1Research Scholar, Department of Studies in Botany, Manasagangotri, University of Mysore, Mysore – 570006, Karnataka, India.
2Professor, 1Department of Studies in Botany, Manasagangotri, University of Mysore, Mysore- 570006, Karnataka, India.
Corresponding author email: mssharada.botany@gmail.com
Article Publishing History
Received: 01/10/2019
Accepted After Revision: 10/12/2019
Antimicrobial activity of aqueous and solvent extracts of Adina cordifolia, Careya arborea, Careya arborea, Hiptage benghalensis and Lannea coromandelica were tested against different bacteria (Bacillus cereus, Bacillus subtilis, Staphylococcus aureus, Streptococcus pyogenes, Enterobacter aerogenes, Escherichia coli, Proteus mirabilis, Klebsiella pneumonia) and fungi (Fusarium oxysporum, Aspergillus niger, Curvularia lunata and Alternaria alternata). Out of the tested plants, Cassia angustifolia and Adina cordifolia were found effective in inhibiting the growth of test microorganisms. Ethanol extract of C. angustifolia exhibited the maximum antibacterial potential with 24.66 mm inhibition zone of against S. aureus followed by E.coli (22.62 mm), B. subtilis (21.63 mm), E. aerogenes (18.32 mm), K. Pneumonia (17.67 mm) and B. cereus with 15.00 mm of inhibition. Aqueous extract of C. angustifolia showed maximum growth inhibition against B. cereus with inhibition zone of 21.55 mm followed by B. subtilis (19.47 mm) and E. coli (16.32 mm). A. cordifolia was found effective against B. cereus (15.52 mm), S. aureus (10.49 mm) and E.coli (11.32 mm). Against test fungi, C.angustifolia was found potent against F. oxysporum with 60 % of mycelial inhibition followed 13.16 % of inhibition against A. alternate. Ethanol extract of C. angustifolia exhibited the strong antifungal potential against C. lunata with 22.93 mm zone of inhibition.
Antibacterial, Antifungal, Cassia angustifolia, Adina cordifolia, Aqueous extract, Ethanol extract.
Tahamtan K, Sharada M. S. Evaluation of Antimicrobial Activity of Some Traditional Medicinal Plants. Biosc.Biotech.Res.Comm. 2019;12(4).
Tahamtan K, Sharada M. S. Evaluation of Antimicrobial Activity of Some Traditional Medicinal Plants. Biosc.Biotech.Res.Comm. 2019;12(4). Available from: https://bit.ly/2EbMKCZ
INTRODUCTION
Mankind is blessed with medicinal plants, an expensive gift from nature. Medicinal plants have been used widely as alternative therapeutic tools for the prevention or treatment of many diseases (Cartea et al., 2010, Nagavani and Rao, 2010).The active principles present in the medicinal plants are unknowingly exploited in traditional methods for the treatment of numerous ailments (Adebajo et al., 1983).Researchers are gradually increasing their attention towards the natural products and looking for new active principles, which lead to develop better drugs against microbial infections(Lopez et al., 2001, Ibrahim, 1997, Philip et al., 2009).The antimicrobial compounds obtained from plants have more preventive and curative effect than the commercial antibiotics, hence antimicrobial compounds of plants have more clinical value than the commercial ones against the infectious strains of resistant microorganisms, (Jamshiya, 2017).
About 800 plants have been in use in Ayurveda, whereas Siddha system of medicine makes use of 600 plants in different formulations and Unani and Amchi system together are practicing about 700 plants in their various preparations. Development of resistance by microbes against the available antibiotics has deepened the concerns for the identification of new and natural antibiotics. Plants are considered as the rich sources of secondary metabolites and have been reported to have potential therapeutic values (Farnsworth, 1973, Farnsworth and Soejarto, 1985). Medicinal plants are gradually gaining more priority and interest from microbiologists, pharmacologists and drug developing institutions/industries of India and abroad for last few decades owing to their vast medicinal values. Antimicrobial activity of medicinal plants have been reported by several workers from in India and other parts of the world, (Bhawasar et al., 1965, Shah and Qadry, 1971, Radhakrishnan et al., 1976, Rastogi et al., 1990, Asolkar et al., 1992, Mehmood et al., 1997, Ahmad et al., 1998, Grainger Bisset, 2000, Behl and Srivastava, 2002, Sofowora et al., 2013 and Gaudillière, 2019).
In the present investigation, five medicinal plants viz. Adina cordifolia, Careyaarborea, Cassia angustifolia, Hiptage benghalensis and Lanneacoro mandelica have been selected based on their traditional medicinal properties like chronic cough, jaundice, stomach ache, swelling in stomach, antiamoebic, anti inflammatory, antifertility, antityphoid, anticholera, anti anaemia, in treating burning sensation of the body, rheumatism, hyperdipsia, obesity, instrinsic hemorrhage, elephantiasis, inflammation, neuralgia, sprains and bruises as reported by many researchers, ( de Saravia and Gaylarde, 1998, Arya et al., 2003, Costa et al., 2009, Sharma et al., 2009, Siddique et al., 2010a, Parveen and Shahzad, 2011). Hence the above plants were evaluated for their antibacterial and antifungal activity against both gram positive and gram negative bacteria and phyto-pathogenic fungi.
MATERIALS AND METHODS
Collection of plant material
Leaves of the selected plants were used for antimicrobial activity. The selected plant materials were collected from in and around region of Mysore. The samples were collected freshly in the air tight clean polythene bags and brought to laboratory, washed under running water to remove the dust and debris.
Microorganisms used in study
Bacteria: Bacillus cereus, Bacillus subtilis, Staphylococcus aureus, Streptococcus pyogenes, Enterobacter aerogenes, Escherichia coli, Proteus mirabilis, Klebsiella pneumonia.
Fungi: Fusarium oxysporum, Aspergillus niger, Curvularia lunata and Alternaria alternata.
Preparation of extracts
Aqueous extraction: Fresh and washed samples were used for the aqueous extraction and extraction was made at 1:1 volume. About 50 gm of sample was weighed and macerated with 50 ml of distilled water in the mortar and pestle. The macerate was kept overnight for exudation of bio chemicals and were filtered through double layered muslin cloth followed by Whatman No. 1 filter paper. Obtained aqueous extracts were stored at 4 °C for further use.
Solvent extraction: The test plants which gave effective results against tested pathogens in aqueous extracts were subjected to solvent extraction with the help of Soxhlet apparatus. Shade dried samples of test plant leaf materials were coarse powdered. The plant materials were exhaustively extracted by solvents with an increasing polarity. The solvent series which are used in extraction based on increasing polarity are as follows; Petroleum ether, chloroform, ethyl acetate and ethanol. The extracted solvents were kept for evaporation and obtained extracts were stored at 5 °C for further use.
Antibacterial Assay
Agar well diffusion method: This method was carried out in-vitro with nutrient agar medium ((HiMedia, Mumbai). About 25 ml of sterilized, autoclaved nutrient agar medium was poured on to the sterilized petri-plates.in a laminar air flow to avoid contamination. The spore suspension was uniformly spread over the sterilized media in Petri-plates with the help of ‘L’ shaped glass rod. The 6 mm of wells were made on the media with the help of stainless steel cork borer. The wells were filled with the extracts of 100 μl each. Plates were incubated at 27 ± 2˚C, three replicates were maintained for each treatment. Inhibition zone was observed after 24 h of inoculation and inhibition zone was measured with zone scale. The results were tabulated.
Agar disc diffusion method: In this method, Sterilized disks were impregnated with extracts solutions of the substances to be examined, the test compound at a known concentration. About 20 ml of sterilized, autoclaved Muller Hinton agar (Hi Media, Mumbai) media was poured into sterile petri-plates and were allowed to solidify inside laminar air flow. About 100 µl of bacterial cultures were spread over solidified, media using ‘L’ shaped glass rod. The test extracts of 100 µl (100 mg/ml) was impregnated onto a sterile disc. Each test plates contained a disc impregnated with extract, one disc as positive control (Gentamicin) and one disc served as negative control(solvent), all discs were placed at equidistant from each other. The inoculated plates were incubated at 37°C for 24 h. The experiment was carried out in triplicates and the zone of inhibition was recorded with zone scale.
Antifungal Assay
Poison Food Technique: Antifungal activity was carried out In-vitro at 10% of concentration. About 2 ml of 100% extract from the prepared stock solution (100%) was added to 20 ml of sterilized, autoclaved Potato Dextrose Agar (PDA) (HiMedia, Mumbai) media to bring up 10% of concentration, poisoned media in the plate were allowed to solidify. A 5 mm diameter of actively growing mycelium disc of the pathogen of 7days old culture was placed in the center of the Petri plates containing poisoned media plates. And plates were incubated at 27 °C. Three replicates were maintained for each treatment. Radial growth of mycelium was measured after seven days after inoculation. The percent inhibition of the fungus in treatments was calculated using following formula
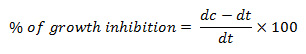
Where;
dc= Average increase in mycelial growth in control, dt= Average increase in mycelial growth in treatment.
Agar disc diffusion method
In this method, sterilized disks impregnated with extracts of the substances to be examined, the test compound at a known concentration. About 20 ml of sterilized, autoclaved Potato Dextrose Agar (PDA) media was poured into sterile petri-plates and were allowed to solidify in laminar air flow. About 100 µl of fungal cultures were spread over solidified media using ‘L’ shaped glass rod. The test extracts of 100 µl (100 mg/ml) was impregnated onto a sterile disc. The inoculated plates were incubated at 27°C for 7 days. The experiments were carried out in triplicates and the zone of inhibition was recorded with zone scale.
Statistical analysis
All the experiments were carried out in triplicates and the data were subjected to one- way analysis of variance (ANOVA), followed by Tukey’s post test at P£0.05 level of significance using Graph pad prism 5 software.
RESULTS AND DISCUSSION
Antibacterial activity of aqueous extracts
In the present study five medicinal plants have been screened for antibacterial activity. Out of tested plants Cassia angustifolia has effectively inhibited growth of the pathogenic bacteria B. cereus with inhibition zone of 21.55 mm, B. subtilis (19.47 mm), S. aureus (15.76 mm), E. coli (16.32 mm) as compared to others followed by Adina cordifoliaB. cereus (11.52 mm), S. aureus (10.49 mm) and E. coli (11.32 mm). The plant Lanneacoromandelica was effective against B. subtilis (11.66 mm) only. Whereas Careyaarborea and Hiptagebenghalensis has shown no activity on any bacterium (Table1, Fig. 1).
Table 1. Antibacterial activity of aqueous plant extracts against bacteria
| Sl. No | Name of the plant | Parts used | Inhibition of Bacteria in mm | |||||||
| Gram Positive Bacteria | Gram negative Bacteria | |||||||||
| B.
cereus |
B. subtiltis | S. aureus | S. pyogenes | E. aerogenes | E.
coli |
P. mirabilis | K. pneumonia | |||
| 1 | Adina cordifolia | Leaves | 11.52 ± 0.06* | 0.00 ± 0.0 | 10.49 ± 0.08* | 0.00 ± 0.0 | 0.00 ± 0.0 | 11.32 ± 0.04* | 0.00 ± 0.0 | 0.00 ± 0.0 |
| 2 | Careyaarborea | Leaves | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 |
| 3 | Cassia angustifolia | Leaves | 21.55 ± 0.04** | 19.47 ± 0.06** | 15.76 ±0.07** | 0.00 ± 0.0 | 0.00 ± 0.0 | 16.32 ± 0.05** | 0.00 ± 0.0 | 0.00 ± 0.0 |
| 4 | Hiptagebenghalensis | Leaves | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 |
| 5 | Lanneacoromandelica | Leaves | 0.00 ± 0.0 | 11.65 ± 0.04* | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 |
Note: Asterisk marks *, **, *** indicate significance of values at P 6 0.05 level according to ANOVA, Tukey’spost test.
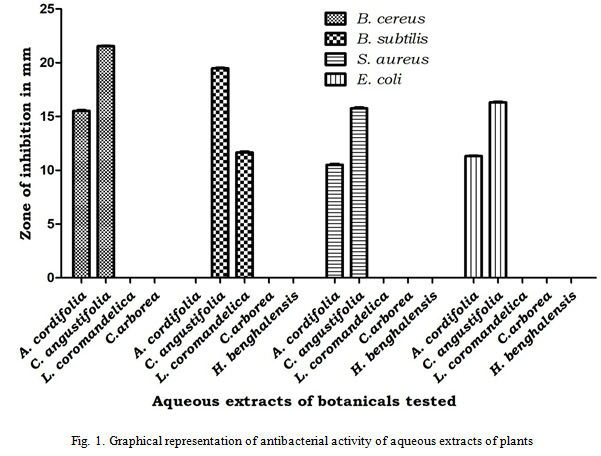 |
Figure 1: Graphical representation of antibacterial activity of aqueous extracts of plants |
Antifungal activity of aqueous extracts
Antifungal activity was carried out by poisoned food technique at 10 %. The results revealed that Cassia angustifolia with percentage inhibition of 60% (Fusarium oxysporum), 13.16 % (Alternaria alternata), 11.20% (Aspergillusniger) and 09% (Curvularialunata) was the only plant which inhibited the growth of the tested fungi and whereas least inhibition was observed in Lanneacoromandelica with 7% inhibition of Aspergillusniger, Hiptagebenghalensisshowed inhibition of Fusarium oxysporumand Aspergillusnigerwith inhibitionpercentage of 6% and 2.22% respectively.Adina cordifolia showed least inhibition against Fusarium oxysporum (2.22%) only. Whereas no inhibition was observed in the Careyaarborea for all the tested fungi (Table 2, Fig. 2).
Table 2. Antifungal activity of aqueous plant extracts against fungi
| Sl. No | Name of the plant | Parts used | Percentage inhibition of Fungi | |||
| Fusarium oxysporum | Aspergillusniger | Curvularialunata | Alternaria alternata | |||
| 1 | Adina cordifolia | Leaves | 2.22 ± 0.64 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 |
| 2 | Careyaarborea | Leaves | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 |
| 3 | Cassia angustifolia | Leaves | 60.00 ± 0.60*** | 11.20 ± 0.15 | 09.00±0.14 | 13.16 ± 0.16* |
| 4 | Hiptagebenghalensis | Leaves | 06 ± 0.11 | 2.22 ± 0.64 | 0.00 ± 0.0 | 0.00 ± 0.0 |
| 5 | Lanneacoromandelica | Leaves | 0.00 ± 0.0 | 07.00 ± 0.08 | 0.00 ± 0.0 | 0.00 ± 0.0 |
Note: Asterisk marks *, **, *** indicate significance of values at P 6 0.05 level according to ANOVA, Tukey’spost test.
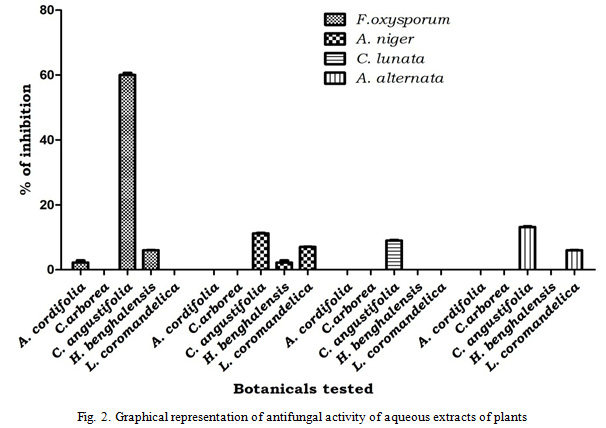 |
Figure 2: Graphical representation of antifungal activity of aqueous extracts of plants |
Antibacterial activity of solvent extracts
Based on the results obtained from the aqueous extracts two medicinal plants were selected for further activity. Adina cordifolia and Cassia angustifolia have been selected for further studies as they shown good activity against both bacteria and fungi as compared to others. These two plants were subjected to solvent extraction and the obtained extracts of solvents were examined for antibacterial activity. The results revealed that, both Petroleum ether and Chloroform of both plants shown no activity on all tested pathogenic bacteria. Ethyl acetate extract of Adina cordifolia effective only on S. aureus with inhibition zone of 15.31mm. Whereas ethanol extract of Adina cordifolia has shown inhibition of S. aureus, B. Cereus and E. coli with inhibition zone of 23.61 mm, 15.33 mm and 14.63 mm respectively. Ethyl acetate extract of Cassia angustifolia was effective on S. aureus and E. coli with inhibition zone of 22.31 mm and 13.65 mm respectively while ethanol extract shown effective inhibition of pathogenic bacteria B.cereus, B. subtilis, S. aureus, E. aerogenes, E. coli, and K. pneumonia with inhibition zone of 15 mm, 21.63 mm, 24.66 mm, 18.32 mm, 22.62 mm, 17.67 mm respectively (Table 3, Fig. 3).
Table 3. Antibacterial activity of solvent plant extracts against bacteria
| Sl. No | Name of the plant | Solvent extracts | Inhibition of Bacteria in mm | |||||||
| Gram Positive Bacteria | Gram negative Bacteria | |||||||||
| B.cereus | B. subtilis | S. aureus | S. pyogenes | E. aerogenes | E.coli | P. mirabilis | K. pneumonia | |||
| 1 | Adina cordifolia | Petroleumether | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 |
| Chloroform | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Ethyl acetate | 0.00 ± 0.0 | 0.00 ± 0.0 | 15.31± 0.02** | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Ethanol | 15.33 ± 0.02** | 0.00 ± 0.0 | 23.61 ± 0.02** | 0.00 ± 0.0 | 0.00 ± 0.0 | 14.63 ± 0.05** | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| 2 | Cassia angustifolia | Petroleumether | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 |
| Chloroform | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Ethyl acetate | 0.00 ± 0.0 | 0.00 ± 0.0 | 22.31 ± 0.02** | 0.00 ± 0.0 | 0.00 ± 0.0 | 13.65 ± 0.03* | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Ethanol | 15 ± 0.17** | 21.63 ± 0.05** | 24.66 ± 0.06*** | 00.00 | 18.32 ± 0.03** | 22.62 ± 0.06** | 0.00 ± 0.0 | 17.67 ± 0.07** | ||
| 3 | Gentamycin | 28.60 ± 0.11*** | 24.29 ± 0.09*** | 27.26 ± 0.07*** | 21.60 ± 0.11** | 25.59 ± 0.08*** | 26.53 ± 0.06*** | 22.21 ± 0.09** | 28.21 ± 0.10*** | |
Note: Asterisk marks *, **, *** indicate significance of values at P 6 0.05 level according to ANOVA, Tukey’spost test.
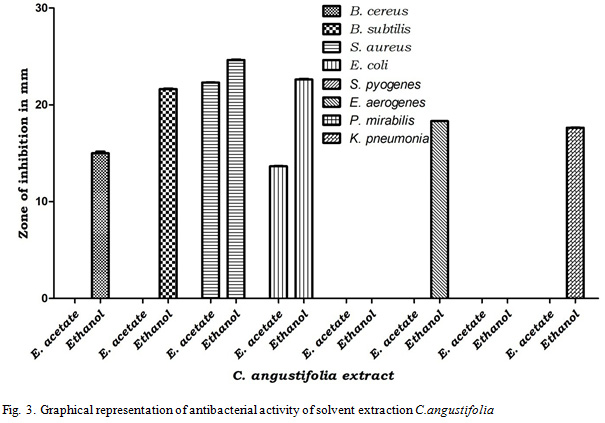 |
Figure 3: Graphical representation of antibacterial activity of solvent extraction C.angustifolia |
Antifungal activity of solvent extracts
Results of antifungal activity revealed that solvent extracts of Cassia angustifolia was more effective than Adina cordifolia. Out of tested four solvent extractions ethyl acetate and ethanol extractions were effective against tested pathogens whereas petroleum ether and chloroform not shown any inhibition against any tested pathogens. Ethanol extract of Cassia angustifolia was more effective with inhibition zone of 14.33 mm (Fusarium oxysporum), 20.63 mm (Aspergillusniger), 22.93 mm (Curvularialunata) and 10.16 mm (Alternaria alternata) and ethyl acetate extract shown that 9.2 mm (Fusarium oxysporum), 15.43 mm (Aspergillusniger), 17.53 mm (Curvularialunata) and 14.26 mm (Alternaria alternata). Ethanol extract of Adina cordifolia has shown inhibition zone of 8.9 mm (Fusarium oxysporum), 10.00 mm (Aspergillusniger) and 8.00 mm (Curvularialunata) whereas ethyl acetate extract of Adina cordifolia was effective only against Fusarium oxysporum and Alternaria alternata with inhibition zone of 7.03 mm and 8.76 mm respectively (Table 4, Fig. 4, 5).
Table 4. Antifungal activity of solvent plant extracts against fungi
| Sl. No | Name of the plant | Solvent extracts | Percentage inhibition of Fungi | |||
| Fusarium oxysporum | Aspergillusniger | Curvularialunata | Alternaria alternata | |||
| 01 |
Adina cordifolia |
Petroleumether | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 |
| Chloroform, | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Ethyl acetate | 7.03 ± 0.20* | 0.00 ± 0.0 | 0.00 ± 0.0 | 8.76 ±0.18* | ||
| Ethanol | 8.9 ± 0.11* | 10.00 ± 0.18* | 08 ± 0.15* | 0.00 ± 0.0 | ||
| 2 |
Cassia angustifolia |
Petroleumether | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 |
| Chloroform, | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Ethyl acetate | 9.2 ± 0.17* | 15.43 ± 0.20** | 17.53 ± 0.20** | 14.26 ± 0.12** | ||
| Ethanol | 14.3 ± 0.20** | 20.63 ± 0.17** | 22.93 ± 0.14*** | 10.16 ± 0.17* | ||
Note: Asterisk marks *, **, *** indicate significance of values at P 6 0.05 level according to ANOVA, Tukey’spost test
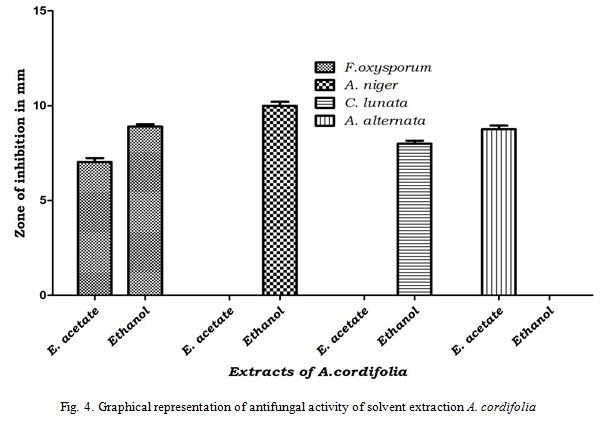 |
Figure 4: Graphical representation of antifungal activity of solvent extraction A. cordifolia |
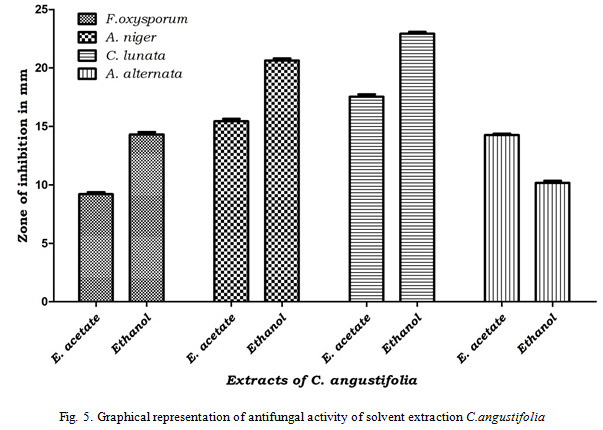 |
Figure 5: Graphical representation of antifungal activity of solvent extraction C.angustifolia |
Natural products such as botanical extracts have bioactive principles which are produced as secondary metabolites; their main function is to protect host plants from pathogenic microbial invaders which affect the health of the host plants. As science and research has progressed, the scientific community has found to be aware of antimicrobial activity of botanicals and started to explore active principles of secondary metabolites from the plants. As a result of this huge number of plants around the world found to have antimicrobial properties, (Bhawasar et al., 1965, Behl and Srivastava, 2002, Chopra and Chopra, 1969, Iyengar, 1981, Mehmood et al., 1997, Grainger Bisset, 2000, Radhakrishnan et al., 1976, Shah and Qadry, 1971, Zafar, 1994 and Dahanukar and Hazra, 2009).
In the present investigation in vitro study was carried out to evaluate the aqueous and solvent extractions five medicinal plants against pathogenic bacteria and fungi. In aqueous extracts significant result was observed in C. angustifolia, it has inhibited B. cereus with 21.55 mm of inhibition zone followed by B. subtilis (19.47 mm), E. coli (16.32 mm), S. aureus (15.76 mm), another plant A. cordifolia has shown inhibition of B. Cereus (15.52 mm), S. aureus (10.49 mm) and E. coli (11.32 mm) and L. coromandelica was effective only against B. subtilis (11.65 mm). Whereas, C. arborea and H. benghalensis not shown any activity against any bacteria. When aqueous extracts of plants tested against fungi only C. angustifolia shown effective results against F. oxysporum with inhibition percentage of 60%. Among solvent extracts ethyl acetate extraction of A. cordifolia was effective against S. aureus with inhibition zone of 15.31 mm and C. angustifolia against S. aureus and E. coli, with inhibition zone of 22.31 mm and 13.65 mm respectively. Ethanol extract of A. cordifolia was effective against B. cereus, S.aureus and E. coli with inhibition zone of 15.33 mm, 23.61 and 14.63 mm and C. angustifolia against S.aureus24.66 mm. whereas against A. niger, C. lunata and A. alternata not shown much inhibition, inhibition percentage are 11.20 %, 9.00% and 13.16% respectively. Some of the earlier reports corroborates with antibacterial and antifungal activity of extracts of the botanicals(Chen and Dai, 2012, de Saravia and Gaylarde, 1998, Goussous et al., 2013). Also, other studies also confirms the antimicrobial capability of C. angustifolia of various extracts against S. mercescens, A. junni, E. cloacae, Aspergillus niger, Aspergillus terreus, Aspergillus flavus, and Aspergillus fumigates, (Al-Marzoqi et al., 2016, Ahmed et al., 2016).
Based on the results of the aqueous extracts the plants which given significant results, two plants were selected for further solvent extraction, the results revealed that only ethyl acetate and ethanol solvent extraction showed activity where as others not shown any activity on any tested pathogens either on bacteria or fungi. Ethyl acetate extraction of A. cordifolia was only effective against S.aureus with inhibition zone of 15.31 mm whereas ethyl acetate extraction of C. angustifolia was effective against S.aureus and E. coli, with inhibition zone of 22.31 mm and 13.65 mm. Ethanol extract of A. cordifolia was effective against B.cereus, S.aureus and E. coli with inhibition zone of 15.33 mm, 23.61 and 14.63 mm whereas ethanol extract of C. angustifolia was effective against maximum tested bacteria except S. pyogenes and P. mirabilis and shown maximum inhibition zone of 24.66 mm against S.aureus and 22.62 mm, 21.63 mm 18.32 mm, 17.67 mm and 15.00 mm against E. coli, B. subtilis, E. aerogenes, K. pneumonia and B. cereus respectively. There are earlier reports that solvent extracts of botanicals has antibacterial activity, (Abas et al., 2006, Shan et al., 2007, Kang et al., 2011, Hosamath, 2011, Parveen and Shahzad, 2011,).
In the antifungal activity of solvent extraction against ethyl acetate and ethanol extractions shown activity whereas petroleum ether and chloroform not shown any activity against all tested phytopathogenic fungi. Ethyl acetate extraction of A. cordifolia shown inhibition of F. oxysporum and A. alternate with inhibition zone of 7.03 mm and 8.76 mm whereas ethyl acetate extraction of C. angustifolia was effective against all the tested fungi with inhibition zone of 17.53 mm (C. lunata), 14.26 mm (A. alternata) and 9.2 mm (F. oxysporum). Ethanol extraction of both plants shown effective inhibition of the phytopathogenic fungi. Ethanol extraction of A. cordifolia was effective against F. oxysporum with inhibition zone of 8.9 mm, A. niger (10.00 mm) andC. lunata (8.00 mm). Ethanol extract of C angustifolia has shown more effective than any other tested solvents with inhibition zone of 22.93 mm against C. lunata, A. niger (20.63 mm), F. oxysporum (14.3 mm) and A. alternata (10.16 mm).
The present investigation came out with medicinal plant C. angustifolia which was effective against both tested bacteria and fungi in both aqueous extracts and solvent extractions. There were many reports of medicinal values of C. angustifolia from various researchers. The plant has been in use as traditional medicine, (Wu et al., 2009), the plant is also used in the treatment of amoebic dysentery, typhoid, cholera, anemia(Siddique et al., 2010b, Wu et al., 2009, Parveen and Shahzad, 2011).
CONCLUSION
The study confirms the antimicrobial potential of leaf extracts of Adina cordifolia, Careya arborea, Cassia angustifolia, Hiptage benghalensis and Lannea coromandelica and thereby confirms their traditional medicinal use. Bioprospection of ethnomedicinal plants paves a way for the identification of natural and novel drugs. Further identification and characterization of biologically actives principles needs to be done which may lead to the discovery of potent antimicrobial agents.
ACKNOWLEDGEMENT
Authors are grateful to University of Mysore for providing all the necessary facilities to carry out this research.
CONFLICT OF INTEREST
Authors do not have any conflict of interest
FUNDING SOURCES
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
AUTHORS’ CONTRIBUTIONS
Kh. Tahamtan and M.S Sharada developed ideas and drafted the manuscript. Dr.M.S Sharada provided the required facilities. Kh. Tahamtan conducted the experiments. Kh .Tahamtan and Dr. M.S Sharad had contributed to the design and analysis of data. Authors contributed to revise the manuscript and approved the final version for publication.
REFERENCES
Abas, F., Lajis, N. H., Israf, D., Khozirah, S. & Kalsom, Y. U. 2006. Antioxidant And Nitric Oxide Inhibition Activities Of Selected Malay Traditional Vegetables. Food Chemistry, 95, 566-573.
Adebajo, A., Adewumi, C. & Essein, E. Anti-Infective Agents Of Higher Plants. International Symposium Of Medium Plants. 5th Ed. University Of Ife, Nigeria, 1983. 152-158.
Ahmad, I., Mehmood, Z. & Mohammad, F. 1998. Screening Of Some Indian Medicinal Plants For Their Antimicrobial Properties. Journal Of Ethnopharmacology, 62, 183-193.
Ahmed, S. I., Hayat, M. Q., Tahir, M., Mansoor, Q., Ismail, M., Keck, K. & Bates, R. B. 2016. Pharmacologically Active Flavonoids From The Anticancer, Antioxidant And Antimicrobial Extracts Of Cassia angustifolia Vahl. Bmc Complementary And Alternative Medicine, 16, 460.
Al-Marzoqi, A. H., Hadi, M. Y. & Hameed, I. H. 2016. Determination Of Metabolites Products By Cassia angustifolia And Evaluate Antimicobial Activity. Journal Of Pharmacognosy And Phytotherapy, 8, 25-48.
Arya, R., Lehman, D., Hunt, K. J., Schneider, J., Almasy, L., Blangero, J., Stern, M. P. & Duggirala, R. Evidence For Bivariate Linkage Of Obesity And Hdl-C Levels In The Framingham Heart Study. BMC Genetics, 2003. Biomed Central, S52.
Asolkar, L., Kakkar, K., Chakre, O., Chopra, R. N., Nayar, S. & Chopra, I. C. 1992. Glossary Of Indian Medicinal Plants, Publications & Information Directorate.
Behl, P. N. & Srivastava, G. 2002. Herbs Useful In Dermatological Therapy, CBS Publishers & Distributors.
Bhawasar, G., Guru, L. & Chadda, A. 1965. Antibacterial Activity Of Some Indigenous Medicinal Plants. Medicine And Surgery, 5, 11-14.
Cartea, M., Francisco, M., Lema, M., Soengas, P. & Velasco, P. 2010. Resistance Of Cabbage (Brassica oleracea Capitata Group) Crops To Mamestra Brassicae. Journal Of Economic Entomology, 103, 1866-1874.
Chen, Y. & Dai, G. 2012. Antifungal Activity Of Plant Extracts Against Colletotrichum lagenarium, The Causal Agent Of Anthracnose In Cucumber. Journal Of The Science Of Food And Agriculture, 92, 1937-1943.
Chopra, R. N. & Chopra, R. N. 1969. Supplement To Glossary Of Indian Medicinal Plants.
Costa, R. M., Magalhães, A. S., Pereira, J. A., Andrade, P. B., Valentão, P., Carvalho, M. & Silva, B. M. 2009. Evaluation Of Free Radical-Scavenging And Antihemolytic Activities Of Quince (Cydonia oblonga) Leaf: A Comparative Study With Green Tea (Camellia sinensis). Food And Chemical Toxicology, 47, 860-865.
Dahanukar, S. & Hazra, A. 2009. Heal With Herbs.
De Saravia, S. G. G. & Gaylarde, C. C. 1998. The Antimicrobial Activity Of An Aqueous Extract Of Brassica Negra. International Biodeterioration & Biodegradation, 41, 145-148.
Farnsworth, N. 1973. Importance Of Secondary Plant Constituents As Drugs. Miller, Lawrence Peter. Phytochemistry.
Farnsworth, N. R. & Soejarto, D. D. 1985. Potential Consequence Of Plant Extinction In The United States On The Current And Future Availability Of Prescription Drugs. Economic Botany, 39, 231-240.
Gaudilliere, J.-P. 2019. From Crisis To Reformulation: Innovation In The Global Drug Industry And The Alternative Modernization Of Indian Ayurveda. Innovation Beyond Technology. Springer.
Goussous, S., Mas’ Ad, I., Abu El-Samen, F. & Tahhan, R. 2013. In Vitro Inhibitory Effects Of Rosemary And Sage Extracts On Mycelial Growth And Sclerotial Formation And Germination Of Sclerotinia sclerotiorum. Archives Of Phytopathology And Plant Protection, 46, 890-902.
Grainger Bisset, N. 2000. Max Wichtl Herbal Drugs And Phytopharmaceuticals. Boca Raton: Crc Press.
Hosamath, P. 2011. Evaluation Of Antimicrobial Activity Of Litsea glutinosa. Int J Pharm Appl, 2, 105-114.
Ibrahim, M. 1997. Anti-Microbial Effects Of Extract Leaf, Stem And Root Bark Of Anogeissus leiocarpus On Staphylococcus aureaus, Streptococcus pyogenes, Escherichia coli And Proteus vulgaris. J. Pharma. Devpt, 2, 20-30.
Iyengar, M. A. 1981. Study Of Crude Drugs, Manipal College Of Pharmaceutical Sciences.
Jamshiya, S. 2017. Formulation And Evaluation Of Herbal Skin Cream For Wound Healing. Rvs College Of Pharmaceutical Sciences, Coimbatore.
Kang, C.-G., Hah, D.-S., Kim, C.-H., Kim, Y.-H., Kim, E. & Kim, J.-S. 2011. Evaluation Of Antimicrobial Activity Of The Methanol Extracts From 8 Traditional Medicinal Plants. Toxicological Research, 27, 31.
Lopez, A., Hudson, J. & Towers, G. 2001. Antiviral And Antimicrobial Activities Of Colombian Medicinal Plants. Journal Of Ethnopharmacology, 77, 189-196.
Mehmood, Z., Ahmad, S. & Mohammad, F. 1997. Antifungal Activity Of Some Essential Oils And Their Major Constituents. Indian Journal Of Natural Products, 13, 10-13.
Nagavani, V. & Rao, T. R. 2010. Evaluation Of Antioxidant Potential And Qualitative Analysis Of Major Polyphenols By Rp-Hplc In Nymphaea nouchali Burm Flowers. International Journal Of Pharmacy And Pharmaceutical Sciences, 2, 98-104.
Parveen, S. & Shahzad, A. 2011. A Micropropagation Protocol For Cassia angustifolia Vahl. From Root Explants. Acta Physiologiae Plantarum, 33, 789-796.
Philip, K., Malek, S. N., Sani, W., Shin, S. K., Kumar, S., Lai, H. S., Serm, L. G. & Rahman, S. N. 2009. Antimicrobial Activity Of Some Medicinal Plants From Malaysia. American Journal Of Applied Sciences, 6, 1613.
Radhakrishnan, N., Hamsveni, R., Uma, R. & Thyagarajan, R. 1976. Antifungal Activity Of Medicinal Plants. J. Res. Ind. Med. Yoga And Homoeopathy, 11, 70-74.
Rastogi, R. P., Mehrotra, B., Sinha, S., Pant, P. & Seth, R. 1990. Compendium Of Indian Medicinal Plants: 1985-1989, Central Drug Research Institute And Publications & Information Directorate
Shah, C. & Qadry, J. 1971. A Textbook Of Pharmacognosy, Messrs BS Shah.
Shan, B., Cai, Y.-Z., Brooks, J. D. & Corke, H. 2007. The In Vitro Antibacterial Activity Of Dietary Spice And Medicinal Herb Extracts. International Journal Of Food Microbiology, 117, 112-119.
Sharma, R., Singh, B. & Singh, D. 2009. Ethnomedicinal, Pharmacological Properties And Chemistry Of Some Medicinal Plants Of Boraginaceae In India. Journal Of Medicinal Plants Research, 3, 1153-1175.
Siddique, A., Nair, G., Alam, M., Sack, D. A., Huq, A., Nizam, A., Longini, I., Qadri, F., Faruque, S. & Colwell, R. 2010a. El Tor Cholera With Severe Disease: A New Threat To Asia And Beyond. Epidemiology & Infection, 138, 347-352.
Siddique, I., Anis, M. & Aref, I. 2010b. In Vitro Adventitious Shoot Regeneration Via Indirect Organogenesis From Petiole Explants Of Cassia angustifolia Vahl.—A Potential Medicinal Plant. Applied Biochemistry And Biotechnology, 162, 2067-2074.
Sofowora, A., Ogunbodede, E. & Onayade, A. 2013. The Role And Place Of Medicinal Plants In The Strategies For Disease Prevention. African Journal Of Traditional, Complementary And Alternative Medicines, 10, 210-229.
Wu, Q. P., Wang, Z. J., Tang, L. Y., Fu, M. H. & He, Y. 2009. A New Flavonoid Glucoside From Cassia angustifolia. Chinese Chemical Letters, 20, 320-321.
Zafar, R. 1994. Medicinal Plants Of India, CBS Publishers & Distributors.


