Department of Biotechnology, Sant Hirdaram Girls College, Bhopal, 462030- (M.P), India.
Corresponding Author Email: darakhshan66@gmail.com
Article Publishing History
Received: 12/03/2025
Accepted After Revision: 25/06/2025
Arsenic contamination is a major global health concern, primarily due to its presence in drinking water from natural and industrial sources. Chronic arsenic exposure is associated with severe toxicity, particularly affecting the kidneys, which serve as a major site for arsenic accumulation and excretion. While the nephrotoxic effects of heavy metals such as cadmium, lead, and mercury are well-documented, arsenic-induced kidney toxicity remains less understood. Kidney Injury Molecule-1 (KIM-1) is a highly specific and non-invasive biomarker for renal injury, making it a valuable tool for assessing arsenic-induced nephrotoxicity. This study investigates the toxic effects of sodium meta-arsenate on human kidney ACHN cells by evaluating cytotoxicity and relative KIM-1 gene expression. ACHN cells were treated with 20 µM and 30 µM sodium meta-arsenate for 24 hours. Cytotoxicity was assessed microscopically and spectrophotometrically using the Crystal Violet assay, while KIM-1 gene expression was analyzed to determine arsenic-induced renal injury. The results demonstrated cytopathic effects in arsenic-treated cells. Gene expression analysis revealed that arsenic exerts a dose-dependent toxic effect on human kidney cells, as reflected by cytotoxicity and alterations in KIM-1 expression. This study highlights the significance of KIM-1 as a potential biomarker for arsenic-related kidney damage and underscores the need for further research into the molecular mechanisms underlying arsenic toxicity.
Evaluating KIM-1; Arsenic Contamination; Kidney Injury; Crystal Violet Assay.
Khan D. Evaluating KIM-1 as a Biomarker for Arsenic-Induced Nephrotoxicity: A Cytotoxic and Molecular Approach. Biosc.Biotech.Res.Comm. 2025;18(2).
Khan D. Evaluating KIM-1 as a Biomarker for Arsenic-Induced Nephrotoxicity: A Cytotoxic and Molecular Approach. Biosc.Biotech.Res.Comm. 2024;18(2). Available from: <a href=”https://shorturl.at/TnfJ4“>https://shorturl.at/TnfJ4</a>
INTRODUCTION
Arsenic contamination remains a critical global health concern, primarily due to its widespread presence in groundwater used for drinking and agriculture (Levin et al., 2025; Rai et al., 2023). Chronic exposure to inorganic arsenic, particularly through contaminated water sources, has been linked to a spectrum of adverse health outcomes, including carcinogenesis, cardiovascular disease, neurotoxicity, and nephrotoxicity (Ganie et al., 2023; Joardar et al., 2021). The kidneys, as primary organs for arsenic accumulation and excretion, are especially vulnerable to its toxic effects (Khaleda et al., 2025; Liu et al., 2021). While the nephrotoxic profiles of heavy metals such as cadmium, lead, and mercury have been extensively characterized, the molecular mechanisms underlying arsenic-induced renal injury remain comparatively underexplored (Mishra et al., 2022).
Previous studies also suggests that arsenic compounds disrupt mitochondrial function, induce oxidative stress, and impair autophagic and mitophagic pathways in renal tubular cells (Wan et al., 2021; Li et al., 2022). However, the identification of sensitive and specific biomarkers for early detection of arsenic-induced nephrotoxicity is still evolving. Traditional markers such as serum creatinine and blood urea nitrogen (BUN) often fail to detect subclinical or early-stage renal damage (Griffin et al., 2019; Vaidya et al., 2008).
Kidney Injury Molecule-1 (KIM-1) has emerged as a promising biomarker for proximal tubular injury due to its high specificity and early upregulation in response to nephrotoxic insults (Ichimura et al., 2004; Jana et al., 2022). KIM-1 is minimally expressed in healthy renal tissue but is markedly elevated in both urine and tissue following toxic or ischemic injury. Elevated urinary KIM-1 levels have been reported in populations exposed to environmental arsenic, suggesting its potential utility in detecting early, subclinical renal damage (Karmakova et al., 2021; Baro et al., 2025).
Although several in vivo studies have demonstrated arsenic-induced nephrotoxicity and the involvement of mitochondrial and oxidative stress pathways, there is a lack of in vitro data specifically evaluating the dose-dependent cytotoxicity of sodium meta-arsenate and its impact on KIM-1 gene expression in human renal epithelial cells (Ramadan et al., 2022; Song et al., 2025). Moreover, the mechanistic link between arsenic-induced cellular injury and KIM-1 upregulation remains insufficiently defined in human cell models.
To address this gap, the present study investigates the cytotoxic and molecular effects of sodium meta-arsenate on human renal ACHN cells. By employing both microscopic and spectrophotometric cytotoxicity assays and quantitative gene expression analysis of KIM-1, this study aims to elucidate the dose-dependent relationship between arsenic exposure and renal epithelial injury. The findings are expected to enhance our understanding of arsenic-induced nephrotoxicity and support the validation of KIM-1 as a sensitive and early biomarker for arsenic-related kidney damage. This work may also contribute to the development of improved screening tools for environmental nephrotoxins and inform public health strategies in arsenic-endemic regions.
MATERIAL AND METHODS
Cell Culture: Human renal adenocarcinoma (ACHN) cells, originally derived from a metastatic renal carcinoma, were obtained from the National Centre for Cell Science (NCCS), Pune. Culturing of cells have been done as per the procedure of Pal et al. (2017). Cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), sodium bicarbonate, and antibiotics. Cultures were grown in a humidified incubator at 37 °C with 5% CO₂ and subcultured twice weekly upon reaching 10–90% confluency.
Cytotoxicity Assay: Cytotoxicity was evaluated using the Crystal Violet assay as per protocol of Saotome et al. (1989). ACHN cells were seeded in 96-well plates and treated with 20 µM and 30 µM sodium meta-arsenate for 24 hours. Following treatment, cells were fixed with methanol and stained with 0.2% crystal violet in 20% methanol. Excess dye was washed off with water, and bound dye was solubilized using Sorenson’s buffer. Absorbance was measured at 540 nm using a spectrophotometer to determine cell viability.
RNA Isolation: Total RNA was extracted using TRIzol reagent as per the protocol of Rio et al. (2010). Briefly, cells were homogenized and lysed in TRIzol, followed by chloroform extraction and isopropanol precipitation. The RNA pellet was washed with 75% ethanol, air-dried, and resuspended in DEPC-treated water. RNA quality and concentration were determined spectrophotometrically at A260/280.
cDNA Synthesis and Real-Time RT-PCR: Reverse transcription of RNA into cDNA was performed as per the procedure of Carlini et al. (2010). The reaction included random primers and MultiScribe™ Reverse Transcriptase in a total volume of 20 µL. The thermal profile included incubation at 25 °C for 10 min, 37 °C for 120 min, and 85 °C for 5 min, followed by a hold at 4 °C. Gene expression was quantified by real-time PCR using TaqMan primer probes and master mix on a StepOnePlus™ Real-Time PCR System. GAPDH was used as the endogenous control. The thermal cycling conditions were 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 sec and 60 °C for 1 min. All samples were run in triplicate, and relative expression levels were calculated using the 2^–ΔCT method.
RESULT
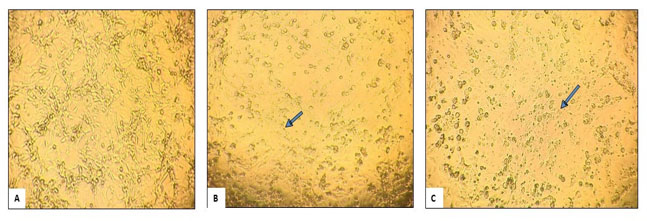
Cytopathic Effect: ACHN cell lines were cultured in DMEM supplemented with 10% fetal bovine serum (FBS). After 24 hours of incubation at 37 °C in a 5% CO2 atmosphere, the cells reached 80% confluence in the culture flask. Following this, the cells were treated with sodium meta-arsenate (NaAsO3) at concentrations of 20 µM and 30 µM, respectively. After another 24 hours, the cells were observed for cytopathic effects under an inverted microscope. In the control, healthy cells were visible. In the cells treated with 20 µM and 30 µM, cytopathic effects were observed. In the 30 µM, most cells were lysed, and the surviving cells appeared rounded and were mostly detached from the surface of the flask. In contrast, the 20 µM treatment showed some cytopathic effects, but to a lesser extent than the 30 µM treatment. Even at 20 µM, the morphology of the cells had altered compared to the control group. The number of living cells was also significantly lower in the 30 µM and 20 µM treated wells compared to the control. Figure 1 illustrates the cell morphology in the control and those treated with 20 µM and 30 µM sodium meta-arsenate, respectively. The arrows indicate the rounded dead cells.
Figure 1: Photomicrographs of ACHN cell lines exposed to Sodium meta-arsenate (NaAsO3):
(A) Control; (B) 20μM NaAsO3; (C) 30 μM NaAsO3
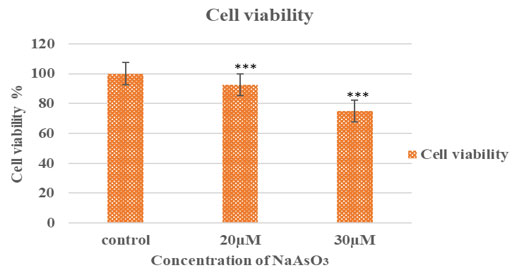
Crystal Violet Assay for Cell Viability: To determine the cytotoxic effects of sodium meta-arsenate on ACHN cells, a crystal violet staining assay was performed. Following microscopic observation, treated and control cells were stained, and optical density (O.D.) was measured at 540 nm to quantify the number of viable, adherent cells.As shown in Table 1, arsenic treatment significantly reduced cell viability in a dose-dependent manner. The mean O.D. value for the untreated control group was 2.449 ± 0.437, whereas cells treated with 20 µM and 30 µM sodium meta-arsenate showed significantly reduced O.D. values of 2.261 ± 0.013 and 1.832 ± 0.027, respectively (p < 0.001 for both compared to control).
Table 1. Optical density of ACHN cells treated with sodium meta-arsenate (NaAsO₃) measured at 540nm:
Data are presented as mean ± SEM.***p < 0.001 compared to control.
| S.No. | Concentration of NaAsO3 | O.D. at 540 nm Mean± SEM |
| 1. | Control | 2.44925±0.437124 |
| 2. | 20µM | 2.2605±0.013211*** |
| 3. | 30µM | 1.832±0.027057*** |
These reductions in optical density reflect a significant loss of adherent, viable cells following arsenic exposure. This trend is visually supported by Figure 2, which displays the stained wells: (1) control cells with dense crystal violet staining, (2) cells treated with 30 µM, and (3) cells treated with 20 µM sodium meta-arsenate (NaAsO3), showing noticeably reduced cell density and dye retention. The corresponding Figure 3 illustrates the percentage of cell viability relative to the untreated control group, further confirming the dose-dependent cytotoxic effect of sodium meta-arsenate in treated groups.
Figure 2: Crystal violet-stained ACHN cells: (A) Control, (B) 20 µM NaAsO₃, and (C) 30 µM NaAsO₃.
Reduced staining indicates decreased cell viability with increasing dose.
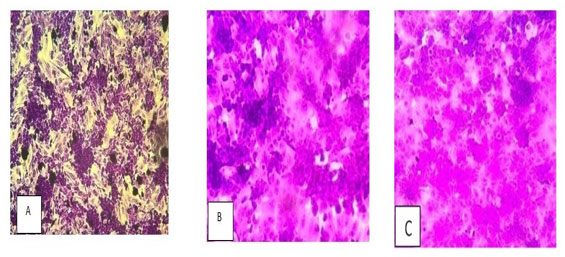
Figure 3: Cell viability of ACHN cells after sodium meta-arsenate treatment. ACHN cells were treated
with 20 µM and 30 µM NaAsO₃ for 24 hours. Cell viability was measured by crystal violet assay and
expressed as a percentage of control. Data represent mean ± SEM (***p < 0.001 vs. control).
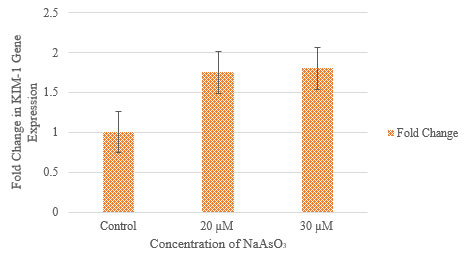
Gene expression studies: Quantitative real-time PCR (qRT-PCR) was performed to assess the expression of KIM-1 in ACHN cells following treatment with sodium meta-arsenate (NaAsO3) at concentrations of 20 µM and 30 µM. Expression levels were normalized to GAPDH, and relative fold changes were calculated using the 2^(-ΔΔCt) method with the control group as reference.
As shown in Table 2 and Figure 4, KIM-1 expression increased in a dose-dependent manner in response to NaAsO3 treatment. At 20 µM, the ΔCt value decreased to 10.599, corresponding to a ΔΔCt of –0.8052 and a 1.75-fold increase in KIM-1 expression compared to control. A further decrease in ΔCt was observed at 30 µM (ΔCt = 10.5518), resulting in a ΔΔCt of –0.8524 and a 1.80-fold increase. These results indicate that NaAsO₂ induces upregulation of KIM-1 expression in ACHN cells, suggesting a potential role in arsenic-induced cellular response.
Table 2. Relative Expression of KIM-1 Normalized to GAPDH in Response to Sodium Arsenate (NaAsO₂) Treatment.
ΔCt, ΔΔCt, and fold change were calculated using the 2^(-ΔΔCt) method relative to the control.
| Concentrations | GAPDH (Ct) | KIM-1 (Ct) | ΔCt | ΔΔCt | Fold Change |
| Control | 16.6149 | 28.0191 | 11.4042 | 0 (reference) | 1 |
| 20 µM NaAsO3 | 14.5119 | 25.1109 | 10.599 | -0.8052 | 1.75 |
| 30 µM NaAsO3 | 14.3836 | 24.9354 | 10.5518 | -0.8524 | 1.8 |
Figure 4: Effect of sodium meta-arsenate (NaAsO3) on KIM-1 gene expression in ACHN cells.
Concentration v/s Relative fold change in KIM-1 gene expression.
Error bars represent standard deviation (n=3).
DISCUSSION
Arsenic, a metalloid, is a naturally occurring environmental contaminant to which humans are frequently exposed through food, water, air, and soil. Approximately 200 million people worldwide have been exposed to high levels of arsenic in groundwater, particularly in regions of Mexico, the United States, and China (Podgorski and Berg, 2020). In some areas, such as parts of Taiwan and Bangladesh, arsenic concentrations have reached as high as 3,000 ppb (IARC, 2012). In India, significant examples of arsenic contamination can be found in Bihar, West Bengal, and various coastal regions, where it is estimated that nearly 230 million people have been drinking water contaminated with arsenic (Mondal et al., 2021; Banerjee et al., 2023; Biswas et al., 2023). The World Health Organization (WHO) recommends a limit of 10 μg/L for arsenic in drinking water (WHO, 2001), yet concentrations ranging from 50 to 3,200 μg/L have been reported (Sadee et al., 2025).
In our research, we investigated the cytotoxicity and molecular effects of sodium meta-arsenate (NaAsO3) on ACHN cells, a human renal carcinoma cell line. Our findings indicate that acute arsenic exposure (over 24 hours) leads to significant cytopathic changes, reduces cell viability in a dose-dependent manner, and upregulates KIM-1, a biomarker associated with early kidney injury and stress response. These results are consistent with those of Wei et al. (2022), who demonstrated that kidney-derived CAK cells from Cromileptes altivelis exhibit dose-dependent cytotoxicity when exposed to heavy metals like mercury, cadmium, and copper. Similarly, our study found that acute arsenic exposure induces notable cytopathic effects and decreases cell viability in a dose-dependent manner.
Additionally, we observed morphological changes in ACHN cells after treatment with NaAsO3, revealing progressive alterations. Cells treated with 20 μM exhibited early signs of cytopathic effects, including partial detachment and rounding. At 30 μM, these effects intensified, leading to widespread cell lysis and loss of adherence, indicating that NaAsO3 induces severe structural damage to renal epithelial cells. These qualitative findings are supported by de Almeida et al. (2023), who reported that alkylphenols caused dose-dependent cytotoxicity and structural damage in RTG-2 cells, marked by compromised membranes and oxidative stress. Notably, the optical density values decreased significantly at both 20 μM and 30 μM concentrations, suggesting substantial cytotoxicity. This aligns with prior studies indicating that arsenic exposure leads to cellular stress, mitochondrial dysfunction, and apoptosis in various renal and epithelial cell types (Cantoni et al., 2022; Liu et al., 2023).
The structural alterations we observed were accompanied by decreased cell viability and upregulation of KIM-1, a marker of early kidney injury. Molecular analysis revealed that KIM-1 expression significantly increased in response to arsenic exposure. The 2^(-ΔΔCt) analysis showed a 1.75-fold increase at 20 μM and a 1.80-fold increase at 30 μM, indicating a dose-responsive activation of this kidney injury marker. Similar mechanisms were noted in the study by Hu et al. (2025), where chromium-induced nephrotoxicity in rats was mediated through oxidative stress, inflammation, and apoptosis, with significant activation of the TLR4/MyD88, HMGB1/RAGE, and NF-κB pathways.
KIM-1 is a well-established biomarker for renal epithelial cell damage, commonly overexpressed during toxic or ischemic renal injury. Its upregulation in our study supports the hypothesis that arsenic induces nephrotoxic stress in ACHN cells and highlights the utility of KIM-1 as an early marker for arsenic-induced renal damage. Interestingly, while the increase in KIM-1 expression between 20 μM and 30 μM was relatively modest, the morphological damage and loss of cell viability were significantly more pronounced at the higher concentration. This suggests that KIM-1 induction may occur early in the injury process, even before extensive cell death, potentially serving as a predictive marker of cytotoxic progression. It is also possible that beyond a certain threshold, additional molecular pathways, such as oxidative stress, DNA damage, or apoptosis, are activated, contributing to more severe cytopathic outcomes.
Overall, our data provide mechanistic insights into the nephrotoxic effects of sodium meta-arsenate, reinforcing its potential to induce renal epithelial injury at sublethal concentrations. These findings are particularly relevant considering the environmental and occupational exposure risks associated with arsenic compounds, especially in regions with contaminated drinking water.
CONCLUSION
Sodium meta-arsenate exposure leads to significant cytotoxic and morphological alterations in ACHN cells, accompanied by dose-dependent upregulation of KIM-1. These results suggest that KIM-1 may serve as a sensitive biomarker for early detection of arsenic-induced renal injury. Further studies investigating oxidative stress markers, apoptotic pathways, and long-term gene expression responses will enhance our understanding of arsenic toxicity mechanisms in renal tissues.
Data availability: All data generated and analysed during this study are included in this published article.
Conflict of interest: None.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
ACKNOWLEDGEMENT
This work was conducted as part of my MSc dissertation at ICMR-National Institute of Occupational Health (NIOH), Ahmedabad, Gujrat. I thank my supervisor, Dr. Mugdha Tiwari (Scientist D, ICMR-NIOH) for guidance and necessary support. I am currently affiliated with Sant Hirdaram Girls College, Bhopal, India as an Assistant Professor.
REFERENCES
- Baro MR, Das M, Das L, Dutta A. (2025). Molecular docking, dynamics simulations, and in vivo studies of gallic acid in adenine-induced chronic kidney disease: targeting KIM-1 and NGAL. Journal of Computer-Aided Molecular Design, 39(1), 11.
- Ganie SY, Javaid D, Hajam YA, Reshi MS. (2024). Arsenic toxicity: sources, pathophysiology and mechanism. Toxicology Research, 13(1), tfad111.
- Griffin BR, Faubel S, Edelstein CL. (2019). Biomarkers of drug-induced kidney toxicity. Therapeutic drug monitoring, 41(2), 213-226.
- Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. (2004). Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. American Journal of Physiology-Renal Physiology, 286(3), F552-F563.
- Jana S, Mitra P, Roy S. (2022). Proficient novel biomarkers guide early detection of acute kidney injury: a review. Diseases, 11(1), 8.
- Joardar M, Das A, Mridha D, De A, Chowdhury NR, Roychowdhury T. (2021). Evaluation of acute and chronic arsenic exposure on school children from exposed and apparently control areas of West Bengal, India. Exposure and Health, 13, 33-50.
- Karmakova ТА, Sergeeva NS, Kanukoev КY, Alekseev BY, Kaprin АD. (2021). Kidney injury molecule 1 (KIM-1): a multifunctional glycoprotein and biological marker. Современные технологии в медицине, 13(3 (eng)), 64-78.
- Khaleda L, Alam MM, Tasnim Z, Ezaj MM, Apu MA, Akter R, Bakar MA, Alam MJ, Chowdhury RH, Datta A, Shawon II.(2025). Detrimental effects of chronic arsenic exposure through daily diet on hepatic and renal health: An animal model study. Toxicology Reports, 14, 101993.
- Levin R, Villanueva CM, Beene D, Cradock AL, Donat-Vargas C, Lewis J, Martinez-Morata I, Minovi D, Nigra AE, Olson ED, Schaider LA.(2024). US drinking water quality: exposure risk profiles for seven legacy and emerging contaminants. Journal of exposure science & environmental epidemiology, 34(1), 3-22.
- Li J, Guo C, Liu Y, Han B, Lv Z, Jiang H, Li S, Zhang Z.(2025). Chronic arsenic exposure-provoked biotoxicity involved in liver-microbiota-gut axis disruption in chickens based on multi-omics technologies. Journal of Advanced Research, 67, 373-386
- Li Z, Liu Z, Luo M, Li X, Chen H, Gong S, Zhang M, Zhang Y, Liu H, Li X. (2022). The pathological role of damaged organelles in renal tubular epithelial cells in the progression of acute kidney injury. Cell death discovery, 8(1), 239.
- Liu W, Wang B, Zhao Y, Wu Z, Dong A, Chen H, Lin L, Lu J, Hai X. (2021). Pharmacokinetic characteristics, tissue bioaccumulation and toxicity profiles of Oral arsenic trioxide in rats: Implications for the treatment and risk assessment of acute Promyelocytic leukemia. Frontiers in Pharmacology, 12, 647687.
- Mishra M, Nichols L, Dave AA, Pittman EH, Cheek JP, Caroland AJ, Lotwala P, Drummond J, Bridges CC. (2022). Molecular mechanisms of cellular injury and role of toxic heavy metals in chronic kidney disease. International Journal of Molecular Sciences, 23(19), 11105.
- Rai PK, Sonne C, Kim KH. (2023). Heavy metals and arsenic stress in food crops: Elucidating antioxidative defense mechanisms in hyperaccumulators for food security, agricultural sustainability, and human health. Science of The Total Environment, 874, 162327.
- Ramadan SS, Almeer R, Albasher G, Abdel Moneim AE. (2022). Lycopene mitigates arsenic-induced nephrotoxicity with activation of the Nrf2 pathway in mice. Toxin reviews, 41(2), 446-456.
- Rio DC, Ares M, Hannon GJ, Nilsen TW. (2010). Purification of RNA using TRIzol (TRI reagent). Cold Spring Harbor Protocols, 2010(6), pdb-prot5439.
- Song Z, Hei TK, Gong X. (2025). Tetramethylpyrazine attenuates sodium arsenate-induced acute kidney injury by improving the autophagic flux blockade via a YAP1-Nrf2-p62-dependent mechanism. International Journal of Biological Sciences, 21(3), 1158.
- Vaidya VS, Ferguson MA, Bonventre JV. (2008). Biomarkers of acute kidney injury. Annual Review of Pharmacology and Toxicology, 48, 463–493.
- Wan F, Zhong G, Wu S, Jiang X, Liao J, Zhang X, Zhang H, Mehmood K, Tang Z, Hu L. (2021). Arsenic and antimony co-induced nephrotoxicity via autophagy and pyroptosis through ROS-mediated pathway in vivo and in vitro. Ecotoxicology and Environmental Safety, 221, 112442.
- Pal D, Sharma U, Singh SK, Kakkar N, Prasad R. (2017). Inhibition of hTERT expression by MAP kinase inhibitor induces cell death in renal cell carcinoma. In Urologic Oncology: Seminars and Original Investigations, 35, 401-408.
- Saotome K, Morita H, Umeda M. (1989). Cytotoxicity test with simplified crystal violet staining method using microtitre plates and its application to injection drugs. Toxicology in vitro, 3(4), 317-321.
- Carlini F, Ridolfi B, Molinari A, Parisi C, Bozzuto G, Toccacieli L, Formisano G, De Orsi D, Paradisi S, Grober OM, Ravo M. (2010). The reverse transcription inhibitor abacavir shows anticancer activity in prostate cancer cell lines. PloS one, 5(12), e14221.
- Mondal D, Rahman MM, Suman S, Sharma P, Siddique AB, Rahman MA, Bari AF, Kumar R, Bose N, Singh SK, Ghosh A. (2021). Arsenic exposure from food exceeds that from drinking water in endemic area of Bihar, India. Science of the Total Environment, 754, 142082.
- Banerjee S, Dhar S, Sudarshan M, Chakraborty A, Bhattacharjee S, Bhattacharjee P. (2023). Investigating the synergistic role of heavy metals in Arsenic-induced skin lesions in West Bengal, India. Journal of Trace Elements in Medicine and Biology, 75, 127103.
- Biswas T, Pal SC, Saha A, Ruidas D. (2023). Arsenic and fluoride exposure in drinking water caused human health risk in coastal groundwater aquifers. Environmental Research, 238, 117257.
- Sadee BA, Zebari SM, Galali Y, Saleem MF. (2025). A review on arsenic contamination in drinking water: sources, health impacts, and remediation approaches. RSC advances, 15(4), 2684-2703.
- Podgorski J, Berg M. (2020). Global threat of arsenic in groundwater. Science, 368(6493), 845-850.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. (2012). Arsenic and arsenic compounds. In Arsenic, Metals, Fibres and Dusts. International Agency for Research on Cancer.
- Wei C, Yang X, Kang M, Cao Z, Sun Y, Zhou Y. (2022). An established kidney cell line from humpback grouper (Cromileptes altivelis) and its susceptibility to bacteria and heavy metals. Fish Physiology and Biochemistry, 48(3), 521-533.
- Liu S, Liu Y, Li J, Wang M, Chen X, Gan F, Wen L, Huang K, Liu D. (2023). Arsenic exposure-induced acute kidney injury by regulating sirt1/pink1/mitophagy axis in mice and in hk-2 cells. Journal of Agricultural and Food Chemistry, 71(42), 15809-15820.
- de Almeida W, Matei JC, Kitamura RS, Gomes MP, Leme DM, de Assis HC, Vicari T, Cestari MM. (2023). Alkylphenols cause cytotoxicity and genotoxicity induced by oxidative stress in RTG-2 cell line. Chemosphere, 313, 137387.
- Cantoni O, Zito E, Guidarelli A, Fiorani M, Ghezzi P. (2022). Mitochondrial ROS, ER stress, and Nrf2 crosstalk in the regulation of mitochondrial apoptosis induced by arsenite. Antioxidants, 11(5), 1034.


