1Faculty of Biotechnology, Ho Chi Minh city Open University, 35 Ho Hao Hon, Ho Chi Minh city, Vietnam
2Faculty of Applied Sciences, Ton Duc Thang University, 19 Nguyen Huu Tho street, Ho Chi Minh city, Vietnam
3Faculty of Biotechnology, Nguyen Tat Thanh University, 298A-300A Nguyen Tat Thanh Street, Ward 13, District 4, Ho Chi Minh 72820, Vietnam
Corresponding author email: buiphunamanh@yahoo.com
Article Publishing History
Received: 06/04/2020
Accepted After Revision: 21/05/2020
Cotton is valued for its fiber and requires yield improvement to compete with synthetic textiles and for sustainable cotton production. Cotton fiber production from seed coat epidermal cells can be categorized into two stages: initiation and development. While a great deal of research has been emphasized on the cotton fiber elongation and secondary cell wall biosynthesis, fiber initiation understanding is still in its infant stage due to the difficulty in studying the mechanism in cotton, namely lengthy transformation, lack of characterized mutants, and large and complex genomes. The Arabidopsis trichome, the most studied cell type, differentiates from the leaf epidermal cells and presents an excellent model system to elucidate the cotton fiber initiation mechanism. Knowledge gained from the initiation mechanism of Arabidopsis trichomes will facilitate, as a comparative model system, in understanding of the cotton fiber initiation mechanisms.
Trichome, fiber initiation, trimeric complex, diploid, tetraploid
Bui A. P. N, Tran T. T. H, Tran H-D. K. Employing Arabidopsis Trichome Model In Studying Fiber Initiation In Improving Cotton Yield. Biosc.Biotech.Res.Comm. 2020;13(2).
Bui A. P. N, Tran T. T. H, Tran H-D. K. Employing Arabidopsis Trichome Model In Studying Fiber Initiation In Improving Cotton Yield. Biosc.Biotech.Res.Comm. 2020;13(2). Available from: https://bit.ly/3d1Qira
Copyright © Bui et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Cotton, (Gossypium spp ) fiber is the primary material for a textile industry, and currently, there is an immense interest in understanding the process of fiber initiation and development. With the recently published reference genome sequences of cotton species, the cotton fiber initiation and development has become an essential field of study. Cotton fibers are unicellular trichomes originating from seed coat epidermal cells. Of these cells, approximately 30% are differentiated into fiber cells resulting in the production of roughly 20,000 fibers/ovule (Berlin, 1986). Increasing the number of fiber initials will result in additional fiber yield, which will ultimately benefit the cotton producers and allied industries A mere 10% increase in initials results in about a 30% increase in the final fiber yield, ( Chen et al., 2020, Patel et al., 2020).
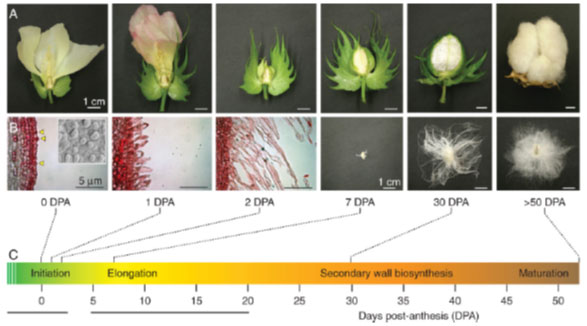
Cotton fibers, which are highly elongated and thickened cells, are one of the few cells in the plant kingdom that can significantly expand in size (up to 6.0cm) or composition during growth and development. These fibers, also known as seed trichomes, will quasi-synchronously undergo four distinct yet overlapping stages of development (Guan & Chen, 2013). Fiber initiation stage begins at approximately -3 days post-anthesis (DPA) to 5 DPA, in which ovular epidermal cells emerge and differentiate into fiber initials. Subsequently, from 3 to 21 DPA, morphologically-distinct fiber cells continue to expand up to 6 cm in length without further cell division (Wilkins & Arpat, 2005). From 14 to 40 DPA, a massive amount of cellulose is deposited which is known as the secondary cell wall biosynthesis stage. Finally, fiber cells mature at 50 to 60 DPA, and at this stage, the cotton fibers and seeds are ready for harvesting and industrial applications (Basra & Malik, 1984). Elucidating the molecular mechanism of fiber initiation will provide specific information on the genes involved in epidermal cell differentiation, and will facilitate the design of novel genetic and molecular strategies to improve the number of initials, thereby improving cotton fiber yield.
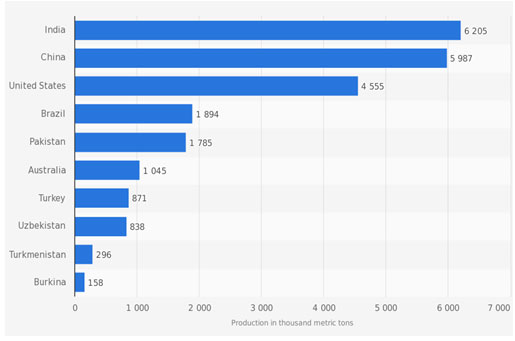
Figure 1: Leading cotton-producing countries worldwide in 2018/2019 (in 1,000 metric tons). Source: US Department of Agriculture 2019 (www.statista.com 2019)
Economic, Environmental And Scientific Importance Of Cotton
Cotton is an essential raw material used to produce numerous commodities, including textile fabrics, medical applications, fine paper, computer screens and automobile brakes; it is also used for cooking oil, cattle feed, and biodiesel fuel. Although additional commercial value can be captured from cottonseed and its associated products, the fundamental economic value originates from cotton fiber (Campbell & Hinze, 2010). Of over fifty documented species in the Gossypium genus (Wendel, 1989), four species (G. hirsutum, G. barbadense, G. arboreum, G. herbaceum) are widely cultivated around the world. They have had a significant impact on global trade and economy (Zhang & Feng, 2000). China, the United States (US), and India produce most of the world’s cotton comprising more than 15.9 million metric tons of cotton lint and 30.4 million metric tons of cottonseed, which was approximately equivalent to 22.8 billion and 6 billion dollars, respectively (Bowman et al., 2013). In 2019, these three countries contributed about 13.5 metric tons of cotton corresponding to 40 to 100 million US dollars annually, (Figure 1). Globally, the economic impact of the cotton industry is estimated to be $500 billion (US) per year, with more than 100 million families from approximately 150 countries directly or indirectly dependent on cotton crop (Bowman et al., 2013, USDA 2019 ).
Currently, the cotton fiber industry is facing fierce competition from companies producing synthetic textile fibers such as polyester, nylon, and polypropylene. Compared to cotton, synthetic fibers are not environmentally friendly as they are made from fossil fuel sources, are non-biodegradable, hydrophobic, burn and melt quickly posing health risks (http://www.barnhardtcotton.net/blog/know-fibers-cotton-vs-synthetic-fibers/). Besides, a recent study showed the presence of synthetic textile fibers in sea fish sold for human consumption (Rochman et al., 2015), thus raising concerns about direct effect on human health. Cotton fiber is not only environmentally friendly, but it also helps to clean environmental pollutants such as oil spills and leaks; for example, 1 gram of raw cotton can absorb 30.5gram of crude oil (Singh et al., 2013). Cotton fiber is one of the most critical cell types on earth, which has scientific, economic, and environmental significance; hence, there is a need to make cotton cultivation more profitable for sustainability and meet the demand of the growing world population.
Figure 2: Cotton (Gossypium) fiber initiation and elongation stages (Lee et al., 2007)
Phylogeny, Genetics, And Genomics of Cotton
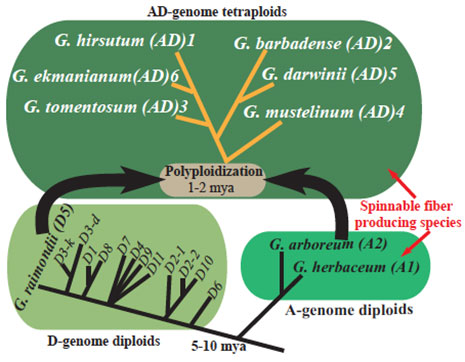
Approximately 1.5 million years ago, the spontaneous interspecific hybridization and genome duplication event of two formerly independent diploid genomes, (2n=2x=26): extant D- genome species closely related to G. raimondii (D5), and A- genome species related to G. arboreum (A2)/G. herbaceum (A1), resulted in allotetraploid species (2n=4x=52) (Wendel & Cronn, 2003). The ancestral A- species produce spinnable fibers while the D- species do not. The polyploidization and subsequent evolution resulted in the emergence of six tetraploid species (Figure 2): G. hirsutum (AD1), G. barbadense (AD2), G. tomentosum (AD3), G. mustelinum (AD4), G. darwinii (AD5) and recently described G. ekmanianum (AD6) (Grover et al., 2015). Among the six allotetraploids, Upland or American cotton, G.hirsutum, represents more than 95% of annual world cotton production, while the remaining 5% is primarily produced from Pima cotton, G. barbadense, known for its finer and longer fiber. Diploid cotton is cultivated in South Asia; however, it contributes as little as 2% to total global cotton production (Wendel et al., 2003).
Figure 3: Phylogenetic framework of cotton (Gossypium)diploid and tetraploid species.
Progress in Understanding the Role of Subgenomes in Fiber Initiation and Development Using Tetraploid Cotton
Though the ancestral D- diploid genome progenitors do not produce spinnable fibers, the QTL (Quantitative Trait Loci) mapping studies have showed that most of the QTLs influencing fiber quality and yield are located on DT subgenome of the tetraploid species. In contrast, differential expression of RNA transcripts during early stages of fiber development in tetraploid species show selective enrichment of AT subgenome specific genes, which is consistent with production of spinnable fibers in ancestral A- diploid species (Samuel Yang et al., 2006). Following this result, systematic mapping of fiber developmental genes in tetraploid species have demonstrated that more genes associated with fiber development are located on AT subgenome, while the DT subgenome provides more transcription factors which regulate the expression of the fiber genes in the AT subgenome (Xu et al., 2010).
In contrast, another study has showed that the ancestral D- genome provides many fiber genes after its hybridization with ancestral A- diploid species (Xu et al., 2015). Additionally, studies using mapped fiber gene-specific Simple Sequence Repeats (SSRs) indicate that both AT and DT subgenomes equally contribute to fiber traits (Han et al., 2006). Overall, the reviews are inconclusive on the subgenome contribution towards fiber development. Moreover, all these studies show expression bias or association of genes with fiber development but do not demonstrate the functional role of these genes. Hence, identification and characterization of individual genes involved in fiber development is essential to understand the specific contribution of different genes.
Since fiber initiation is a result of an interaction of different proteins to form active complex as well as activation of several downstream genes (Guan et al., 2007), a thorough understanding of this mechanism warrants a systematic and dedicated study. Current knowledge of the molecular mechanisms of cotton fiber initiation is in its infancy due to a complex and large genome, polyploidy, gene duplications, recalcitrance to genetic transformation (~1year), long growth cycles and lack of available (genetically characterized) mutants for functional studies (Pang et al., 2013). Of the total seed coat epidermal cells, approximately 30% are differentiated into fiber cells, which further complicate the isolation of pure fiber initial cells for the molecular analysis. To circumvent these complications, the Arabidopsis trichome has been successfully employed as a model system for functional characterization of cotton fiber initiation genes.
Arabidopsis Trichome Initiation is Regulated by Counteracting Positive and Negative Regulators
The first step in the trichome initiation process is the formation of an active trimeric complex composed of an R2R3-MYB, a basic helix-loop-helix (bHLH), and a WD40 protein abbreviated as MBW complex. Because of its simplicity, flexibility and plasticity, the MBW regulatory complex has been utilized extensively by plants (Ramsay & Glover, 2005). The MBW complex plays diverse roles in Arabidopsis such as anthocyanin production, stomatal-cell identity and root-hair formation (Walker et al., 1999). Emerging evidence suggests that the same mechanism as in Arabidopsis may control trichome formation in other plant species. For example, MYB-like genes from Mimulus guttatus and peach mediate trichome formation (Scoville et al., 2011; Vendramin et al., 2014); ectopic expression of a R3 MYB gene from Solanum lycopersicum in Arabidopsis results in glabrous phenotypes (Tominaga-Wada et al., 2013).
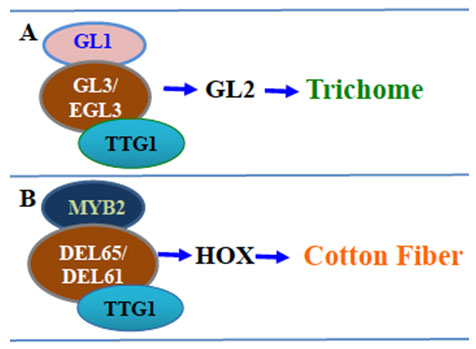
Figure 4: Two MBW complexes is proposed to initiate Arabidopsis trichome and cotton fiber (by authors) A.Trimeric complex involved in the Arabidopsis trichome initiation. B. Proposed model of trimeric complex involved in cotton fiber initiation.
In Arabidopsis, the trichome initiation is positively mediated by a trimeric complex composed of GLABRA1 (GL1) (Oppenheimer et al., 1991), GLABRA3 (GL3), which acts redundantly with its close homolog ENHANCER OF GLABRA3 (EGL3) (Payne et al., 2000), and TRANSPARENT TESTA GLABRA1 (TTG1) (Serna & Martin, 2006). This trimeric activator complex up-regulates the expression of GLABROUS2 (GL2) (Hülskamp, 2004) and a small family of single-repeat MYB proteins lacking the typical transcriptional activation domains, including TRIPTYCHON (TRY) (Schellmann et al., 2002), CAPRICE (CPC), ENHANCER OF TRY & CPC1 (ETC1, 2 and 3) (Tominaga et al., 2008) and TRICHOME-LESS (TCL) (Wang et al., 2007). The GL2 initiates the trichome patterning and differentiation while TRY, CPC, ETC1, 2, 3, TCL are six small, R3-single repeat MYB transcriptional regulators that repress trichome initiation from adjacent cells in a redundant manner
(Tominaga et al., 2008; Wester et al., 2009)
The regulation of trichome initiation is proposed to be mediated in a spatial and dose-dependent manner (Wester et al., 2009). Once a certain threshold level of activator MBW complex has been reached, expression of downstream regulators will be triggered, including positive regulators of trichome cell fate GL2 and inhibitory R3 MYB proteins (Zhao et al., 2008). The activation of GL2 mediates the trichome formation while the activated inhibitor proteins diffuse to adjacent cells and prevent them from becoming trichomes (lateral inhibition mechanism). The inhibitors, due to their smaller sizes and mobility, can laterally spread to neighboring cells to obstruct assembly of trimeric MBW activator complexes, thus preventing the adjacent cells from forming a trichome cell. The inhibitor proteins (TRY or CPC) prevent the formation of active MBW complex by competing with GL1, thus avoiding the trichome formation (Wester et al., 2009). Overall, Arabidopsis trichome is the most studied cell type with a wealth of information and resources that serves as an useful model system to study the cotton fiber initiation mechanism.
Composition of Cotton Fiber Initiation Protein Complex
The current method for rapid characterization of cotton fiber initiation genes is by complementation of cotton homologs in corresponding Arabidopsis mutants and by examining the trichome recovery phenotype (Guan et al., 2008; Guan et al., 2014; Li et al., 2017; Wang et al., 2013). Ectopic expression of MYB2 from G. arboreum, which is homologous to AtGL1, rescues trichomeless phenotype of the Arabidopsis gl1 mutant, confirming MYB2-A is a functional homolog of AtGL1 (Guan et al., 2014). Additionally, homologs of Arabidopsis GL3, TTG1, CPC, TRY, and GL2 have been isolated from G. arboretum (DEL65, TTG1, CPC, TRY, and HOX1, respectively) and functionally characterized using the Arabidopsis trichome model system (Guan et al., 2008; Wang et al., 2013). Formation of active trimeric complex is a prerequisite for the leaf epidermal cell differentiation into trichome in Arabidopsis (Li et al., 2017). Functional characterization and protein-protein interaction of the genes involved in cotton fiber initiation indicate a trimeric protein complex, similar to the Arabidopsis trimeric complex, is involved in cotton fiber initiation
(Figure 2) (Guan et al., 2008; Guan et al., 2014; Wang et al., 2013).
Despite the characterization of individual genes, there is currently no comprehensive understanding of the nature of the cotton trimeric complex and its function. The trichomes initiation follows defined pattern on Arabidopsis leaves while the cotton fibers appear randomly, with no design on the seed coat. The fundamental difference in the patterning mechanism remains unanswered due to lack of suitable tools.
Future Research on Fiber Patterning on Cotton Fiber Initiation
To address this fundamental question, creating a trimeric cotton complex in Arabidopsis is proposed. Currently, Arabidopsis mutant defective for one or two gene(s) is replaced with a cotton homolog for functional characterization of cotton fiber initiation genes. It essentially replaces only one component of Arabidopsis trimeric complex with a cotton homolog while retaining the other parts of the Arabidopsis complex. This approach proved to be highly useful to study individual genes. However, it still reflects the Arabidopsis trimeric complex in its interactions, or complex formation, or activation of downstream genes, which is evident from the patterned trichomes in the Arabidopsis lines complemented with cotton genes. As a result, creating cotton fiber initiation complex in Arabidopsis without trichome initiation complex will be a novel tool to comprehensively understand the molecular basis for lack of fiber patterning on cotton seed. Comparative studies will be performed with leaf trichome and fuzz fiber systems to understand the intrinsic differences in these systems leading to differential pattern formation within cotton.
CONCLUSION
Cotton fiber, also known as seed trichome, is differentiated from the seed coat epidermal cells similar to Arabidopsis leaf trichome, which is differentiated from the leaf epidermal cells. Knowledge gained from the initiation mechanism of Arabidopsis trichomes will facilitate, as a comparative model system in understanding of the cotton fiber initiation mechanisms. Despite functional characterization of individual cotton fiber initiation genes, currently, there is not a comprehensive understanding of the mechanism behind cotton fiber initiation. Though there is a great of deal of resemblance in initiation mechanism, there is a fundamental difference in the pattern formation of Arabidopsis trichomes and cotton fibers. The trichomes are well patterned on Arabidopsis leaves due to the lateral inhibition mechanism, while there is no apparent pattern in fiber formation on cotton seed. We aim to address the fundamental differences in the pattern formation by developing a novel tool, cotton trimeric complex in Arabidopsis. The mechanistic studies will have broader implications in fiber production as they will have tremendous applications in improving the fiber yield.
ACKNOWLEDGMENT
The authors would like to express their gratitude to Dr Venu Mendu for his advice in manuscript preparation.
REFERENCES
Basra, A. S., & Malik, C. P. (1984). Development of the Cotton Fiber. In G. H. Bourne, J. F. Danielli, & K. W. Jeon (Eds.), International Review of Cytology (Vol. 89, pp. 65-113): Academic Press.
Berlin, J. D. (1986). The outer epidermis of the cotton seed. In: J. R. Mauney, J. McD. Steward (Ed.). Cotton Physiology. USA: The Cotton Foundation, Publisher Memphis, 375-414.
Bowman, M. J., Park, W., Bauer, P. J., Udall, J. A., Page, J. T., Raney, J., . . . Campbell, B. T. (2013). RNA-Seq Transcriptome Profiling of Upland Cotton (Gossypium hirsutum L.) Root Tissue under Water-Deficit Stress. PLOS ONE, 8(12), e82634. doi:10.1371/journal.pone.0082634
Campbell, B. T., & Hinze, L. (2010). Cotton production, processing, and uses of cotton. In: B P. Singh. Industrial Crops and Uses. UK: CABI, 259–276.
Chen, Z. J., Sreedasyam, A., Ando, A., Song, Q., De Santiago, L. M., Hulse-Kemp, A. M., . . . Schmutz, J. (2020). Genomic diversifications of five Gossypium allopolyploid species and their impact on cotton improvement. Nature Genetics, 52(5), 525-533. doi:10.1038/s41588-020-0614-5
Grover, C. E., Gallagher, J. P., Jareczek, J. J., Page, J. T., Udall, J. A., Gore, M. A., & Wendel, J. F. (2015). Re-evaluating the phylogeny of allopolyploid Gossypium L. Molecular Phylogenetics and Evolution, 92, 45-52. doi:https://doi.org/10.1016/j.ympev.2015.05.023
Guan, X.-Y., Li, Q.-J., Shan, C.-M., Wang, S., Mao, Y.-B., Wang, L.-J., & Chen, X.-Y. (2008). The HD-Zip IV gene GaHOX1 from cotton is a functional homologue of the Arabidopsis GLABRA2. Physiologia Plantarum, 134(1), 174-182. doi:10.1111/j.1399-3054.2008.01115.x
Guan, X., & Chen, Z. J. (2013). Cotton Fiber Genomics. In: P. W. Becraft (Ed.). Seed Genomics. USA: Willey-Blackwell, 203-216.
Guan, X., Song, Q., & Chen, Z. J. (2014). Polyploidy and small RNA regulation of cotton fiber development. Trends in Plant Science, 19(8), 516-528. doi:10.1016/j.tplants.2014.04.007
Guan, X., Yu, N., Shangguan, X., Wang, S., Lu, S., Wang, L., & Chen, X. (2007). Arabidopsis trichome research sheds light on cotton fiber development mechanisms. Chinese Science Bulletin, 52(13), 1734-1741. doi:10.1007/s11434-007-0273-2
Han, Z., Wang, C., Song, X., Guo, W., Gou, J., Li, C., . . . Zhang, T. (2006). Characteristics, development and mapping of Gossypium hirsutum derived EST-SSRs in allotetraploid cotton. Theoretical and Applied Genetics, 112(3), 430-439. doi:10.1007/s00122-005-0142-9
Hülskamp, M. (2004). Plant trichomes: a model for cell differentiation. Nature Reviews Molecular Cell Biology, 5(6), 471-480. doi:10.1038/nrm1404
Lee, J. J., Woodward, A. W., & Chen, Z. J. (2007). Gene expression changes and early events in cotton fibre development. Annals of botany, 100(7), 1391-1401. doi:10.1093/aob/mcm232
Li, Y.-J., Zhu, S.-H., Zhang, X.-Y., Liu, Y.-C., Xue, F., Zhao, L.-J., & Sun, J. (2017). Expression and functional analyses of a Kinesin gene GhKIS13A1 from cotton (Gossypium hirsutum) fiber. BMC biotechnology, 17(1), 50-50. doi:10.1186/s12896-017-0373-2
Oppenheimer, D. G., Herman, P. L., Sivakumaran, S., Esch, J., & Marks, M. D. (1991). A myb gene required for leaf trichome differentiation in Arabidopsis is expressed in stipules. Cell, 67(3), 483-493. doi:10.1016/0092-8674(91)90523-2
Pang, J., Zhu, Y., Li, Q., Liu, J., Tian, Y., Liu, Y., & Wu, J. (2013). Development of Agrobacterium-Mediated Virus-Induced Gene Silencing and Performance Evaluation of Four Marker Genes in Gossypium barbadense. PLOS ONE, 8(9), e73211. doi:10.1371/journal.pone.0073211
Patel, J. D., Huang, X., Lin, L., Das, S., Chandnani, R., Khanal, S., . . . Paterson, A. H. (2020). The Ligon lintless-2 Short Fiber Mutation Is Located within a Terminal Deletion of Chromosome 18 in Cotton. Plant Physiology, 183(1), 277-288. doi:10.1104/pp.19.01531
Payne, C. T., Zhang, F., & Lloyd, A. M. (2000). GL3 encodes a bHLH protein that regulates trichome development in Arabidopsis through interaction with GL1 and TTG1. Genetics, 156(3), 1349-1362. Retrieved from https://pubmed.ncbi.nlm.nih.gov/11063707
Ramsay, N. A., & Glover, B. J. (2005). MYB–bHLH–WD40 protein complex and the evolution of cellular diversity. Trends in Plant Science, 10(2), 63-70. doi:10.1016/j.tplants.2004.12.011
Rochman, C. M., Tahir, A., Williams, S. L., Baxa, D. V., Lam, R., Miller, J. T., . . . Teh, S. J. (2015). Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Scientific Reports, 5(1), 14340. doi:10.1038/srep14340
Samuel Yang, S., Cheung, F., Lee, J. J., Ha, M., Wei, N. E., Sze, S.-H., . . . Jeffrey Chen, Z. (2006). Accumulation of genome-specific transcripts, transcription factors and phytohormonal regulators during early stages of fiber cell development in allotetraploid cotton. The Plant journal : for cell and molecular biology, 47(5), 761-775. doi:10.1111/j.1365-313X.2006.02829.x
Schellmann, S., Schnittger, A., Kirik, V., Wada, T., Okada, K., Beermann, A., . . . Hülskamp, M. (2002). TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. The EMBO journal, 21(19), 5036-5046. doi:10.1093/emboj/cdf524
Scoville, A. G., Barnett, L. L., Bodbyl-Roels, S., Kelly, J. K., & Hileman, L. C. (2011). Differential regulation of a MYB transcription factor is correlated with transgenerational epigenetic inheritance of trichome density in Mimulus guttatus. The New phytologist, 191(1), 251-263. doi:10.1111/j.1469-8137.2011.03656.x
Serna, L., & Martin, C. (2006). Trichomes: different regulatory networks lead to convergent structures. Trends in Plant Science, 11(6), 274-280. doi:https://doi.org/10.1016/j.tplants.2006.04.008
Singh, V., Kendall, R. J., Hake, K., & Ramkumar, S. (2013). Crude Oil Sorption by Raw Cotton. Industrial & Engineering Chemistry Research, 52(18), 6277-6281.
Tominaga-Wada, R., Nukumizu, Y., Sato, S., & Wada, T. (2013). Control of plant trichome and root-hair development by a tomato (Solanum lycopersicum) R3 MYB transcription factor. PLOS ONE, 8(1), e54019-e54019. doi:10.1371/journal.pone.0054019
Tominaga, R., Iwata, M., Sano, R., Inoue, K., Okada, K., & Wada, T. (2008). Arabidopsis CAPRICE-LIKE MYB 3 (CPL3) controls endoreduplication and flowering development in addition to trichome and root hair formation. Development, 135(7), 1335-1345. doi:10.1242/dev.017947
Vendramin, E., Pea, G., Dondini, L., Pacheco, I., Dettori, M. T., Gazza, L., . . . Rossini, L. (2014). A Unique Mutation in a MYB Gene Cosegregates with the Nectarine Phenotype in Peach. PLOS ONE, 9(3),
United States Department of Agriculture (2019) www.statista.com e90574. doi:10.1371/journal.pone.0090574
Walker, A. R., Davison, P. A., Bolognesi-Winfield, A. C., James, C. M., Srinivasan, N., Blundell, T. L., . . . Gray, J. C. (1999). The transparent testa glabra1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. The Plant cell, 11(7), 1337-1350. doi:10.1105/tpc.11.7.1337
Wang, G., Zhao, G.-H., Jia, Y.-H., & Du, X.-M. (2013). Identification and Characterization of Cotton Genes Involved in Fuzz-Fiber Development. Journal of Integrative Plant Biology, 55(7), 619-630. doi:10.1111/jipb.12072
Wang, S., Kwak, S.-H., Zeng, Q., Ellis, B. E., Chen, X.-Y., Schiefelbein, J., & Chen, J.-G. (2007). TRICHOMELESS1 regulates trichome patterning by suppressing GLABRA 1 in Arabidopsis. Development, 134(21), 3873-3882. doi:10.1242/dev.009597
Wendel, J. F. (1989). New World tetraploid cottons contain Old World cytoplasm. Proceedings of the National Academy of Sciences of the United States of America, 86(11), 4132-4136. doi:10.1073/pnas.86.11.4132
Wendel, J. F., & Cronn, R. C. (2003). Polyploidy and the evolutionary history of cotton. Advances in Agronomy, 78, 139-186.
Wester, K., Digiuni, S., Geier, F., Timmer, J., Fleck, C., & Hülskamp, M. (2009). Functional diversity of R3 single-repeat genes in trichome development. Development, 136(9), 1487-1496. doi:10.1242/dev.021733
Wilkins, T. A., & Arpat, A. B. (2005). The cotton fiber transcriptome. Physiologia Plantarum, 124(3), 295-300. doi:10.1111/j.1399-3054.2005.00514.x
Xu, Z., Yu, J., Kohel, R. J., Percy, R. G., Beavis, W. D., Main, D., & Yu, J. Z. (2015). Distribution and evolution of cotton fiber development genes in the fibreless Gossypium raimondii genome. Genomics, 106(1), 61-69. doi:https://doi.org/10.1016/j.ygeno.2015.03.002
Xu, Z., Yu, J. Z., Cho, J., Yu, J., Kohel, R. J., & Percy, R. G. (2010). Polyploidization Altered Gene Functions in Cotton (Gossypium spp.). PLOS ONE, 5(12), e14351. doi:10.1371/journal.pone.0014351
Zhang, B. H., & Feng, R. (2000). Cotton resistance to insect and pest-resistant cotton. Beijing: Chinese Agricultural Science and Technology Press.
Zhao, M., Morohashi, K., Hatlestad, G., Grotewold, E., & Lloyd, A. (2008). The TTG1-bHLH-MYB complex controls trichome cell fate and patterning through direct targeting of regulatory loci. Development, 135(11), 1991-1999. doi:10.1242/dev.016873