Department of Botany, M.M.V., Banaras Hindu University, Varanasi (Uttar Pradesh), India
Corresponding author email: nuttyshilpam17@gmail.com
Article Publishing History
Received: 08/12/2021
Accepted After Revision: 29/03/2021
Andrographis paniculata (AP) is an annual herbaceous plant commonly known as Kalmegh, belonging to Acanthaceae family. It has enormous use in research in form of herbal preparations and products and hence its crude extract can be studied further. In the present study, phytochemical screening, antioxidant activity, polyphenolic activity and reducing power of Andrographis paniculata plant prepared in different solvents (methanolic, ethanolic and double distilled water) was assessed by different protocols.2,2-Diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity, Hydrogen peroxide (H2O2) radical scavenging activity, Polyphenolic contents and Reducing activity of the plant was evaluated by modified method. Phytochemical screening of plant showed the presence of carbohydrate, cardiac glycosides, amino acids, flavonoids, alkaloids, phenols, saponins, steroids and tannins. In DPPH free radical and H2O2 radical scavenging activity, methanolic extract of plant were most potent in activity with 50% inhibition at 333.34 µg/ml. and 398.12 µg/ml concentration respectively. Total phenolic (309 ± 0.81 mg/g of gallic acid equivalent) and flavonoid content (82.125 ± 0.85 mg/g of rutin equivalent) were maximum in the methanolic extract of plant. High reducing capacity of plant was observed in case of methanolic extract. A significant positive correlation was found between antioxidant activity and polyphenolic content (total phenols and total flavonoids). Moreover, a significant correlation was found between antioxidant activities and reducing potential of plant extract, depicting that reducers are important contributors to antioxidant. The study shows whole plant extract of A. paniculata as an important natural source of antioxidants and phytochemicals. Through this study we could able to determine the results that can act as a milestone supporting future studies in a progressive manner.
Andrographis paniculata, Whole plant extract, Antioxidant activity, Polyphenolic content, Reducing power.
Sinha S, Raghuwanshi R. Evaluation of Phytochemical, Antioxidant and Reducing Activity in Whole Plant Extract of Andrographis paniculata. Biosc.Biotech.Res.Comm. 2021;14(1).
Sinha S, Raghuwanshi R. Evaluation of Phytochemical, Antioxidant and Reducing Activity in Whole Plant Extract of Andrographis paniculata. Biosc.Biotech.Res.Comm. 2021;14(1). Available from: <a href=”https://bit.ly/3tm5mZv”>https://bit.ly/3tm5mZv</a>
Copyright © Sinha and Raghuwanshi This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Medicinal plant is the future of phytomedicines (plant-derived drugs) and serves as a rich source of food, fibres, and drugs. They have been used in folk medicine since ancient times for the prevention and treatment of the numerous diseases as they express a vast array of biological activities. Presently, research is focusing attention on medicinal plants as it is considered as the most sustainable alternative source of antioxidants to supplement the endogenous oxidative stress defense system in humans. Antioxidants obtained from the plants either in the form of crude extracts or their derived products is very effective to inhibit the destructive processes caused by oxidative stress (Zengin et al., 2011; Rahman et al. 2012).Oxidative stress generates free radicals in form of reactive oxygen species (ROS) in the human body through aerobic respiration, ionizing radiation and pollution may increase the risk of chronic and degenerative diseases such as cancer, cardiovascular diseases, ageing and atherosclerosis. The human body generates antioxidant enzymes to neutralize free radicals, a diet rich in edible antioxidants is recommended to assist the human body to protect itself from food borne free radicals (Rimbach et al., 2005; Valko et al., 2007; Taïlé et al., 2020).
Phytochemicals have shown to possess antioxidant properties capable of scavenging free radicals, preventing cellular damages and related diseases via several mechanisms. Hydrogen peroxide (H2O2), superoxide ion (O2−) and hydroxide radical (OH−) are considered as most common ROS. Antioxidants are the molecules which stabilize or deactivate free radicals, before they hit targets in living human cells (Nunes et al., 2012). Plants contain a wide variety of free radical scavenging molecules, such as anthocyanins, carotenoids, flavonoids, glutathione, vitamins, and endogenous metabolites (Zheng and Wang, 2001). The concentration of the phenolic compounds like phenolic acids, flavonoids, anthocyanins, and tannins etc. may be related to the antioxidant activity of medicinal plants (Djeridane et al., 2006). Natural antioxidants have gained interest in pharmaceutical research as an alternative for substitution of synthetic substances showing antioxidant activity (Huang et al., 2005). It is mainly because natural antioxidants are cost effective, easily available, non-toxic, eco-friendly, and sometimes more efficient than synthetic ones. Continuous efforts are required to characterize plants phytochemicals for their antioxidant potentials and mode of action for various therapeutic uses against oxidative stress-related diseases.
Andrographis paniculata (Burm. f.) Wall. ex Nees of Acanthaceae family is commonly known as Kalmegh/King of Bitter. The plant is gregarious and grows abundantly in moist, shady waste area and dry forests. It is extensively cultivated in southern Asia, some parts of Europe and China. Traditionally it is used for treating common cold, bronchitis, diarrhoea, fever, hypertension, liver disease and sinusitis and snake bite (Gabrielian et al., 2002; Premchandran et al., 2011). Major constituents of A. paniculata are diterpenoids i.e., andrographolide,14-deoxy-11,12-didehydroandrographolide. 14-deoxyandrographolide, neoandrographolide, flavonoids and polyphenols reported to possess the most potent hypotensive and vasorelaxing effect (Pholphana et al., 2013). The plant has been reported to exhibit multifarious pharmacological and biological properties like antibacterial, anticancer, antidiabetic, antifungal, anti-inflammatory, anti-HIV and antihepatotoxic. The plant showed potential therapeutic action in curing liver disorders, common cough, and colds in humans (Nanduri et al., 2004; Akhtar et al., 2006; Geethangili et al., 2008; Mishra et al., 2009; Chandrasekaran et al., 2010; Nagalekshmi et al., 2011; Lim et al., 2012; Sule et al., 2012). The present study was therefore performed to study the antioxidant and polyphenols of whole plant extract of A. paniculata in three different solvents, which may prove to be beneficial against free radical generated disorders. Reducing potential of the plant was evaluated for the first time in methanolic, ethanolic and aqueous extracts derived from the plant.
MATERIAL AND METHODS
The chemicals used are, ascorbic acid, 2,2-Diphenyl,1-picryl hydrazyl (DPPH), gallic acid, rutin, trichloroacetic acid (TCA), potassium ferricyanide (K3Fe (CN)6), ferric chloride (FeCl3), Folin-Ciocalteu reagent, aluminium chloride (AlCl3), rutin, sodium potassium tartarate (Na-K tartarate), sodium carbonate (Na2CO3) was purchased from Hi-Media Ltd and solvent ethanol and methanol used were of analytical grade and purchased from Merck (Darmstadt, Germany).
For the flora collection and preparation of extracts, A. paniculata plant was collected from the campus of Banaras Hindu University, Varanasi. The plant was washed under running tap water to remove the soil and dust particles. The plant was authenticated at Botanical Survey of India (BSI), Allahabad. Collection number BHU-173 and voucher number-91924 was given by BSI to plant flora. Whole plant consisting of (root, stem, leaf, seed, flower) was shade dried for one week and kept in an oven at 40-45°C for 24 h, and then grinded in an electrical grinder to make coarse powder. Extraction was done from 20 g of plant powder in 200 ml of solvent by using a Soxhlet apparatus for 12 h. Methanol, ethanol and double distilled water were used as extraction solvents for extraction purpose. Extracts were then filtered and dried at 40°C in a rotary evaporator. Extracts were stored at 4°C till use. Percentage yield {PY, expressed in (w/w)} of crude plant extract was calculated by given.
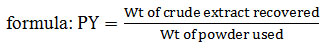
One gram of each extract was dissolved in 10 ml of respective extraction solvents to obtain a stock solution of concentration 100 mg/ml. Test plant samples were diluted in various concentrations according to the experiments.For the phytochemical screening, testing of the plant for various solvent extract was carried using a standard protocol (Harborne, 1973; Sofowora, 1993).
For the antioxidant assay through DPPH, the free radical scavenging activity of the extracts, based on the scavenging activity of the stable DPPH free radical, was determined by the method given by McCune and Johns, (2008) with some modifications. One ml sample of various concentrations (100-600 µg/ml) of plant extract (PE) was added to 3 ml methanolic solution of DPPH (0.004%) and shaken vigorously. The mixtures were incubated in the dark for 15 min at room temperature. Ascorbic acid was used as standard and methanol served as blank. The solution without sample was served as control. The absorbance of the samples was recorded at 517 nm by using a spectrophotometer (UV1, Thermo Scientific, US). The experiment was expressed as the percent inhibition of free radicals by the sample and was
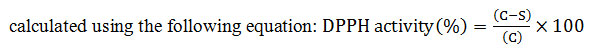
(C =Absorbance of control, S = Absorbance of sample)
For the hydrogen peroxide (H2O2) scavenging assay, the radical scavenging activity of methanolic, ethanolic and aqueous extracts of the plants to scavenge hydrogen peroxide (H2O2) was evaluated by the method of Ruch et al., (1989) with slight modifications. One ml sample of various concentrations (100-600 µg/ml) of plant extract (PE) were added to 2 ml of H2O2 (40 mM) prepared in (50 mM, pH-7.4) phosphate buffer. The test samples were incubated for 10 min at room temperature. The absorbance was measured at 230 nm (Thermo Scientific UV 1). Phosphate buffer without H2O2 was used for blank and hydrogen peroxide solution without extract served as control. Ascorbic acid was used as a standard.Hydrogen peroxide scavenging activity was calculated by following
![]()
Where, C = absorbance of control, T= absorbance of test sample
For the estimation of total phenolic content, the total phenolic content (TPC) was measured by Folin-Ciocalteu assay (McDonald et al., 2001). In brief, 0.5 ml Folin reagent (1:10 diluted with DDW) was added to 0.5 ml (200 µg ml-1) PE and finally 4 ml (1M) aqueous sodium carbonate (Na2CO3) was added to this reaction mixture and incubated for 15 min at room temperature. Absorbance was recorded at 650 nm. Gallic acid was prepared in methanol and DDW (1:1) and used as standard. Total phenolic content was expressed in terms of gallic acid equivalent (GAE, mg/g of dry mass), which is a common reference compound (McDonald et al., 2001). The total flavonoid content (TFC) was determined using the method of aluminium chloride (AlCl3). The plant extract (1 ml, different concentration) prepared with different solvent (methanol, ethanol and water) was taken in which 100 μl AlCl3 (10% w/v), 100 μl Na-K tartrate and 2.8 ml distilled water were added and kept for 30 min. Finally, the reaction mixture was diluted to 10 ml with double distilled water and the absorbance was measured at 415 nm. The results were expressed as mg rutin (RE)/g plant material (Chang et al., 2002).
For the estimation of reducing power capacity (RPC) of methanolic, ethanolic and aqueous plant extract was estimated by the method of Athukorala et al., (2006) with some modifications. In brief, 1ml of PE (50-300 µg/ml) prepared in different solvents were mixed with 2.5 ml of phosphate buffer solution (PBS, 0.2 M, pH- 6.6) and 2.5 ml potassium ferricyanide (30mM). The above reaction mixture was incubated at 50°C for 20 min. After that, 2.5 ml trichloro acetic acid (TCA, 0.6M) was added to the mixture to stop the reaction and centrifuged at 3000 rpm for 10 min. Then, 2.5 ml of supernatant was taken out and mixed with 2.5 ml double distilled water and 0.5 ml ferric chloride (FeCl3) solution. Absorbance was recorded at 700 nm. Ascorbic acid was used as standard. For the statistical analysis, all the above experiments were performed in quadriplate (n=4) and repeated thrice (x=3). Data were analyzed as mean ± SE by applying one way analysis of variance (ANOVA). Tukey’s multiple range tests were used for separation of means when ANOVA was significant (p< 0.001) (SPSS 16.0; Chicago, IL, USA). IC50 was calculated through linear regression analysis. The graphs were drawn in sigma plot 11.0.
RESULTS AND DISCUSSION
Percentage yield and phytochemical screening in different solvents: Percentage yield of A. paniculata extract was found maximum (22%) in aqueous followed by methanol (18.4%) and ethanol (17.6%) was obtained. The percentage yield of extract differed in various extraction solvents and this may be due to various degrees of solubility of plant materials depending on polarity of solvents. A similar trend was seen in leaves extract of A. paniculata (Banji et al., 2018). Our results highlight that methanolic and ethanolic extracts whole plant were enriched in phytochemicals like alkaloids, amino acids, carbohydrate, flavonoids, phenols, saponins, steroids and tannins while aqueous extract shows presence of alkaloids and amino acids only (Table 1). It may be due to poor solubility of these phytochemicals in the aqueous extract.
Table 1. Screening of plant in different solvents
| Phytochemicals | Test performed | Methanolic extract | Ethanolic extract | Aqueous extract |
| Carbohydrate | Fehling test | + | + | – |
| Phenols | Ferric chloride test | + | + | – |
| Flavonoids | Ammonia test | + | + | – |
| Alkaloids | Wagner’s test | + | + | + |
| Steroids | Salkowski test | + | + | – |
| Tannins | Lead acetate test | + | + | – |
| Saponins | Frothing test | + | + | – |
| Glycosides | Nitroprusside test | + | – | – |
| Amino acids | Ninhydrin test | + | + | + |
Note: + = Presence; – = Absence of phytochemicals
Antioxidant activity of plant extract by through DPPH assay:In the present study, the free radical scavenging ability of the crude methanolic, ethanolic and water extracts were determined through the degree of discoloration of the methanol solution of DPPH (Table 2). In A. paniculata, methanolic extract showed higher scavenging activity (IC50 = 398.31 µg/ml) than ethanolic (IC50 = 404 µg/ml) and aqueous extracts (IC50 = 483.29 µg/ml). The present study reveals that the best antioxidant activity in terms of DPPH scavenging strength was displayed by methanol extract followed by ethanol and aqueous extract. The higher antioxidative capacity of methanolic extract followed by ethanolic extract may be explained via the higher content of biologically active substances, such as e.g., polyphenol (Zwolan et al., 2020).
The antioxidant activity of the extract is first estimated based on their capacity to trap free radical DPPH. In the presence of an active free radical scavenger, the absorption vanishes and the resulting discoloration from deep violet to light yellow. The solution fades colour with increase in concentration of antioxidant as electrons are taken up by DPPH radical from the antioxidant of the extract (Calliste et al., 2001). Ascorbic acid was used as a standard antioxidant as used as a standard to determine the IC50 value of the extract in other plants (Sreekala et al., 2013). Ethanolic extract was characterized by higher free radical antioxidant activity than water extract in Argyreia pierreana, Matelea denticulata and Nigella sativa (Gudise et al., 2019; Zwolan et al., 2020).
Table 2. Antioxidant activity of A. paniculata by DPPH free radical scavenging method in different solvents
| Concentration
(µg/ml) |
Percentage inhibition (Mean±SE) | |||
| Methanolic | Ethanolic | Aqueous | Ascorbic Acid | |
| 100 | 23.89±0.68f | 25.77±0.60f | 17.07±0.34f | 25.12±0.29f |
| 200 | 30.52±0.63e | 35.01±0.23e | 24.71±1.9e | 39.34±0.20e |
| 300 | 43.09±0.68d | 42.88±0.18d | 34.10±0.59d | 56.25±0.22d |
| 400 | 49.92±0.82c | 51.58±0.53c | 43.69±0.72c | 65.15±0.14c |
| 500 | 59.11±1.04b | 58.84±0.12b | 51.94±0.51b | 86.47±0.38b |
| 600 | 63.81± 0.49a | 63.65±0.19a | 60.12±0.80a | 95.22±0.32a |
| IC50 | 398.31 | 404.00 | 483.29 | 271.47 |
Data represented as mean ±SE (n=4). One way ANOVA followed by Tukey’s test. All data are significant at p<0.001; a, b, c, d, e, f = different letter shows significant difference between means.
Antioxidant activity by Hydrogen peroxide (H2O2) scavenging assay: Hydrogen peroxide (H2O2) scavenging activity of A. paniculata plant was observed higher in methanolic (IC50= 377.074 µg/ml) followed by ethanolic (IC50= 379.06 µg/ml) extract and aqueous extract (IC50= 467.65 µg/ml) (Table-3). H2O2 scavenging activity relies upon the phenolic content of the plant extract by donating electrons to H2O2, thereby neutralizing it into water. The study suggests that aqueous extract will be required in relatively high concentration to show its effectiveness. The ethanolic extract of the Aesculus hippocastanum was capable of scavenging H2O2 in a dose dependent manner (Geetha et al., 2013). H2O2 radical scavenging activity was also reported from different extracts of E. prostrata (Sinha and Raghuwanshi, 2016a).
Table 3. Antioxidant activity of A. paniculata by H2O2 radical scavenging in different solvents
| Concentration
(µg/ml) |
Percentage inhibition (mean±SE) | |||
| Methanolic | Ethanolic | Aqueous | Ascorbic Acid | |
| 100 | 21.07±0.79f | 27.66±0.50f | 20.57±0.70f | 25.86±0.38f |
| 200 | 31.68±0.63e | 35.63±0.62e | 27.88±0.62e | 36.80±0.30e |
| 300 | 43.17±0.56d | 43.68±0.60d | 36.00±0.34d | 48.33±0.31d |
| 400 | 51.81±0.83c | 51.80±0.38c | 44.62±0.49c | 57.07±0.29c |
| 500 | 56.69±0.50b | 55.67±1.98b | 54.75±0.85b | 61.87±0.39b |
| 600 | 62.87±0.78a | 63.01±0.49a | 59.62±0.87a | 74.16±0.38a |
| IC50 | 377.07 | 379.06 | 467.65 | 342.56 |
Data represented as mean ±SE (n=4). One way ANOVA followed by Tukey’s test. All data is significant at p <0.001. a, b, c, d, e, f = different letters show significant difference between means.
Total phenolic and flavonoid content: Total phenolic content was reported as mg/g of GAE in reference to standard curve (y=0.001x+0.05, R2=0.997). In A. paniculata plant, maximum TPC (309.00±0.816 mg/g) was found in methanolic extract followed by ethanolic (290.5±1.29 mg/g) and aqueous extracts (189.25±0.957 mg/g) respectively. Total flavonoid content was calculated by standard curve (y=0.0008x+0.198, R2=0.994) and reported as mg/g of RE. A. paniculata plant showed maximum TFC (82.125±0.853 mg/g) in methanolic extract followed by ethanolic (61.375±1.10 mg/g) and aqueous extracts (37.80±0.731 mg/g) (Table 4). Methanol extract of A. paniculata shows important antioxidant activity because it contains phenols and flavonoids (Kurzawa et al., 2014). Similar, higher phenolic content in organic solvent has also been reported (Zaman et al., 2011). Presence of active metabolites like phenol and flavonoid contents in plant extract depend on solvent used (Sulaiman et al., 2011; Kurzawa et al., 2014).
Phenolic compounds present in plant contain an aromatic ring bearing one or more hydroxyl groups. Flavonoids are the largest group of naturally occurring phenolic compounds, which occurs in different plant parts in form as aglycone and glycosides. It has two benzene rings separated by a propane unit. Their ideal structural chemistry nature helps them to scavenge injurious free radicals such as super oxide and hydroxyl radicals (Younes and Siegers, (1981). Therefore, acting as antioxidants for their scavenging activity (Das and Pereira, 1990) or chelating process, inhibition of hydrolytic and oxidative enzymes and anti-inflammatory actions and giving protection against cardiovascular disease, certain forms of cancer and age-related degeneration of cell components. Flavonoids might show higher antioxidant activity in organic solvent due to structure and substitution pattern of hydroxyl group (Clavin et al., 2007; Kurzawa et al., 2014).
Table 4: Total phenolic and flavonoid content of A. paniculata in different solvents
| Total polyphenolic content (A. paniculata) | ||
| Plant extract TPC (mg/g GAE) TFC (mg/g RE) | ||
| Methanol | 309 ± 0.816a | 82.125±0.853a |
| Ethanol | 290.5±1.290b | 61.375±1.108b |
| Aqueous | 189.25±0.957c | 37.805±0.731c |
Data represented as Mean ±SE (n=4); One way ANOVA followed by Tukey’s test. All data is significant at p <0.001; a, b, c letters show significant difference between means.
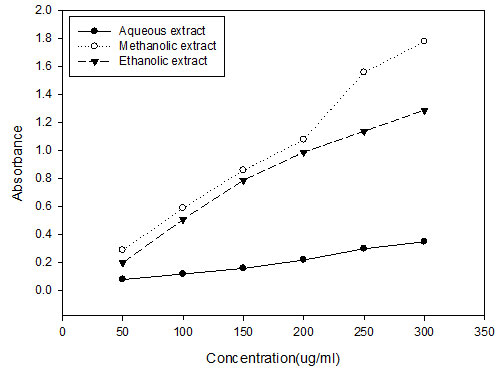
Reducing Potential: The reducing power of the extracts (methanolic, ethanolic and aqueous) of A. paniculata (Fig. 1) plant increased in a concentration dependent manner from lower to higher concentrations. Similar results reported by in which the reducing power of Ziziphus mauritiana extract increased with the increase of their concentrations. Maximum reducing power was observed in the methanolic extract the plant. In reducing potential assay, after the addition of the extract, the yellow colour of the test solution changes from yellowish green to blue. The colour change of sample solution indicates the reducing power of extract of plants. High absorbance shows high reduction potential of the plant. These reducers show their antioxidant action by breaking the free radical chain by donating a hydrogen atom (Gordon, 1990). Thus, it is concluded that both polyphenolic compounds and reducers present in the extracts are major determinants of antioxidant capacity of extracts (Abdallah et al., 2016).
Figure 1 : Reducing potential of A. paniculata plant extracts in different solvents
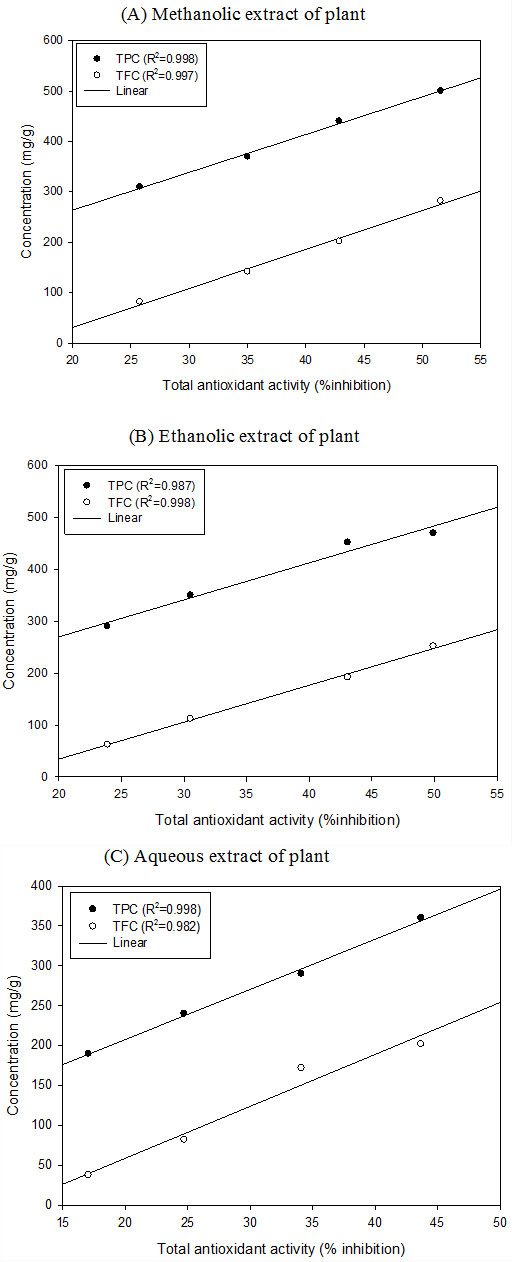
Correlation between antioxidant activity and reducing potential of A. paniculata plant: Correlation between total antioxidant activity and reducing power was obtained through linear regression analysis. A significant correlation was found between total antioxidant activities and reducing potential in A. paniculata extract (Fig. 2). In, A. paniculata, correlation coefficient (R2) between antioxidant activity and reduction potential was (R2 =0.989) for methanolic, (R2 =0.992) for ethanolic and (R2 =0.992) for aqueous extract. In our result, there is significant positive correlation between antioxidant activity and reducing power of the plant. Koleva et al., (2002) also reported positive correlation between antioxidant activity and reducing potential. Similar studies are seen in E. prostrata and Ocimum americanum (Sinha and Raghuwanshi, 2016a; Jaiswal et al., 2019).
Figure 2: Correlation between antioxidant and reducing potential in (A) Methanolic, (B) Ethanolic and (C) Aqueous extract of A. paniculata plants
Correlation between antioxidant activity and polyphenolic compounds: A positive, significant, and linear correlation was found between total antioxidant activity and polyphenolic contents (TPC & TFC) of various extracts. Correlation coefficient (𝑅2) values of different extracts showed a very close correlation between antioxidant activities and polyphenolic contents (TPC and TFC content). Positive and linear correlation (R2, ranges from 0.982-0.998) was found in A. paniculata in the present experiment (Fig. 3). In the present work, we found a strong correlation between antioxidant activity and total phenolic contents (TPC & TFC). High correlation coefficient (R2≥0.946) values showed close correlation between them. Correlation coefficient (R2) between antioxidant activity and polyphenolic contents (TPC & TFC) of aqueous and methanolic extracts of Chinese medicinal plant and Jordanian plant species are well reported (Cai et al., 2004; Tawaha et al., 2007; Akilandeswari et al., 2020). Phenolic compounds play an important role as antioxidants and a good correlation exists between the concentration of plant phenolics and the total antioxidant capacity (Sinha and Raghuwanshi, 2016a).
The phytochemicals present in the plant and food products are generally nontoxic and contain many medicinal properties. Generally, antioxidants and polyphenolic compounds are mutually related with each other for their activities. A. paniculata is a good source of phytochemicals like phenolics, flavonoids, antioxidants, alkaloids, and tannins etc. These phytochemicals play an important role in promoting pharmaceutical drug preparation and are used for curing various health ailments (Usman and Osuji, 2007; Akilandeswari et al., 2020).
Figure 3: Correlation between antioxidant activity and polyphenols (TPC and TFC) (A) Methanolic (B) Ethanolic (C) Aqueous extract of A. paniculata
CONCLUSION
Our study reports that the whole plant extract of A. paniculata plant is a rich source of natural antioxidants. The antioxidant property, reducing potential and polyphenolic components like total phenols and flavonoids varied significantly in the different extraction solvent. The organic solvent i.e., methanol and ethanol gave better results than aqueous one. This revealed that the whole plant extract contains rich number of antioxidants i.e., phenolic, and flavonoid contents with good free radical scavenging activity. Thus, bioactive compounds present in the extract of this plant may develop into antioxidant agents in the form of plant-based drugs that may have applications in human health in form of food additive or nutraceutical and biopharmaceutical industries.
ACKNOWLEDGEMENTS
The authors would like to thank the University Grants Commission for research support
Conflict of Interests: The authors declare that they have no competing interests.
REFERENCES
Abdallah, EM., Elsharkawy, ER. and Ed-dra, A. (2016) Biological activities of methanolic leaf extract of Ziziphus mauritiana. Bioscience Biotechnology Research Communications, 9(4), pp.605-614.
Akhtar, MT., Bin Mohd Sarib, MS., Ismail, IS., Abas, F., Ismail, A., Lajis, NH. and Shaari, K. (2016) Anti-Diabetic Activity and Metabolic Changes Induced by Andrographis paniculata Plant Extract in Obese Diabetic Rats. Molecules, 21(8), 1026.
Akilandeswari G, Bupesh G, Vijaya Anand A, Saradhadevi K M, Mayur Mausoom Phukan and Meenakumari K. (2020). Invitro Efficacy of antioxidant activity in ethanolic and aqueous leaf extracts of Andrographis paniculata Nees and Rhinacanthus nasutus Kurz. International Journal of Research in Pharmaceutical Sciences, 11(4), pp.6301-6306.
Athukorala, Y., Jeon, Y. and Kim, K. (2006) Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga, Ecklonia cava. Food and Chemical Toxicology, 44(7), pp.1065-74.
Banji, A., Goodluck, B., Oluchi, O. and Stephen, F. (2018) Antimicrobial and Antioxidant Activities of Crude Methanol Extract and Fractions of Andrographis paniculata leaf (Family: Acanthaceae) (Burm. f.) Wall. Ex Nees. Jordan Journal of Biological Sciences, 11(1), pp.23-30.
Cai, Y., Luo, Q., Sun, M. and Corke, H. (2004). Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sciences, 74(14), pp.2157-2184.
Calliste, CA., Trouillas, P., Allais, DP., Simon, A. and Duroux, JL. (2001) Free radical scavenging activities measured by electron spin resonance spectroscopy and b16 cell antiproliferative behaviors of seven plants, Journal of Agricultural and Food Chemistry, 49(7), pp.3321-3327.
Chandrasekaran, CV., Gupta, A., and Agarwal, A. (2010) Effect of an extract of Andrographis paniculata leaves on inflammatory and allergic mediators in vitro. Journal of Ethnopharmacology, 129(2), pp.203-207.
Chang, C., Yang M, Wen H and Chern J. (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food and Drug Analysis, 10(3), pp.178-82.
Clavin, M., Gorzalczany, S., Macho, A., Munoz, E., Ferraro, G., Acevedo, C. and Martino, V. (2007) Anti-inflammatory activity of flavonoids from Eupatorium arnottianum. Ethnopharmacological communication, 112, pp.585–589.
Das, NP. and Pereira, TA. (1990) Effects of flavonoids on thermal autoxidation of palm oil: Structure-activity relationships. Journal of the American Oil Chemists’ Society, 67(4), pp.255-258.
Djeridane, A., Yousfi, M., Nadjemi, B., Boutassouna, D., Stocker, P. and Vidal, N. (2006) Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chemistry, 97(4), pp.654-660.
Engwa, G.A. (2018) Free radicals and the role of plant phytochemicals as antioxidants against oxidative stress-related diseases. In: Phytochemicals-Source of Antioxidants and Role in Disease Prevention (Edited by) Toshiki Asao and Md Asaduzzaman, InTech: London UK
Gabrielian, ES., Shukarian, AK., Goukasova, GI., Chandanian, GL. and Panossian, AG. (2002) A double blind, placebo-controlled study of Andrographis paniculata fixed combination Kan Jang in the treatment of acute upper respiratory tract infections including sinusitis. Phytomedicine, 9(7), pp.589-597.
Geetha, RV., Roy, A. and Sitalakshmi, T. (2013) In Vitro Antioxidant and free Radical Scavenging activity of the Ethanolic extract of Aesculus hippocastanum. International Journal of Drug Development and Research, 5(3), pp.403-407.
Geethangili, M., Rao, YK., Fang, SH. and Tzeng, YM. (2008) Cytotoxic constituents from Andrographis paniculata induce cell cycle arrest in jurkat cells. Phytotherapy Research, 22(10), pp.1336-1341.
Gordon, MH. (1990). The mechanism of antioxidant action in vitro, In Food Antioxidants, BJF. Pp 1-18 Hudson, Ed., Elsevier Applied Science, London, UK,
Gudise, V., Chowdhury, B. and Manjappa, AS. (2019) In vitro free radical scavenging and antidiabetic activity of aqueous and ethanolic leaf extracts: a comparative evaluation of Argyreia pierreana and Matelea denticulata. Future Journal of Pharmaceutical Sciences, 5(13).
Harborne, JB. (1973) A guide to modern techniques of plant analysis; phytochemical methods. Pp 49-188. Chapman and Hall, Ltd. London.
Huang, D., Boxin, O. and Prior, RL. (2005) The chemistry behind antioxidant capacity assays. Journal of Agricultural and Food Chemistry, 53(6), pp.1841-1856.
Jaiswal, P., Yadav, A. and Kumari, N. (2019) Phytochemical and Antioxidant Activities of Leaf extracts of Ocimum americanum, International Journal of Pharmacy and Biological Sciences 9(2), pp.388-396.
Koleva, II., Van Breek, TA., Linssen, JPH., De Groot, A and Evstatieva, LN. (2002) Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochemical Analysis, 13(1), pp.8-17.
Kurzawa, M., Filipiak-Szok, A., Kłodzińska, E and Szłyk, E. (2015) Determination of phytochemicals, antioxidant activity and total phenolic content in Andrographis paniculata using chromatographic methods. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, 995-996, pp.101–106.
Lim, JC., Chan, TK., Ng, DS., Sagineedu, SR., Stanslas, J., and Wong, WS. (2012) Andrographolide and its analogues: versatile bioactive molecules for combating inflammation and cancer. Clinical and Experimental Pharmacology & Physiology, 39(3), pp. 300-310.
McCune, LM. and Johns T. (2002) Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the indigenous peoples of the North American boreal forest. Journal of Ethnopharmacology, 82(2-3), pp.197–205.
McDonald, S., Prenzler, PD., Autolovich, M. and Robards, K. (2001) Phenolic content and antioxidant activity of olive extracts. Food Chemistry, 73(1), pp.73-84.
Mishra, US., Mishra, A., Kumari, R., Murthy, PN., and Naik, BS. (2009) Antibacterial Activity of Ethanol Extract of Andrographis paniculata. Indian Journal of Pharmaceutical Sciences, 71(4), pp.436-438.
Nagalekshmi, R., Menon, A., Chandrasekharan, DK. and Nair, CK. (2011) Hepatoprotective activity of Andrographis paniculata and Swertia chirayita. Food and Chemical toxicology, 49(12), pp.3367-3373.
Nanduri, S., Nyavanandi, VK., Thunuguntla, SSR. (2004) Synthesis and structure-activity relationships of andrographolide analogues as novel cytotoxic agents. Bioorganic and Medicinal Chemistry Letters, 14(18), pp.4711–4717.
Nunes, PX., Silva, SF., Guedes, RJ. and Almeida, S. (2012) Biological oxidations and antioxidant activity of natural products, In: Phytochemicals as Nutraceuticals – Global Approaches to Their Role in Nutrition and Health. Pp 1-20 (Edited by) Rao V Tech publisher, Croatia.
Pholphana, N., Rangkadilok, N., Saehun, J., Ritruechai, S. and Satayavivad, J. (2013) Changes in the contents of four active diterpenoids at different growth stages in Andrographis paniculata (Burm.f.) Nees (Chuanxinlian). Chinese Medicine, 8(2), pp.1-12.
Premendran, SJ., Salwe, KJ., Pathak, S., Brahmane, R., and Manimekalai, K. (2011) Anti-cobra venom activity of plant Andrographis paniculata and its comparison with polyvalent anti-snake venom. Journal of Natural Science, Biology and Medicine, 2 (2), pp.198-204.
Rahman, T., Hosen, I., Islam, TM. and Shekhar, HU. (2012) Oxidative stress and human health. Advances in Bioscience and Biotechnology, 3(07), pp.997-1019.
Rimbach, G., Fuchs, J. and Packer, L. (2005) Application of nutrigenomics tools to analyze the role of oxidants and antioxidants in gene expression, In: Rimbach G, Fuchs J, Packer L (eds.) Nutrigenomics, Pp 1-12 Taylor and Francis Boca Raton Publishers, FL USA.
Roy, S., Rao, K., Bhuvaneswari, C., Giri, A. and Mangamoori, LN. (2010) Phytochemical analysis of Andrographis paniculata extract and its antimicrobial activity. World Journal of Microbiology and Biotechnology, 26(1), pp.85-91.
Ruch, RJ., Cheng, SJ. and Klaunig, JE. (1989) Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis, 10(6), pp.1003–8.
Sinha, S. and Raghuwanshi, R. (2016a) Phytochemical screening and antioxidant potential of Eclipta prostrata (L) L-a valuable herb. International Journal of Pharmacy and Pharmaceutical Sciences, 8(3), pp.255-260.
Sofowora, A. (1993) Medicinal plants and traditional medicine in Africa. Pp 289.Spectrum Books Ltd. (Pub.), Ibandan, Nigeria.
Sreekala Devi, R., Radhamany, PM. and Gayathri Devi, V. (2013) Investigation of the antioxidant principles from Psilanthus travancorensis (WT.& ARN.) Leroy – an unexplored taxon of rubiaceae. International Journal of Pharmacy and Pharmaceutical Sciences, 5(1), pp.13–17.
Sulaiman, SF., Sajak AAB., Supriatno, KLO. And Seow, EM. (2011) Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. Journal of Food Composition and Analysis. 24(4-5), pp.506-515.
Sule, A., Ahmed, QU., Latip, J., Samah, OA., Omar, MN., Umar, A. and Dogarai, BBS. (2012) Antifungal activity of Andrographis paniculata extracts and active principles against skin pathogenic fungal strains in vitro. Pharmaceutical Biology, 50(7) pp.850-856.
Taïlé, J., Arcambal, A., Clerc, P., Gauvin-Bialecki, A., Gonthier, MP. (2020) Medicinal Plant Polyphenols Attenuate Oxidative Stress and Improve Inflammatory and Vasoactive Markers in Cerebral Endothelial Cells during Hyperglycemic Condition, Antioxidants (Basel) pp. 9(7): 573.
Tawaha, K., Alali, FQ., Gharaibeh, M., Mohammad, M. and El-Elimat, T. (2007) Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chemistry, 104(4), pp.1372-1378.
Tiwari, P., Kumar, B., Kaur, M., Kaur, G. and Kaur, H. (2011) Phytochemical Screening and Extraction: A Review. Internationale Pharmaceutica Sciencia, 1(1), pp.98-106.
Usman, H. and Osuji, J.C. (2007). Phytochemical and in vitro antimicrobial assay of the leaf extract of Newbouldia leavis. African Journal of Traditional, Complementary and Alternative Medicines, 4(4), pp.476-480.
Valko, M., Leibfritz, D., Moncol, J., Cronin, MT., Mazur, M. and Telser, J. (2007) Free radicals and antioxidants in normal physiological functions and human disease. The International Journal of Biochemistry & Cell Biology, 39(10), pp.44-84.
Younes, M. and Siegers, CP. (1981) Inhibitory action of some flavonoids on enhanced spontaneous lipid peroxidation following glutathione depletion. Planta Medica, 43, pp.240-245.
Zaman, RU., Ghaffar, M., Fayyaz, T. and Mehdi, S. (2011) In vitro evaluation of total phenolics and antioxidant activities of Withania somnifera, Eclipta prostrata L., Gossypium herbasceum L. Journal of Applied Pharmaceutical Science, 1, pp.133-44.
Zengin, G., Cakmak, YS., Guler, GO. and Aktumsek, A. (2011) Antioxidant properties of methanolic extract and fatty acid composition of Centaurea urvillei DC. subsp. hayekiana Wagenitz. Records of Natural Products, 5(2), pp.123-132.
Zheng, W. and Wang, SY. (2001) Antioxidant activity and phenolic compound in selected herbs. Journal of Agricultural and Food Chemistry, 49(11), pp.5165-5170.
Zwolan, A., Pietrzak, D., Adamczak, L., Chmiel, M., Kalisz, S., Wirkowska-Wojdyła, M., Florowski, T. and Oszmiański, J. (2020) Effects of Nigella sativa L. seed extracts on lipid oxidation and color of chicken meatballs during refrigerated storage, LWT – Food Science and Technology, 130(109718).