1Centre for Biological Sciences, Department of Biochemistry, K.S. Rangasamy
College of Arts and Science, Tiruchengode, Tamil Nadu, India.
2Department of Pharmacology, Nandha College of Pharmacy, Erode, Tamil Nadu, India.
3Department of Biochemistry, PGP College of Arts and Science, Namakkal, Tamil Nadu, India.
Corresponding author email: sarabioc@gmail.com
Article Publishing History
Received: 25/10/2021
Accepted After Revision: 15/12/2021
In diabetes, the postprandial phase is characterized by a rapid and large increase in blood glucose levels, and the possibility that the postprandial “hyperglycemic spikes” may be relevant to the onset of cardiovascular complications has recently received much attention. Medicinal use of herbal medicine in the treatment and prevention of diseases including diabetes has a long history compared to conventional medicine. These plants have no side effects and many existing medicines are derived from the plants. Hence, the current investigation was planned to make a poly herbal drug (PHD) through Punica granatum (fruits), Illicium verum (flowers) and Nyctanthes arbor (leaves) and assess their antioxidant and antidiabetic activities in vitro and in HepG2 cell line. The respective plant methanolic extracts and PHD are exposed to phytochemical assessment and to discriminate the bioactive factors by Gas Chromatography–Mass Spectrometry.
We evaluated the antioxidant properties 2, 2-diphenyl-1-picrylhydrazyl scavenging, hydrogen peroxide scavenging, thiobarbituric acid reactive substances and total antioxidant activity of individual plant extracts and the PHD. At the same time, In vitro and cell culture approaches were used to assess the anti-diabetic activity. The PHD has a higher concentration of secondary metabolites than individual plant extracts, according to our findings. On the other hand, we also notice that PHD demonstrated higher antioxidant capability and considerable in vitro glucose-lowering effects along with noteworthy inhibition of ɑ-amylase, glucosidase, lipase, dipeptidyl peptidase-IV, collagenase and protein glycation in HepG2 cell line. In conclusion, this study clearly demonstrated the significant antioxidant and antidiabetic activities of the PHD. Hence, PHD may be used as a potential source in the management of diabetes, hyperglycemia and the related state of oxidative stress.
Antioxidants, Free Radicals, Medicinal Plants, Phytochemicals, Postprandial Diabetes
Chandrasekaran, Uddandrao V.V.S, Sethumathi P.P, Tamilmani P, Vadivukkarasi S, Saravanan G. Evaluation of Antioxidant and Antidiabetic Potential of Polyherbal Drug Made with Three Herbs: In Vitro and Cell Culture Model. Biosc.Biotech.Res.Comm. 2021;14(4).
Chandrasekaran, Uddandrao V. V. S, Sethumathi P. P, Tamilmani P, Vadivukkarasi S, Saravanan G. Evaluation of Antioxidant and Antidiabetic Potential of Polyherbal Drug Made with Three Herbs: In Vitro and Cell Culture Model. Biosc.Biotech.Res.Comm. 2021;14(4). Available from: <a href=”https://bit.ly/32aI3IX“>https://bit.ly/32aI3IX</a>
Copyright © Chandrasekaran et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Diabetes mellitus (DM) is a multifaceted metabolic disorder attributable to a complete deficiency of insulin or comparative be short of insulin as an outcome of defection in insulin production, action or a combination of both. The clinical feature of DM is glucose intolerance, which results in hyperglycemia. Type 1 DM (T1DM) and type 2 DM (T2DM) are the two main types of DM. T1DM, which is linked with full or near-total insulin insufficiency and autoimmune destruction of pancreatic β-cells, affects around 5-10% of people with DM. T2DM, on the other hand, has evolved into a virulent illness marked by β-cell dysfunction, various degrees of insulin resistance, and increased hepatic glucose production (American Diabetes Association 2015). Nearly 425 million persons have DM; by 2045, this number is expected to rise to 629 million. 79 percent of adults with DM live in low- and middle-income countries, 1 of every 2 (212 million) people with DM is undiagnosed, and 352 million people are at risk of developing T2DM (IDF 2018). In most developing countries, such as India, DM is widespread (Uddandrao et al. 2019; Cole and Florez 2020).
According to statistics, India has the world’s highest number of DM patients and has been termed the “diabetes capital of the world”. At the same time, the limitation of the accessible treatments and also the harmful side effects, the look for new medication continues to be directed against DM (Abate and Chandalia 2007; Parim et al. 2019). The researchers have an interest in looking out new medication to treat DM because of impending hypoglycemic activity and negligible side effects of healing plants (Kavishankar et al. 2011; Cole and Florez 2020).
One technique of a therapy to reduce hyperglycemia, the inhibition of carbohydrate hydrolyzing enzymes in the digestive organs, slows down and reduces the digestion and absorption of ingested carbohydrates, especially after a meal. As a result, these inhibitors may be able to minimize the postprandial rise in blood glucose levels (Narkhede, 2011; Gurung et al. 2020). Consequently, natural inhibitors derived from dietary plants have a reduced inhibitory impact on carbohydrate hydrolyzing enzyme activities and can be employed as a possible postprandial hyperglycemia treatment with little adverse effects (Uddandrao et al., 2020).
On the other hand, oxidative stress plays a crucial function in DM physiopathology; thus, the interest of using natural antioxidants as beneficial tools exists (Uddandrao et al. 2018). There is a rising interest in using therapeutic plants and their phytoconstituents as natural sources because of their well-known capability to scavenge free radicals. Plants are effective sources of natural antioxidant molecules with a variety of pharmacological activities and few or no side effects that protect human health from a variety of diseases (Omari et al. 2019). The idea of poly herbal drug (PHD) is well known in the therapeutic system. Plant formulations and concentrated concentrations of plants are employed as a contrast to individual ones in traditional Indian drug procedures. The Ayurvedic herbals are set up in a variety of measuring structures in India’s traditional helpful framework called Ayurveda, with the most of them including PHD (Parasuraman et al. 2014; Hill-Briggs et al. 2020).
Since PHD is a result of nature, they are modestly less expensive, environment-friendly, and promptly introduced than allopathic medications (Parasuraman et al. 2014; Hill-Briggs et al. 2020). Because of the rapid rise in DM, safe and efficient treatments are required, and one potential strategy is to minimize postprandial hyperglycemia by delaying glucose absorption by inhibiting carbohydrate-hydrolyzing enzymes and scavenging free radicals (Uddandrao et al. 2020). There was no scientific data available about the PHD made with Punica granatum (fruits), Illicium verum (flowers) and Nyctanthes arbor (Leaves) and their antioxidant and anti-diabetic properties so far. Therefore, in this study we made an effort to prepare PHD with three herbs as mentioned above and evaluated their antioxidant and antidiabetic activities through in vitro and cell culture models.
MATERIAL AND METHODS
For the sample collection, The P. granatum fruits were taken from a local garden in Erode, Tamil Nadu, the I. verum flowers was taken from the neighborhood garden, Tiruchengode, Namakkal, Tamil Nadu and N. arbor (Leaves) was procured from local garden, Salem, Tamil Nadu, India. The plants P. granatum (Voucher No: BSI/SRC/5/23/2020/Tech/510), I. verum (Voucher No: BSI/SRC/5/23/2020/Tech./509) and N. arbor (Voucher No: BSI/SRC/5/23/2020/TECH/511) were authenticated by the Southern Regional Centre, Botanical Survey of India, TNAU Campus, Coimbatore, Tamilnadu, India. For sample preparation, the P. granatum (fruits), I. verum (flowers), and N. arbor (leaves) were rinsed with cleaned water and on a fresh sheet, air-dried for seven days at room temperature.
The dry plant materials were made of powder and extraction was carried out with the help of soxhlet apparatus in the presence of methanol. Then the samples were evaporated to waterlessness at decreased pressure at 40oC by rotary evaporator (Superfit, Precision Scientific, India). A PHD was made through extracts of P. granatum, I. verum and N. arbor. A variety of concentrations and combinations were tried and a last valuable formulation with equal volume of each one plant extracts (1:1:1 ratio) was fixed for experiments.
For better formulation stability during shelf life, several additives such as Tween-80, Sodium CMC, sweetening agent (Sodium saccharin), and flavouring agent (Lemon oil) were incorporated. Preliminary phytochemical screening was done by Kokate’s techniques to test the plant individual extracts and PHD for the presence of a range of phytoconstituents (Kokate 1986). For quantitative estimation of phytochemicals, we used the individual concentrates and PHD to quantify secondary metabolites based on the elemental phytochemical examination results; for example, total phenolic content, total flavonoids, and total tannins were measured in accordance with the guidelines (Folin and Ciocalteu 1927; Singleton and Rossi 1965; Ordonez et al. 2006).
GC-MS (Gas Chromatography-Mass Spectrophotometry) analysis, the plant individual extracts and PHD were subjected to identify bioactive compounds by GC-MS as described in the previous studies (Uddandrao et al. 2020). Analysis of the mass spectrum the compounds were identified using GC-MS and the National Institute of Standards and Technology (NIST) database. For evaluation of antioxidant activity, plant individual extracts and PHD was determined using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity, Hydrogen peroxide (H2O2) scavenging assay, total antioxidant activity as determined by the phosphomolybdate assay proposed in the previous studies and Thiobarbituric acid reactive substances (TBARS) assay (Ruch et al. 1989; Shimada et al. 1992; Kikuzaki and Nakatani 1993; Prieto et al. 1999).
For assessment of in vitro antidiabetic effects, the inhibitory activities of α-amylase, α-glucosidase (Becerra-Jimenez et al. 2008) and glucose diffusion inhibition were measured as per customary protocols. All experiments were performed in triplicate, with results expressed as a percentage of inhibition (Gallagher et al. 2003; Thalapaneni et al. 2008). For determination of antidiabetic activity by cell culture model, the HepG2 cell culture was incubated at 37°C in a humidified environment with 5% CO2, and growth media, consisting of RPMI 1640 media supplemented with 10% foetal calf serum, was replaced every 2-3 days. Venter et al. (2008) described a technique for determining glucose consumption in HepG2 cells (Venter et al. 2008).
The difference between the cell-free and cell-containing wells was used to compute the amount of glucose utilized. The percentage of glucose intake was calculated in contrast to the untreated controls. For Assay of metabolic enzymes inhibition potential of PHD in HepG2 cell line, the inhibition of ɑ–amylase, ɑ-glucosidase, lipase, dipeptidyl peptidase-4 (DPP-IV), and collagenase were measured as per respective protocols (Sancheti and Seo 2010; Lewis and Liu 2012; Odeyemi 2015; Idowu et al. 2018). The percentage of inhibitory activity was calculated by using the following formulae: % Inhibition= (1 – A/B) × 100, A= Absorbance of the untreated (Control), B= Absorbance of the test well.
For protein glycation assay, the protein glycation assay was performed according to the methodology described by Idowu et al. (2018). The studies were done three times, and the percentage of inhibition was estimated using the formula below: % Inhibition= (1 – A/B) × 100
A= Fluorescence of test well, B= Fluorescence of negative control. For Statistical analysis, the investigational data were presented as the average of three replicates’ standard deviations (SD). Wherever relevant, the results be treated to a unidirectional analysis of variance (ANOVA) and the significant difference (p<0.05, 0.01) between means was decided by the least significant difference (LSD) using Statistical Package for Social Sciences (SPSS) version 15.0 for Windows.
RESULTS AND DISCUSSION
DM is a metabolic disorder characterized by hyperglycemia and glucose intolerance, as well as abnormalities in insulin production and insulin action to increase glucose uptake. The high prevalence of morbidity and death, as well as higher health-care costs for management and treatment, make this condition a global burden. Herbal medications are made from a variety of plant sources, either in its natural state or after processing with various ingredients.
Despite the fact that a variety of oral hypoglycemic medications, as well as insulin, are used to treat DM, there is a revived curiosity among patients in using natural medicines with anti-diabetic properties (Brahmanaidu et al. 2017; Vadivel et al. 2018; Unuofin and Lebelo 2020). A large number of conservative or native medications are also driving the cutting-edge prescription arrangement. In comparison to a single herb, the PHD offers a greater and broader therapeutic potential, particularly for DM (Petchi et al. 2014; Williams et al. 2020).
Figure 1: Quantitative estimation of secondary metabolites such as (A) phenolics, (B) flavonoids, and (C) tannins present in the P. granatum, I. verum, N. arbor and the PHD. Values are mean ± SD, n=3. Values are significant at *P<0.05, **P<0.01
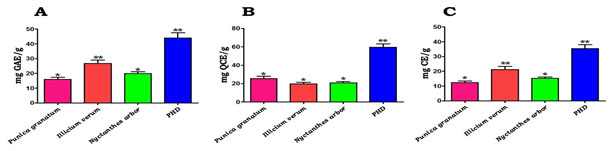
In this study, we attempted to make PHD from P. granatum (fruits), I. verum (flowers), and N. arbor (leaves) and evaluated their antioxidant and anti-diabetic properties. Preliminary phytochemical screening: The methanolic extract of PHD contained secondary metabolites such as alkaloids, carbohydrates, phenols, flavonoids, amino acids, proteins, terpenoids, Saponins and Tannins. Quantitative estimation of phytochemicals: The quantification of secondary metabolites such as phenolics, flavonoids, and tannins in P. granatum, I. verum, N. arbour, and the PHD is shown in Figure 1. PHD has the highest concentration of phenolics (Fig. 1A), flavonoids (Fig. 1B), and tannins (Fig. 1C) when compared to individual plan extracts.
The levels of phenolics were found to be larger than flavonoids and tannins, according to these calculations. As shown in figure 1A-C, the individual extracts of P. granatum, I. verum, and N. arbour extracts showed a noticeable quantity of these secondary metabolites. Since DM may pose a hazard to our populace, scientists all around the world are scouring phytochemicals for natural active agents (Pimple et al. 2011; Chan et al. 2021).
Figure 2: GC-MS chromatogram of the methanolic extract of PHD.
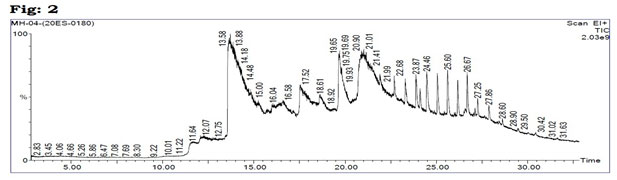
The antioxidant efficacies of phenolic compounds have been shown to be robust, and they play an important role in the management of chronic diseases like DM. Flavonoids are polyhydroxy polyphenolic chemicals with a wide range of applications in herbal medicine. Flavonoids, which are divided into flavanols, flavones, and flavanones, have a variety of therapeutic benefits, including anti-diabetic qualities (Roman et al. 1995; Adefegha et al. 2013). GC-MS analysis: GC-MS analysis of the PHD (Table 1, Fig 2) revealed that several compounds as exposed tables 1. Antioxidant activity: For antioxidant activity, when compared to the plant extracts individually, PHD had the DPPH radical scavenging activity is greatest at doses ranging between 100µg/mL to 1000µg/mL (Fig. 3A).
Similarly, PHD demonstrated H2O2 decomposition activity in a dose-dependent manner, with the maximum activity reported at 1000µg/mL. At the same time, Individual plant extracts were shown to have moderate H2O2 scavenging capabilities when compared to PHD (Fig. 3B). Figure 3C shows the % inhibition of P. granatum, I. verum, N. arbour, and PHD at various doses using the TBARS test. When compared to separate plant extracts, our results clearly demonstrated that the PHD might possibly prevent lipid peroxidation in a dose-dependent manner. Phosphomolybdate test was used to investigate the total antioxidant capabilities of P. granatum, I. verum, N. arbour, and PHD at various doses, and the findings revealed dose-dependent antioxidant activity (Fig. 3D) (Zhang et al. 2020).
Table 1. Compounds identified in the methanolic extract of PHD with GC-MS
| RT | Name of the Compound | Molecular Formula | Molecular Weight | Peak Area % |
| 11.662 | 1,2-Cyclohexanedione | C6H8O2 | 112 | 1.197 |
| 12.252 | Cyclohexanone, 2-Methyl- | C7H12O | 112 | 0.977 |
| 12.307 | Cyclohexanone, 2-Methyl- | C7H12O | 112 | 1.051 |
| 12.882 | 5-Trans-Methyl-1r,3-Cis-Cyclohexanediol | C7H14O2 | 130 | 0.844 |
| 13.713 | Benzene, 1-Methoxy-4-(1-Propenyl)- | C10H12O | 148 | 27.781 |
| 16.619 | Benzene, (1-Methoxyethyl)- | C9H12O | 136 | 12.014 |
| 17.534 | Phenol, 4-(2-Propenyl)-, Acetate | C11H12O2 | 176 | 8.983 |
| 18.615 | 2-Cyclopenten-1-One, 2-(2-Butenyl)-4-Hydroxy-3-Methyl-, (Z)- | C10H14O2 | 166 | 4.328 |
| 19.690 | N-Hexadecanoic Acid | C16H32O | 256 | 8.856 |
| 21.011 | Dodecanoic Acid, 9-Decen-1-Yl Ester | C22H42O2 | 338 | 17.146 |
| 21.901 | 3-(5-Benzyloxy-3-Methylpent-3-Enyl)-2,2-Dimethyloxirane | C17H24O2 | 260 | 17.146 |
| 22.681 | Oxalic Acid, Isohexyl Nonyl Ester | C17H32O4 | 300 | 3.813 |
| 23.291 | Octane, 3,4,5,6-Tetramethyl- | C12H26 | 170 | 2.387 |
| 23.867 | Octadecane, 3-Ethyl-5-(2-Ethylbutyl)- | C26H54 | 366 | 1.183 |
| 24.097 | Cyclopropyl 2-(5′-Methyl-2′-Furyl)Cyclopropyl Ketone | C12H14O2 | 190 | 0.974 |
| 24.462 | Hexatriacontane | C36H74 | 506 | 1.595 |
| 25.042 | Heptacosane | C27H56 | 380 | 0.883 |
| 25.602 | Ethanol, 2-(Octadecyloxy)- | C20H42O2 | 314 | 0.836 |
When compared to individual plant extracts, the PHD demonstrated much higher activity. Free radicals, such as reactive oxygen species (ROS), have been linked to DM, obesity, inflammation, cardiovascular disease, atherosclerosis, ageing, and cancer (Meriga et al. 2017; Brahmanaidu et al. 2017). It’s critical to strike stability between the pace of free radical creation and exclusion. Excessive cell radical formation is typically harmful; in any event, oxidative cellular stress occurs when there is a significant increase in excessively radical formation or a reduction in radical clearance from the cell (Valko et al. 2007; Li et al. 2019).
According to research and scientific data, oxidative stress is linked to the onset and development of DM (Rösen et al. 2001). Using DPPH radical and H2O2 scavenging action, TBARS test, and total antioxidant activity, we evaluated the antioxidant properties of PHD and individual plant extracts in this study. DPPH is a stable, nitrogen-focused free radical that is transformed to diphenylpicryl hydrazine after absorbing hydrogen from the polyphenolic extract’s antioxidants (Vladimir et al. 2011). The drop in DPPH seen by the PHD was caused by either a hydrogen atom exchange or an electron exchange. Additionally, phenolic compounds and flavonoids are abundant hydrogen benefactors, making them excellent antioxidants (Michalak 2006; Yaribeygi et al. 2020).
Figure 3: (A) DPPH radical scavenging activity, (B) H2O2 scavenging activities, (C) TBARS and (D) total antioxidant capacities of P. granatum, I. verum, N. arbor and PHD at different concentrations, Values are mean ± SD, n=3.
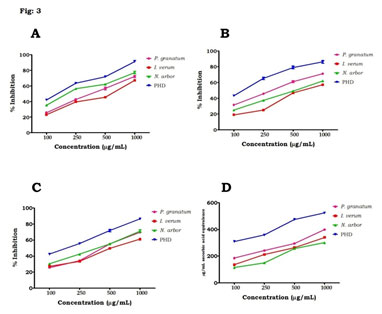
H2O2 is a mild oxidising agent that may inactivate a few enzymes directly, often by oxidising crucial thiol groups. H2O2 may quickly permeate cell membranes, and once inside, it interacts with Fe2+ and perhaps Cu2+ ions to produce hydroxyl radicals, which are harmful to cells. Because of their hydrogen-giving and scavenging capacities, secondary metabolic products may act as free radical scavengers (Beyhan et al. 2010). PHD had a substantially greater overall antioxidant capacity than individual plant extracts, according to our research. The total phenolic content and antioxidative activity have been linked in several studies. Natural antioxidants’ efficiency is determined in part by the chemical composition and chemical structures of the extract’s active components (Hossain et al. 2015; Jiao et al. 2019).
Figure 4: In vitro anti-diabetic activities of individual plant extracts and PHD, (A) ɑ-amylase inhibitory activity, (B) α-glucosidase inhibitory activity and (C) glucose diffusion, Values are mean ± SD, n=3.
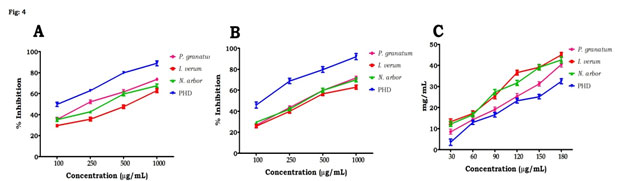
We observed high quantities of total phenolic content, flavonoids, tannins, and bioactive components in PHD using GC-MS throughout this investigation. PHD’s high antioxidant action might be attributed to these components. In vitro antidiabetic activity of PHD: the current research found a dose-dependent increase in % inhibitory efficacy against the α-amylase (Fig. 4A) and α-glucosidase enzymes (Fig. 4B). The PHD demonstrated considerable inhibitory action (p < 0.05) against these enzymes, with the maximum dosage of 1000g/mL having the maximum inhibitory action. The individual extracts of P. granatum, I. verum and N. arbor did not demonstrate substantial inhibitory activity against α-glucosidase and α-amylase as compared to PHD.
The current study used a variety of biochemical and cell-based tests to determine the probable mechanism(s) of P. granatum, I. verum, N. arbour, and PHD extracts antidiabetic effects. Glucose absorption inhibition activity: When compared to metformin, the results of this study on glucose uptake in HepG2 cells indicated that PHD increased glucose uptake in HepG2 cells. This suggests that the PHD works similarly to metformin in that it increases glucose absorption in the liver. Metformin can have a hypoglycemic effect by activating the AMP activated protein kinase in the liver, which can lead to a variety of pharmacologic effects, such as glucose inhibition, lipid synthesis inhibition, and enhanced hepatic insulin sensitivity (Viollet et al. 2012). Phytochemicals such as phenols, terpenoids, flavonoids, and flavanols have been shown to limit glucose release from the liver while also increasing glucose absorption in the hepatic cells, therefore altering the intracellular signalling pathway (Hanhineva et al. 2010; Peng et al. 2019).
Figure 5: Effect of PHD and individual extracts on (A) glucose utilization, (B) α-amylase inhibition and (C) α-glucosidase inhibition in HepG2 cell line. Values are mean ± SD, n=3. Values are significant at *P<0.05, **P<0.01.
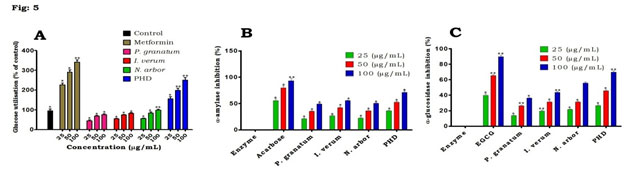
Effect of PHD on glucose utilization in HepG2: when compared to the control, PHD had the greatest potential for preventing the flow of glucose molecules over the dialysis membrane (Fig. 4C). In comparison to PHD, individual plant extracts were less efficient at stopping glucose molecules from diffusing. Figure 5A shows the findings for glucose uptake in HepG2 cells in the presence of the plant extract at concentrations of 25, 50 and 100 µg/mL.
When compared to the untreated control, the PHD generated a significant (p < 0.05) greater increase in glucose absorption in HepG2 cells at all concentrations in a concentration-dependent manner, tests were conducted. Phytochemicals have beneficial effects through a variety of pathways, including glucose and lipid metabolism modulation, insulin secretion, cell stimulation, the NF-kB signalling pathway, inhibition of gluconeogenic enzymes, and ROS protection. Insulin deficiency has an impact on glucose, protein, and fat metabolism, as well as water and electrolyte balance (Frier et al. 2006; Saeedi et al. 2020).
A high postprandial blood glucose response is connected to micro- and macrovascular issues in people with DM, and is more strongly linked to cardiovascular disease risk than fasting blood glucose. α-glucosidase enzymes in the intestinal lumen and the brush border membrane convert starch and oligosaccharides to monosaccharides before absorption, which is essential for carbohydrate digestion. Inhibiting the activity of these digestive enzymes was thought to cause a delay in the decomposition of starch and oligosaccharides, leading in a decrease in glucose absorption and, as a result, a decrease in postprandial blood glucose levels rising (Puls et al. 1977; Lachin et al. 2021).
Figure 6: Effect of PHD and individual extracts on (A) Lipase inhibition, (B) DPP-IV inhibition, (C) Collagenase inhibition and (D) Protein glycation in HepG2 cell line. Values are mean ± SD, n=3. Values are significant at *P<0.05, **P<0.01.
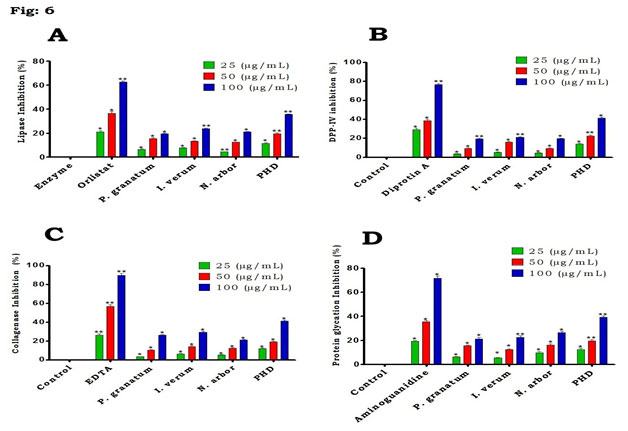
Effect of PHD on α-amylase in HepG2: when compared to various plant extracts, the results showed that PHD had the most significant impact on α-amylase at all doses tested (Fig. 5B). Both PHD and individual extracts had a considerable impact on α-amylase at the maximum concentration (100µg/ml) tested. α-glucosidase inhibition potential of PHD in HepG2: figure 5C depicts that PHD demonstrated that a highest significant effect on α-glucosidase at all the tested concentrations and highest at 100𝜇g/ml than the individual plant extracts tested. However, as positive controls, EGCG was somewhat more efficacious than the extract and untreated control in the respective experiments. Lipase inhibition assay in HepG2: PHD exhibited significant inhibition against pancreatic lipase in a concentration dependent manner (Fig. 6A).
On the other hand, individual plant extracts demonstrated less lipase inhibition activity when compared to PHD and orlistat. This shows that PHD (100𝜇g/ml) may have anti-diabetic properties through processes involving lipase inhibition (Lachin et al. 2021). Dipeptidyl peptidase-4 inhibition assay in HepG2: the PHD exhibited highest significant inhibition against Dipeptidyl peptidase-4 at all the tested concentrations as similar like standard diprotin A (Fig. 6B). When compared to the untreated control and individual plant extracts, there was a high significant inhibition of the PHD. Collagenase inhibition activity of PHD in HepG2: In this study, the extracts of P. granatum, I. verum, N. arbor exhibited moderate inhibition activity of collagenase when compared to PHD (Fig. 6C). On the other hand, PHD demonstrated that the highest activity at 100𝜇g/ml concentration similar like EDTA.
Protein glycation assay in HepG2: the findings demonstrated that the PHD extract inhibited protein glycation in a dose-dependent manner at all concentrations tested (Fig. 6D). At the same time, individual plant extracts demonstrated only moderate activity when compared to PHD and aminoguanidine. The inhibition of lipase and collagenase enzymes by secondary plant chemicals has the potential to manage postprandial hyperglycemia and hyperlipidemia, while inhibiting enzyme catalysis will reduce the number of monosaccharides and fatty acids accessible for absorption (Villa-Rodriguez et al. 2018). Pancreatic lipase is accountable for the hydrolysis of dietary lipids and its inhibition outcome in reduced fat absorption, contributing to DM (Conforti et al. 2012). Anti-diabetic medicines are used to treat or manage DM, and their mechanisms of action are known. Lipase and DPP-IV enzyme inhibition are among them (Saeedi et al. 2020).
When compared to the individual positive controls, our results showed that the extract inhibited lipase and DPP-IV significantly. This suggests that the anti-diabetic mechanism of PHD is as a result of through the inhibition of these enzymes. On the other hand, protein glycation has been established in studies to be one of the outcomes of abnormally high blood glucose in DM individuals (Ulrich and Cerami 2001; Mckay et al. 2019). Protein glycation is a reversible process in which a reducing sugar and a free amino group of a protein form adducts that yield glycation products over time. These responses play a crucial role in the aetiology of DM complications. When compared to relevant standards, at the concentrations investigated, the PHD showed significant suppression of collagenase and protein glycation. However, this is the first study to look at both the PHD’s protein glycation and anticollagenase properties (Kiho et al. 2000; Eckel et al. 2021).
CONCLUSION
The findings of the present study demonstrated that the PHD has contained abundant phytoconstituents and showed a significant free radical scavenging activity and glucose lowering effects in vitro. On the other hand, PHD exhibited noteworthy inhibition of α-amylase, α-glucosidase, lipase, DPP-IV, collagenase and protein glycation in HepG2 cell line. Hence the results obtained from this recommended that PHD may be considered for the treatment of postprandial glycemia in people with type 2 diabetes mellitus. However, further preclinical studies are crucial in advanced animal models to validate its antioxidant and anti-diabetic activities.
ACKNOWLEDGEMENTS
The study was financially supported by the management of K.S. Rangasamy College of Arts and Science, Tiruchengode, Namakkal, Tamil Nadu, India. Authors thank the college for providing necessary facilities.
Conflict of Interests: Authors declare no conflicts of interests to disclose.
REFERENCES
Abate, N., and Chandalia, M. (2007). Ethnicity, type 2 diabetes & migrant Asian Indians. The Indian journal of medical research, 125(3): 251–258.
Adefegha, S.A., and Oboh, G. (2013). Phytochemistry and mode of action of some tropical spices in the management of type-2 diabetes and hypertension. African Journal of Pharmacy and Pharmacology, 7: 332–346.
American Diabetes Association. (2015). Standards of medical care in diabetes-2015: summary of revisions. Diabetes Care, 38(4):67-70. doi: 10.2337/dc15-S003.
Amzad Hossain, M., and Muhammad, D.S. (2015). A study on the total phenols content and antioxidant activity of essential oil and different solvent extracts of endemic plant Merremia borneensis. Arabian Journal of Chemistry, 8: 66–71.
Becerra-Jimenez, J., Andrade-Cetto, A.A. and Cárdenas-Vázquez, R., (2008). Alfa-glucosidase-inhibiting activity of some Mexican plants used in the treatment of type 2 diabetes. Journal of ethnopharmacology, 116(1): 27–32. https://doi.org/10.1016/j.jep.2007.10.031
Beyhan, O., Elmastas, M., and Gedikli, F. (2010). Total phenolic compounds and antioxidant capacity of leaf, dry fruit and fresh fruit of fei-joa (Acca sellowiana, Myrtaceae). Journal of Medicinal Plant Research, 4: 1065–1072.
Brahmanaidu, P., Uddandrao, V.S., Sasikumar, V., et al. (2017). Reversal of endothelial dysfunction in aorta of streptozotocin-nicotinamide-induced type-2 diabetic rats by S-Allylcysteine. Molecular and cellular biochemistry, 432(1-2): 25–32. https://doi.org/10.1007/s11010-017-2994-0.
Chan, J.C., Lim, L.L., Wareham, N.J., et al. (2021). The Lancet Commission on diabetes: using data to transform diabetes care and patient lives. Lancet (London, England), 396(10267), 2019–2082. https://doi.org/10.1016/S0140-6736(20)32374-6.
Cole, J.B., and Florez, J.C. (2020). Genetics of diabetes mellitus and diabetes complications. Nature Reviews Nephrology, 16: 377–390 https://doi.org/10.1038/s41581-020-0278-5.
Conforti, F., Perri, V., Menichini, F., et al. (2012). Wild Mediterranean dietary plants as inhibitors of pancreatic lipase. Phytotherapy research: PTR, 26(4): 600–604. https://doi.org/10.1002/ptr.3603.
Eckel, R. H., Bornfeldt, K. E., and Goldberg, I. J. (2021). Cardiovascular disease in diabetes, beyond glucose. Cell metabolism, 33(8): 1519–1545. https://doi.org/10.1016/j.cmet.2021.07.001
Folin, O., and Ciocalteu, V. (1927). On tyrosine and tryptophane determinations in proteins. Journal of Biological Chemistry, 73: 627–650.
Frier, B.M. (2006). Davidson’s principle and practice of medicine, Churchill Livingstone Elsevier: Ediburgh, (20), 805–845.
Gallagher, A.M., Flatt, P.R., Duffy, G.A.W.Y. et al. (2003). The effects of traditional antidiabetic plants on in vitro glucose diffusion. Nutrition Research, 23: 413–424.
Gurung, M. (2020). Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine, 51, 102590. https://doi.org/10.1016/j.ebiom.2019.11.051
Hanhineva, K., Törrönen, R., Bondia-Pons, I., et al. (2010). Impact of dietary polyphenols on carbohydrate metabolism. International journal of molecular sciences, 11(4): 1365–1402. https://doi.org/10.3390/ijms11041365
Hill-Briggs, F., Adler, N.E., Berkowitz, S.A., et al. (2020). Social Determinants of Health and Diabetes: A Scientific Review. Diabetes care, 44(1): 258–279.https://doi.org/10.2337/dci20-0053
Jiao, W. (2019). Activation of the Notch‑Nox4‑reactive oxygen species signaling pathway induces cell death in high glucose‑treated human retinal endothelial cells. Molecular medicine reports, 19(1): 667–677. https://doi.org/10.3892/mmr.2018.9637
Kavishankar, G.B., Lakshmidevi, N., Murthy, S.M., et al. (2011). Diabetes and medicinal plants-A review. Journal of Pharmaceutical and Biomedical Sciences, 2: 65–80.
Kiho, T., YAMANE, A., HUI, J., et al. (1996). Polysaccharides in fungi. XXXVI. Hypoglycemic activity of a polysaccharide (CS-F30) from the cultural mycelium of Cordyceps sinensis and its effect on glucose metabolism in mouse liver. Biological & pharmaceutical bulletin, 19(2): 294–296. https://doi.org/10.1248/bpb.19.294
Kikuzaki, H., and Nakatani, N. (1993). Antioxidant effects of some ginger constituents. Journal of Food Science, 58: 1407–1410.
Kokate, C.K. (1986). Preliminary phytochemical screening, practical pharmacognosy. Vallabh Prakashan; 1: 111.
Lachin, J. M., and Nathan, D. M., (2021). Understanding Metabolic Memory: The Prolonged Influence of Glycemia During the Diabetes Control and Complications Trial (DCCT) on Future Risks of Complications During the Study of the Epidemiology of Diabetes Interventions and Complications (EDIC). Diabetes care, dc203097. https://doi.org/10.2337/dc20-3097
Lewis, D. R., and Liu, D. J. (2012). Direct Measurement of Lipase Inhibition by Orlistat Using a Dissolution Linked In Vitro Assay. Clinical pharmacology & biopharmaceutics, 1: 1000103. https://doi.org/10.4172/2167-065X.1000103.
Li, Z. (2019). Asthma-Alleviating Potential of 6-Gingerol: Effect on Cytokines, Related mRNA and c-Myc, and NFAT1 Expression in Ovalbumin-Sensitized Asthma in Rats. Journal of environmental pathology, toxicology and oncology: official organ of the International Society for Environmental Toxicology and Cancer, 38(1): 41–50. https://doi.org/10.1615/JEnvironPatholToxicolOncol.2018027172
McKay, T. B., Priyadarsini, S., and Karamichos, D. (2019). Mechanisms of Collagen Crosslinking in Diabetes and Keratoconus. Cells, 8(10): 1239. https://doi.org/10.3390/cells8101239
Meriga, B., Parim, B., Chunduri, V.R., et al. (2017). Antiobesity potential of Piperonal: promising modulation of body composition, lipid profiles and obesogenic marker expression in HFD-induced obese rats. Nutrition & metabolism, 14: 72. https://doi.org/10.1186/s12986-017-0228-9
Michalak A. (2006). Phenolic compounds and their antioxidant activityin plants growing under heavy metal stress. Polish Journal of Environmental Studies, 15: 523–530.
Narkhede, M.B. (2011). Investigation of in vitro α-amylase and α-glucosidase inhibitory activity of polyherbal extract. International Journal of Pharmaceutical Research and Development, 3(8),97–103.
Odeyemi, S.W. (2015). A comparative study of the in vitro antidiabetic properties, cytotoxicity and mechanism of action of Albuca bracteata and Albuca setosa bulb extracts Doctoral dissertation [Doctoral, thesis], University of Fort Hare.
Omari El, N. (2019). Evaluation of In Vitro Antioxidant and Antidiabetic Activities of Aristolochia longa Extracts. Evidence-based complementary and alternative medicine: eCAM, 2019: 7384735. https://doi.org/10.1155/2019/7384735
Ordon, J.D., Gomez, J.D., Vattuone, M.A. et al. (2006). Antioxidant activities of Sechium edule (Jacq.) Swart extracts. Food Chemistry, 97: 452–458. DOI:10.1016/j.foodchem.2005.05.024
Parasuraman, S., Thing, G.S., and Dhanaraj, S.A. (2014). Polyherbal formulation: Concept of ayurveda. Pharmacognosy Reviews, 8(16): 73–80. https://doi.org/10.4103/0973-7847.134229
Parim, B., Sathibabu Uddandrao, V. V., and Saravanan, G. (2019). Diabetic cardiomyopathy: molecular mechanisms, detrimental effects of conventional treatment, and beneficial effects of natural therapy. Heart failure reviews, 24(2): 279–299. https://doi.org/10.1007/s10741-018-9749-1
Peng, J.J., Xiong, S.Q., Ding, L.X., et al. (2019). Diabetic retinopathy: Focus on NADPH oxidase and its potential as therapeutic target. European journal of pharmacology, 853: 381–387. https://doi.org/10.1016/j.ejphar.2019.04.038
Petchi, R. R., Vijaya, C., and Parasuraman, S. (2014). Antidiabetic activity of polyherbal formulation in streptozotocin – nicotinamide induced diabetic wistar rats. Journal of traditional and complementary medicine, 4(2): 108–117. https://doi.org/10.4103/2225-4110.126174
Pimple, B.P., Kadam, V., and Patil, J. (2011). Antidiabetic and antihyperlipi- demic activity of Luffa acutangula fruit extracts in streptozotocin induced niddm rats. Asian Journal of Pharmaceutical Education and Research, 4: 156–163.
Prieto, P., Pineda, M., and Aguilar, M. (1999). Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Analytical biochemistry, 269(2): 337–341. https://doi.org/10.1006/abio.1999.4019
Puls, W. (1977). Glucosidase inhibition. A new approach to the treatment of diabetes, obesity, and hyperlipoproteinaemia. Die Naturwissenschaften, 64(10): 536–537. https://doi.org/10.1007/BF00483562
Roman-Ramos, R., Flores-Saenz, J.L., and Alarcon-Aguilar, F.L. (1995). Anti-hyperglycemic effect of some edible plants. Journal of Ethnopharmacology, 48: 25–32. https://doi.org/10.1016/0378-8741(95)01279-m
Rösen, P., Nawroth, P.P., King, G., et al. (2001). The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes/metabolism research and reviews, 17(3): 189–212. https://doi.org/10.1002/dmrr.196
Ruch, R. J., Cheng, S. J., and Klaunig, J. E. (1989). Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis, 10(6): 1003–1008. https://doi.org/10.1093/carcin/10.6.1003
Saeedi, P., Salpea, P., Karuranga, S., et al. (2020). Mortality attributable to diabetes in 20-79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes research and clinical practice, 162, 108086. https://doi.org/10.1016/j.diabres.2020.108086
Sagbo, I.J., Venter, M.V., Koekemoer, T. et al. (2018). In vitro antidiabetic activity and mechanism of action of Brachylaena elliptica (Thunb.) DC. Evidence-Based Complementary and Alternative Medicine, 2018.
Sancheti, S., Sancheti, S., and Seo, S. Y. (2010). Evaluation of antiglycosidase and anticholinesterase activities of Boehmeria nivea. Pakistan journal of pharmaceutical sciences, 23(2): 236–240.
Shimada, K., Fujikawa, K., Yahara, K. et al. (1992). Antioxidative properties of xanthan on the anti-oxidation of soybean oil incyclodextrin emulsion. Journal of Agricultural and Food Chemistry, 40: 945–948.
Singleton, V.L., and Rossi, J.A. (1965). Colorimetry of total phenolics with Phosphomolybdic phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16, 144–158.
Thalapaneni, N.R., Chidambaram, K.A., Ellappan, T., et al. (2008). Inhibition of carbohydrate digestive enzymes by Talinum portulacifolium (Forssk) leaf extract. Journal of Complementary and Integrative Medicine, 5(1): 1–10. https://doi.org/10.2202/1553-3840.1120
Uddandrao, V., Brahmanaidu, P., and Ganapathy, S. (2020). Evaluation of the Antioxidant and Antidiabetic Potential of the Poly Herbal Formulation: Identification of Bioactive Factors. Cardiovascular & hematological agents in medicinal chemistry, 18(2): 111–123. https://doi.org/10.2174
Uddandrao, V.S., Brahmanaidu, P., Nivedha, P.R., et al. (2018). Beneficial Role of Some Natural Products to Attenuate the Diabetic Cardiomyopathy Through Nrf2 Pathway in Cell Culture and Animal Models. Cardiovascular toxicology, 18(3): 199–205. https://doi.org/10.1007/s12012-017-9430-2
Uddandrao, V.S., Brahmanaidu, P., Ravindarnaik, R., et al. (2019). Restorative potentiality of S-allylcysteine against diabetic nephropathy through attenuation of oxidative stress and inflammation in streptozotocin-nicotinamide-induced diabetic rats. European journal of nutrition, 58(6): 2425–2437. https://doi.org/10.1007/s00394-018-1795-x
Ulrich, P., and Cerami, A. (2001). Protein glycation, diabetes, and aging. Recent progress in hormone research, 56: 1–21. https://doi.org/10.1210/rp.56.1.1
Unuofin, J. O., and Lebelo, S. L. (2020). Antioxidant Effects and Mechanisms of Medicinal Plants and Their Bioactive Compounds for the Prevention and Treatment of Type 2 Diabetes: An Updated Review. Oxidative medicine and cellular longevity, 2020: 1356893. https://doi.org/10.1155/2020/1356893
Vadivel, V., Ravichandran, N., Rajalakshmi, P., et al. (2018). Microscopic, phytochemical, HPTLC, GC–MS and NIRS methods to differentiate herbal adulterants: pepper and papaya seeds. Journal of Herbal Medicine, 11: 36–45. https://doi.org/10.1016/j.hermed.2018.01.004
Valko, M., Leibfritz, D., Moncol, J., et al. (2007). Free radicals and antioxidants in normal physiological functions and human disease. The international journal of biochemistry & cell biology, 39(1): 44–84. https://doi.org/10.1016/j.biocel.2006.07.001
Venter, M.V.D., Roux, S., Bungu, L.C., et al. (2008). Antidiabetic screening and scoring of 11 plants traditionally used in South Africa. Journal of ethnopharmacology, 119(1): 81–86. https://doi.org/10.1016/j.jep.2008.05.031.
Villa-Rodriguez, J.A., Kerimi, A., Abranko, L., et al. (2018). Acute metabolic actions of the major polyphenols in chamomile: an in vitro mechanistic study on their potential to attenuate postprandial hyperglycaemia. Scientific reports, 8(1): 5471. https://doi.org/10.1038/s41598-018-23736-1
Viollet, B., Guigas, B., Garcia, N.S., et al. (2012). Cellular and molecular mechanisms of metformin: an overview. Clinical science, 122(6), pp.253-270.
Vladimir-Knežević, S., Blažeković, B., Bival Štefan, M., et al. (2011). Antioxidant activities and polyphenolic contents of three selected Micromeria species from Croatia. Molecules, 16(2): 1454–1470. https://doi.org/10.3390/molecules16021454
Williams, R., Karuranga, S., Malanda, B., et al. (2020). Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes research and clinical practice, 162: 108072. https://doi.org/10.1016/j.diabres.2020.108072
Yaribeygi, H., Sathyapalan, T., Atkin, S.L. et al. (2020). Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxidative medicine and cellular longevity, 2020, 8609213. https://doi.org/10.1155/2020/8609213
Zhang, P., Li, T., Wu, X., et al. (2020). Oxidative stress and diabetes: antioxidative strategies. Frontiers of medicine, 14(5), 583–600. https://doi.org/10.1007/s11684-019-0729-1


