soil Properties in the Desert-Steppe Zone of the Southeast Russian Plain during the Second Part of the Holocene (4000 BC-XIII century AC). Arid Ecosystems, 8(1): 38–46. https://doi.org/10.1134/S2079096118010055
Kozlov, A.V., Kulikova, A.H. and Uromova, I.P. (2017). Biological activity of the cespitose-podsolic soil and productivity agrophytocenosis in dependence from use of high-siliceous breeds as soil conditioners. NAUCH VED BELGORO EN, 39(11): 155–166.
Lisetskii, F.N. (1988). Profi le distribution of fertility in soils of the Ukrainian steppe and changes in it as a result of erosion processes. Pochvovedenie, 4: 68–76.
Lisetskii, F.N. (1992). Periodization of antropogenically determined evolution of steppe ecosystems. SOV J ECOL, 23(5): 281–287.
Lisetskii, F.N. (2008). Agrogenic transformation of soils in the dry steppe zone under the impact of antique and recent land management practices. Eurasian Soil Sci., 41(8): 805–817.
Lisetskii, F.N., Goleusov, P.V., Moysiyenko, I.I. and Sudnik- Wojcikowska, B. (2014 a). Microzonal distribution of soils and plants along the catenas of mound structures. CONTEMP PROBL ECOL, 7(3): 282–293. https://doi.org/10.1134/ s1995425514030111
Lisetskii, F.N., Pavlyuk, Ya.V., Kirilenko, Zh.A. and Pichura, V.I. (2014 b). Basin organization of nature management for solving hydroecological problems. RUSS METEOROL HYDRO, 39(8): 550–557. https://doi.org/10.3103/s106837391408007x
Lisetskii, F.N., Zemlyakova, A.V. and Kirichenko, A.D. (2018). Variability of microbiota under diverse conditions of soil moistening.
BIOL BULL, 45(4): 337–344. https://doi.org/10.1134/ S106235901804009X
Mirchink, T.G. (1988). Soil microbiology. Moscow: MGU. 220 p. (in Russian)
Polyanskaya, L.M., Prikhod’ko, V.E., Lomakin, D.G. and Chernov, I.Yu. (2016). The number and biomass of microorganisms in ancient buried and recent chernozems under different land uses. Eurasian Soil Sci., 49(10): 1122–113. https://doi.org/10.1134/S1064229316100100
Prikhod’ko, V.E., Cheverdin, Yu.I. and Titova, T.V. (2013). Changes in the organic matter forms in chernozems of the Kamennaya Steppe under Different Land Uses, Locations,and Hydromorphism Degrees. Eurasian Soil Sci., 46(12): 1166– 1176. https://doi.org/10.1134/S1064229313120065
Prudnikova, E.Y. and Savin, I.Y. (2015). Satellite assessment of dehumifi cation of arable soils in Saratov region. Eurasian Soil Sci., 48(5): 533–539. https://doi.org/10.1134/ S1064229315050075
Shtompel, Y.A., Lisetskii, F.N., Sukhanovskii, Y.P. and Srtelnikova, A.V. (1998). Soil loss tolerance of brown forest soils of northwestern Caucasus under intensive agriculture. Eurasian Soil Sci., 31(2): 185–190.
Tikhomirova, L.D. 1970. A method for determining effective soil fertility. Patent number: 338196. Patent Assignee: Experimental Design Bureau of the Siberian Scientifi c Research Institute of Agriculture. Inventor: Tikhomirova, L.D. http://www. fi ndpatent.ru/patent/33/338196.html
Ulrich, A., Klimke, G. and Wirth, S. (2008). Diversity and activity of cellulose-decomposing bacteria, isolated from a sandy and a loamy soil after long-term manure application. Microbial Ecology, 55(3): 512–522. https://doi.org/10.1007/s00248-007-9296-0
Upton, R.N., Bach, E.M. and Hofmockel, K.S. (2018). Belowground response of prairie restoration and resiliency to drought. Agriculture Ecosystems & Environment, 266: 122–132. https://doi.org/10.1016/j.agee.2018.07.021
Val’kov, V.F. and Kargaltsev, V.I. (1982). Biological activity of typical chernozem and its change under the infl uence of fertilizers in crop rotation. Agrochemistry, 4: 64–69. (in Russian)
Volungevicius, J., Amaleviciute, K., Feiziene, D., Feiza, V., Slepetiene, A., Liaudanskiené, I., Versuliene, A. and Vaisvalavicius, R. (2018). The effects of agrogenic transformation on soil profi le morphology, organic carbon and physico-chemical properties in Retisols of Western Lithuania. ARCH AGRON SOIL SCI, 64(13): 1910–1923. https://doi.org/10.1080/036503 40.2018.1467006.
Webb, N.P., McGowan, H.A., Phinn, S.R. and McTainsh G.H. (2006). AUSLEM (Australian Land Erodibility Model): a tool for identifying wind erosion hazard in Australia. Geomorphology, 78(3-4): 179–200. https://doi.org/10.1016/j.geomorph.2006. 01.012
Widhiya, E.W. and Suharjono Ardiyati, T. (2016). Potency of bacterial consortium from apple crops as production of indole acetic acid (IAA). Int. J. ChemTech Res., 9(6): 718–724.
Yemtsev, V.T. and Mishustin, E.N. (2005). Microbiology. 5th ed. Moscow: Drofa. 445 p. (in Russian) Zakharov, I.S. (1978). Formation of humus substances by cellulose- destroying microorganisms. Chisinau: Shtiintsa. 115 p. (in Russian)
Zavarzin, G.A. (2014). Lectures on naturalists’ microbiology. Moscow: Nauka. 348 p. (in Russian).
Zelenskaya, E., Pichura, V. and Domaratsky, Y. (2018). Priorities of agroecological monitoring of the composition of soil trace elements taking into account the peculiarities of its formation over time. J ENG APPL SCI, 13(14): 5807–5813. https://doi.org/10.3923/jeasci.2018.5807.5813.