Department of Zoology, Maulana Azad College, Kolkata, West Bengal, India
Corresponding author email: rajarshi.ghosh@maulanaazadcollegekolkata.ac.in
Article Publishing History
Received: 15/12/2020
Accepted After Revision: 23/03/2021
The neurological disorders include Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease, stroke, epilepsy, brain tumours, multiple sclerosis etc. which are the leading health concerns in today’s world. The conventional therapies are not yet successful in treating these diseases because of the presence of intracellular and extracellular barriers across the central nervous system (CNS), which poses the major challenge of drug delivery to the CNS. The field of nanotechnology promises revolutionary advances of treating these devastating neuronal human disorders and has shown great potential to overcome the problems related to the conventional treatment approaches. Gold nanoparticles, micelles, quantum dots, polymeric nanoparticles, liposomes, microparticles, carbon nanotubes, fullerenes and several other types of nanoscale materials have been engineered and utilized for various purposes including improvement of diagnosis, delivery of neurotherapeutic agents, treatment-response assessment etc.
The nanomaterials cross those barriers, target specific cell or signalling pathway, respond to endogenous stimuli, act as a vehicle for gene delivery and also support nerve regeneration. Such frameworks may serve as effective drug delivery systems and can pave the way for effective treatments in the neuronal disorders.It has been found that the drugs encapsulated with nanomaterials have better efficacy in eradicating the diseases than the bulk materials used in conventional therapies. But there are several basic concerns related to the therapeutic approach of nanotechnology, including health issues and other problems because of the very small size of nanomaterials. This review mainly aims to focus on the barriers which guard the CNS, the nanomaterials as effective drug delivery systems, their preparation, mechanism of action, nanoformulations of different neuroprotective agents, nano-neurotoxicity and future perspectives.
Blood-Brain Barrier, Nanomaterials, Neuronal Disorders, Therapeutic Drugs
Ghosh R, Bhattacharjee P, Pal A. Emerging Applications of Nanotechnology in Neurological Disorders: Recent meta Review. Biosc.Biotech.Res.Comm. 2021;14(1).
Ghosh R, Bhattacharjee P, Pal A. Emerging Applications of Nanotechnology in Neurological Disorders: Recent Meta Review. Biosc.Biotech.Res.Comm. 2021;14(1). Available from: <a href=”https://bit.ly/3jqhyn6″>https://bit.ly/3jqhyn6</a>
Copyright © Ghosh et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
The term ‘Neurological Disorders’ refers to central and peripheral nervous system diseases including the brain, spinal cord, cranial nerves, peripheral nerves, nerve roots, autonomic nervous system (ANS), neuromuscular junction and muscles. These disorders include cerebrovascular diseases, Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, amyotrophic lateral sclerosis, multiple sclerosis, brain tumour, stroke, neuroinfections, autism spectrum disorder and schizophrenia (Chhabra et al., 2015). Unfortunately, many potent neuropharmaceuticals aimed at providing a treatment for such disorders proved inefficient in large scale clinical trials. The reason, at least in part, is the unsuccessful delivery of substances to their targeted site of action inside the body. A wide spectrum of potential drugs has been investigated to treat several neurological disorders but their therapeutic success is still limited due to range of challenges (Sahoo et al., 2017).
The difficulty of crossing the peripheral barriers viz. the blood-brain barrier (BBB) and blood-cerebrospinal fluid barrier (BCSFB), particularly the BBB, is the key challenge in delivering therapeutic agents such as medicines, nucleic acids, proteins, imaging agents and other macromolecules to the CNS (Sahoo et al., 2017). Nanotechnology is an innovative and promising approach for delivering these neurotherapeutics across BBB. Although the assembly and use of nano-sized particles had taken place many years ago, nanomedicine was first established as an interdisciplinary science within the nineties of the last century. The nanotechnological approach was first framed within the 1950’s and soon the constitutive force to determine nanomedicine gained importance as a paramount section in science and medical treatments (Krukemeyer et al., 2015 Sohail et al., 2020).
In the last few decades, due to its nano-size range, its unique physico-chemical properties and ability to exploit surface engineered biocompatible and biodegradable nanomaterials, drugs loaded inside nanoparticles (Table 1) have shown great potentials for efficient drug delivery to CNS (Aso et al., 2019). These nanoparticles can be made through different approaches which are illustrated in Table 2. Nanotechnology will gain importance in the coming decade in medical field, as it has the capability to improve the quality of life of the patients having neuronal disorders (Sohail et al., 2020). After the successful implementation of the strategies in nanotechnology, the growth of the field of neural circuitry has exponentially accelerated (Sohail et al., 2020).
Recent years have witnessed an explosion of research studies in the field of nanotechnology which opens up new probabilities in drug delivery, theranostics, tissue engineering, magnetofection and gene therapy (Krukemeyer et al., 2015). The effectiveness of nanotechnology is now well established and it has carved path for new and very efficient systems for drug delivery even to the most inaccessible regions such as CNS (Kumar and Singh, 2015). In this review, we strive to explain the applications of nanotechnology in neurological disorders by identifying the key principles, concepts and techniques, which will lead to further understanding in this topic and will call for much more research (Naqvi et al., 2020).
Blood Brain Barrier (BBB) and Blood Cerebrospinal Fluid Barrier (BCSFB): The brain has a very dense microvasculature with the average distance between the blood capillaries to be around 40 microns suggesting that each cell in the brain might have its own capillary (Duvernoy et al., 1983). The diffusion distances from nearest capillary to a neuron are approximately 10-20 nm (Schlageter et al., 1999). The epithelial cells of the choroid plexus (CP) contain tight junctions which limits the penetration of substances from blood to CSF.
Table 1. Preparation of nanoparticles
| Sr. No. | Techniques Used | Preparation Procedure | Types of Nanoparticles |
| 1. | Solvent Evaporation | The polymer solutions are prepared in water-non-miscible, Organic volatile solvents (CHCl3, CHCl2, and C4H8O2). The Emulsion (o/w, w/o/w) undergoes evaporation of the solvent. The NPs are collected, washed, and lyophilized after ultracentrifugation | Poly (lactic-co-glycolic acid) (PLGA) nanoparticle isprepared by this method
(Reis et al., 2006) |
| 2. | Nanoprecipitation | A solution is prepared by dissolving polymer in water miscible organic solvent. For formation of colloidal suspension and its precipitation pipetting is done in stirring aqueous medium | Preparation of cyclosporine A loadedNPs
(Allemann et al., 1998) |
| 3. | Emulsification | The polymer is dissolved in partially water-soluble solvent in the presence of excess of water. This is then dissolved in aqueous solution having surfactant. Nano spheres or Nano capsules are produced depending on the concentration of oil and polymer | Doxorubicin (anti-cancer drug) loaded PLGA NPs is done by this method
(Yoo et al., 1999) |
| 4. | Salting Out | Drug and polymer are dissolved in a solvent(acetone). This is dissolved in an aqueous solution containing as calcium chloride or sucrose which acts as salting out agent and polyvinyl pyrrolidone acting as stabilizing agent. This forms o/w emulsion that is then diluted in excess water resulting in the production of Nano spheres | This technique is employed in formation of lipophilic drugs
(Memisoglu et al., 2003) |
| 5. | Supercritical Fluid Technology
|
In this process, rapid expansion of supercritical solution into liquid solvent (RESOLV) and rapid expansion of super critical fluids (RESS) was used | Submicron particles of cyclosporine, water
insoluble drug (Young et al., 2000) |
| 6. | Emulsion Polymerization | The monomer is dispersed in aqueous or organic non-soluble solvent followed by addition of surfactant. Polymerization is established either by adding an initiation molecule such as a free radical or by producing the radical by the monomer itself with the aid of radiation | Poly (styrene-methyl methacrylate)/SiO2composite NPs
(Mahdavian et al., 2007) |
| 7. | Ionic Gelation | A solution of a biodegradable polymer (chitosan or gelatin) and a di block polymer is produced and is then mixed with a solution of the drug to be incorporated. The molecules undergo electrostatic interactions resulting change of state form liquid to gel, the process is referred to as Gelation | Chitosan nanoparticles are produced by this process
(Memisoglu et al., 2003) |
Table 2. Nano approaches towards CNS drug delivery
| Sr No. | Nanoparticles | Description | Uses |
| 1. | Micelles
|
Micelles are the vesicles ranging from10 to 100 nm with outer hydrophilic portion and inner hydrophobic core (generally polypropylene glycols, phospholipids, fatty acids). They may be made up of either amphiphilic surfactants (non-polymeric micelles) or amphiphilic copolymers (polymeric micelles) | They help in the loading of hydrophobic drugsfor CNS delivery (Torchilin, 2007) |
| 2. | Polymeric Nanoparticles | Polymeric nanoparticles (10-100 nm) are solid colloidal dispersion of biocompatible, biodegradable polymers. These have a core of dense polymer and a hydrophilic outer covering to provide steric stability | Encapsulates lipophilic drugs which may be encapsulated, adsorbed or chemically attached to the surface (Sahoo et al., 2017) |
| 3. | Solid Lipid Nanoparticles (SLN) | They are aqueous colloidal nano-carrier system composed of lipids (triglycerides, fatty acids, steroids, etc.), introduced aqueous surfactant solution or water and generally solidify on cooling | Quercetin loaded to treat AD, Atazanavir loaded against HIV-encephalitis (Chattopadhyay et al., 2008) |
| 4. | Nano Emulsions | Nano emulsions (100-500nm) are o/w or w/o colloidal particulate systems which are made up of edible oils, surfactants and water. | Modification of nano emulsions helps in overcoming the BBB, helping in rapid distribution of drugs to peripheral sites, mainly the brain (Shah et al., 2013) |
| 5. | Dendrimers | Dendrimers have 3-dimensional symmetrical structure having an inner core from which there is a number of hyper branches, (‘generations’) with functional groups at the peripheral terminal surface to be easily functionalized with many ligands | Dendrimers are used for hydrophobic and hydrophilic drug delivery (Tripathy and Das, 2013; Sohail et al., 2020) |
| 6. | Carbon Nanotubes and Fullerenes | These are carbon allotropes which are characterized by a hollow structure and striking thermal, electrical and mechanical properties. Fullerenes are of two types- Spherical Fullerenes and Cylindrical Fullerenes or Nanotubes | These are successfully used in neuronal disorders like AD, PD, and ischemic stroke and in vivo in many diseases like bone implants, rheumatoid arthritis, osteoporosis, and cancer (Boridy et al., 2009) |
But due to its low resistance (Saito, 1983), few substances penetrate from the blood into the CSF. For example, Azidothymidine (AZT) enters CSF through the choroid plexus epithelium but is tightly restricted at the BBB. The entry of a substance into the CSF may not allow its penetration into the brain parenchyma. The circumventricular organs are separated from the rest of the brain by the unique presence of ependymal and glial cells (Abbott et al., 2006) and penetration of substances from CSF to brain parenchyma is facilitated through diffusion. The large distances create a diffusion barrier that can be referred to as the CSF-brain barrier (CBB). The arachnoid barrier (AB) cells are present in subarachnoid space filled with CSF, which surrounds the brain and spinal cord which may act as a barrier and restrict the penetration of substances into the CSF (Yasuda et al., 2013; Sohail et al., 2020).
Strategies for Drug Delivery into CNS: Delivery of drugs through invasive techniques causes a number of problems like immunological inflammatory reactions, damage nervous system and many others. On the contrary, non-invasive technique such as nano drug delivery ensures drug delivery without damaging BBB (Jain, 2007). In this procedure, the targeted drug delivery in required quantity could be achieved by encapsulating the drug within a carrier system specially nanoparticles by the technique of nanotechnology. The nanoparticles should have a sufficient tensile strength to remain in the circulation for a long period without getting degraded. The delivery system may be either polymer based or lipid based (Naqvi et al., 2020).
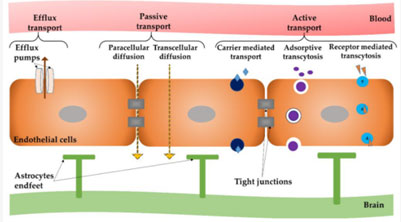
Mechanism of Action of Drug Release with the help of Nanomaterials: Nanomaterials possessing positive surface charge electrostatically interact with the negative surface charge of endothelial cells present in brain, and further the lipophilic nature of nano-carriers facilitates adsorption process. The nanoparticles get absorbed by getting access to low density lipoprotein receptors on the brain capillary endothelial cells following normal endocytosis and transcytosis. Desorption occurs and then it re-enters into the blood stream, then on the surface of the blood brain barrier, the drug loaded nano carrier releases the encapsulated or adsorbed drug and further diffuses into brain parenchyma. Either passive, gradient-dependent (passive targeting) or active, energy-dependent (active targeting) pathways can lead to selective entry into the brain (Figure 1). The nano-carriers having a size less than 500 Da undergo transcellular transportation (Georgieva et al., 2014).
Figure 1: Different ways of crossing the BBB. Source: (Teleanu et al., 2019)
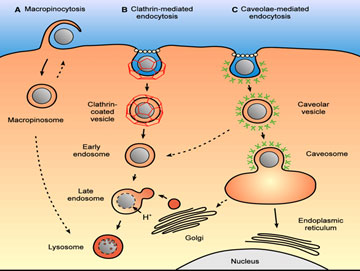
The nanoparticles can enter the cell through macropinocytosis, a vesicle mediated endocytosis or by phagocytosis which can be carried out through the following two pathways (Figure 2):
(i) Clathrin-Mediated Endocytosis: This mechanism occurs on all mammalian cells. The nano-carrier binds with a specific plasma membrane receptor, stimulating the polymerization of clathrin-1, a cytoplasmic protein just below the plasma membrane in order to form an inward budding leading to the engulf of cargo (Rappoport, 2008). The GTPase activity of dynamin pinches off the inward budding resulting into the formation of clathrin-coated vesicles. Actin helps in shedding of clathrin coat leading to the formation of early endosomes which deliver their content to late endosomes and finally to the lysosomes where it is degraded off. During the transition from late endosomes to lysosomes, the pH gradually decreases, causing the release of the drug from the nano-vehicle and finally releasing the drug at the target site (Georgieva et al., 2014).
(ii) Caveolar Pathway for Delivery in the Brain: This pathway escapes lysosomal delivery thus making it different from different from the clathrin-mediated pathway. Caveolae are flask-shaped invaginations in the plasma membrane and three isoforms of caveolin proteins are present in mammalian cells: caveolin-1, caveolin-2, and caveolin-3 helps in transportation through this pathway. The Nano carriers are internalized after binding to caveolar receptor forming a vesicular structure known as cavicle. The cavicle then is drived with the help of energy derived from actin and is ultimately fused with caveosomes which have neutral pH and then moves toward the endoplasmic reticulum penetrating into the cytosol and finally gaining access to the nucleus through the nuclear pore complex (Rappoport, 2008).
Figure 2: Macropinocytosis and phagocytosis pathways for drug delivery into brain. Source: (Hillaireau and Couvreur, 2009)
Applications of Nanotechnology in CNS Disorders: In Alzheimer’s disease, polyethylene glycol (PEG) stabilized nanomicelles made up of phospholipids inhibit Ab-aggregation and attenuate Ab-induced neurotoxicity in SHSY-5Y human neuroblastoma cell line in vitro. Microemulsion nanoparticles loaded with copper chelator d-penicillamine were found to have capability of crossing the BBB and dissolving the pre-existing Ab aggregates in vitro (Vinogradow et al., 2004). The nanoliposomes made up of curcumin not only inhibits Ab aggregation but also enhanced its bioavailability. Besides, Fullerene has a neuroprotective action, has an ability to inhibit Ab peptide fibrillization and prevention of Ab-induced cognitive impairments after intraventricular administration (Taylor et al., 2011). In Parkinson’s disease, the PEG and polyethylenimine nano gels complexes with antisense oligonucleotides can efficiently cross BBB in vitro. When injected intravenously, the oligonucleotides supply the brain more efficiently, particularly when the gels were functionalized with insulin (Mohanraj et al., 2013). The nerve growth factor (NGF) bound polybutylcyanoacrylate (PBCA) nanoparticles and L-Dopa encapsulated nanoparticles cross blood brain barrier (Siddiqi et al, 2018).
In Huntington’s disease, fullerenols have ability to clear free radicals and reduce oxidative stress to cell. Nitrendipine encapsulated in SLNs showed higher uptake of drug in comparison of bulk drug. Short-interfering RNA (siRNA) encapsulated cyclodextrin nanoparticles reduce expression of Huntingtin (HTT) mRNA both in vivo as well as in vitro (Huang et al., 2012). In multiple sclerosis, the interaction of carbon nanotubes along with stem cell is a way for tissue engineering to explore and add to cell behaviour. In a preclinical study, ciliary neurotrophic factor (CNTF) loaded microcapsules demonstrated in situ sustained delivery of CNTF upon encapsulation into polymers. This does not cause any immune response and cytotoxic effect (Godinho et al., 2013). In amyotrophic lateral sclerosis, a superoxide dismutase (SOD)-coated gold nanoparticle along with SOD1 aggregates is used as colorimetric detection system for ALS diagnosis. Sometimes carboxyfullerenenanotubes with SOD can be used. Carbon NPs may be used to effectively and precisely deliver riluzole, a glutamate inhibitor, to the affected sites (Klyachko et al., 2013; Alexander et al., 2019).
In brain tumour, nanoformulations like PBCA nanoparticles filled with methotrexate and temozolamide have resulted in increased intracerebral drug concentration as compared with free drugs. Solid lipid nanoparticles (SLNs) of etoposide and paclitaxel, in vitro, then enhanced inhibitory effect on glioma cell line proliferation was shown to be more effective than the free drug alone (Kohane et al., 2002). SLNs filled with carbamazepine and PLGA nanoparticles loaded with b-carotene are effective in epilepsy. In rat model, liposomal muscimol formulation is found to suppress focal seizures while producing minimal histological alterations (Brioschi et al., 2012). Xenon gas loaded liposomes were found to be successful in rat models administered for up to 5 h after the onset of stroke with an acceptable dosage range of 7-14 mg/kg (Peng et al., 2013). In neuro-AIDS, enhanced targeted delivery of Azidothymidine (AZT) to macrophages is possible using poly (hexylcyanoacrylate) NPs. Poly (hexylcyanoacrylate) NPs can also be used to deliver Saquinavir in human monocytes or macrophages (Chhabra et al., 2015; Alexander et al., 2019).
Nanotechnology Based Delivery of Neuroprotective Drugs: The biologically active and key phenolic constituent of turmeric, Curcumin (diferuloylmethane), obtained from the rhizomes of Curcuma longa, has shown considerable therapeutic efficacy in several diseases (Chattopadhyay et al., 2008). Being a natural antioxidant, curcumin has been found to possess many pharmacological activities including anti-inflammatory, antimicrobial, anticancer, the neuroprotective effect in neurodegenerative disorders, in both preclinical and clinical studies. Despite the wide medicinal applications of curcumin, due to low solubility, physico-chemical instability, poor bioavailability and quick metabolism, its clinical implications are hindered (Chattopadhyay et al., 2008). However, these problems can be resolved by developing efficient delivery systems with the help of nanotechnological approach. Compared to bulk curcumin, curcumin loaded PLGA-PEG nanoparticles, curcumin-loaded polysorbate 80 modified with some (CPC) nanoparticles showed better stability, longer circulation period and higher permeation of curcumin nanoformulation (Naksuriya et al., 2014; Alexander et al., 2019).
Among growth factors, Nerve Growth factors (NGFs) have great therapeutic potential for various CNS disorders. Vascular endothelial growth factor (VEGF) has been shown to participate in the process of post-ischemic brain repair via promoting neurogenesis and cerebral angiogenesis. Successful neuroprotection and promotion of vascular regeneration in the ischemic brain have therefore been achieved by treatment with modified liposomes with VEGF loaded transferrin. Edaravone (EDR), a well-known lipophilic drug, is used as a free radical scavenger for not only neurodegenerative disease, but also cardiovascular disease and cancer (Hudson et al., 2013). In preclinical studies, EDR has shown great efficiency against AD and cerebral aneurysm via oral administration, although oral bioavailability of EDR is very limited (Cruz, 2018). The lipid-based nanosystem (LNS) loaded with EDR was developed to promote its successful oral delivery by increasing the oral bioavailability (Alexander et al., 2019).
Neurotoxicity of Nanomaterials (Nano-Neurotoxicity): While invading the barriers within neural networks, doors are open not only for drug delivery but also to toxicity. The scope and size of toxic events is a part of the challenge in determining nanotoxicology. They interact heavily with components and pathways in both the biochemical environment of the cell and physiological system (Karmakar et al., 2014). Metal oxide NPs are highly useful in various fields such as medicine and engineering. However, these NPs have high chemical reactivity and toxicity as a consequence of their small size and large surface area. These NPs can accumulate in structures of the brain, such as the cerebellum and cortex (Valavanidis and Vlachogianni, 2016). For the use of multi walled carbon nanotubes (MWCNTs) as scaffolds, studies have inferred a substantial degree of genotoxicity (DNA interference) that is symptomatic of a broader problem posed by the use of nanomaterials. Hence, nanotoxicology profiling is a critical component of studies of nanomaterials (Kumar et al., 2017; Alexander et al., 2019).
CONCLUSION
In last few recent years brain-targeted drug delivery systems have been developed and gained large attention. Applications of nanotechnology have been developed in many fields in the last decade such as method of drug delivery, biological sensing, biomedical imaging, targeted anticancer drugs and antibiotic carriers. Within the realm of medicine, nanotechnology has found its way not only in improving drug delivery, but also in improving the required surgical procedures as seen in case of brain tumours. Compared to conventional implants that may cause neuroinflammation due to rigidity of the material, new polymeric implants are advantageous as they provide increased bioavailability with minimal or no neuroinflammation. Though several nanoformulations have shown great efficacy in preclinical and clinical studies, there are several basic concerns which should be addressed in the future to achieve the successful clinical translation of nanoformulations. The nanomaterials should be effective and safe in brain-targeted drug delivery systems, as well as they must be easily biodegradable. The approaches for the development of nanomaterials should be eco-friendly. The physico-chemical properties attached to nanomaterials must be evaluated carefully for the development of effective brain targeted drug delivery systems. To avoid complications associated with invasive routes a non- invasive alternative method for drug delivery should be developed. More studies are required on the basic level to increase the possibility of the use of nanoparticles in clinical settings.
ACKNOWLEDGEMENTS
All the authors in this manuscript have made substantial contributions towards conception, design, acquisition of data, analysis and interpretation of the data, participated in drafting the manuscript and revising it critically for important intellectual content. The authors of this manuscript would like to thank Prof. Subir Chandra Dasgupta, Head, Department of zoology, Maulana Azad College, Kolkata for his constant support and useful insight during the preparation of this review paper.
Conflict of Interest:The authors declare that there is no conflict of interests.
REFERENCES
Abbott NJ, Rönnbäck L and Hansson E (2006). Astrocyte-endothelial interactions at the blood-brain barrier. Nature Reviews Neuroscience, 7(1): 41-53.
Alexander A, Agrawal M, Uddin A, Siddique S, Shehata AM and Shaker MA (2019). Recent expansions of novel strategies towards the drug targeting into the brain. International Journal of Nanomedicine, 14: 5895-5909.
Allemann E, Leroux JC and Gurny R (1998). Polymeric nano-microparticles for the oral delivery of peptides and peptidomimetics. Advanced Drug Delivery Reviews, 34: 171-189.
Aso E, Martinsson I, Appelhans D, Effenberg C, Benseny-Cases N and Cladera J (2019). Poly (propylene imine) dendrimers with histidine-maltose shell as novel type of nanoparticles for synapse and memory protection. Nanomedicine and Nanotechnology, 17: 198-209.
Boridy S, Takahashi H, Akiyoshi K and Maysinger D (2009). The binding of pullulan modified cholesteryl nanogels to Ab oligomers and their suppression of cytotoxicity. Biomaterials, 30: 5583-5591.
Brioschi A, Zenga F, Zara GP, Gasco MR, Ducati A and Mauro A (2007). Solid lipid nanoparticles: could they help to improve the efficacy of pharmacologic treatments for brain tumors? Neurology Research, 29(3): 324-330.
Chattopadhyay N, Zastre J, Wong HL, Wu XY and Bendayan R (2008). Solid lipid nanoparticles enhance the delivery of the HIV protease inhibitor, atazanavir, by a human brain endothelial cell line. Pharmaceutical Research, 25: 2262-2271.
Chhabra R, Grabrucker AM and Tosi G (2015). Emerging use of nanotechnology in the treatment of neurological disorders. Current Pharmaceutical Design, 21: 3111-3130.
Cruz MP (2018). Edaravone (Radicava): a novel neuroprotective agent for the treatment of amyotrophic lateral sclerosis. Physical Therapy, 43: 25-28.
Duvernoy H, Delon S and Vannson JL (1983). The vascularization of the human cerebellar cortex. Brain Research Bulletin, 11: 419-480.
Georgieva J, Hoekstra D and Zuhorn I (2014). Smuggling drugs into the brain: an overview of ligands targeting transcytosis for drug delivery across the blood–brain barrier. Pharmaceutics, 6: 557-583.
Godinho BM, Ogier JR, Darcy R, O’driscoll CM and Cryan JF (2013). Self-assembling modified b-cyclodextrin nanoparticles as neuronal sirna delivery vectors: focus on Huntington’s disease. Molecular Pharmaceutics, 10: 640-649.
Hillaireau H and Couvreur P (2009). Nanocarriers’ entry into the cell: Relevance to drug delivery. Cellular and Molecular Life Sciences, 66(17): 2873-2896.
Huang YJ, Wu HC, Tai NH and Wang TW (2012). Carbon nanotube rope with electrical stimulation promotes the differentiation and maturity of neural stem cells. Small, 8: 2869-2877.
Hudson JS, Hoyne DS and Hasan DM (2013). Inflammation and human cerebral aneurysms: current and future treatment prospects. Future Neurology, 8: 663–676.
Jain K (2007). Nanobiotechnology-based drug delivery to the central nervous system. Neurodegenerative Diseases, 4: 287-291.
Karmakar A, Zhang Q and Zhang Y (2014). Neurotoxicity of nanoscale materials. Journal of Food and Drug Analysis, 22(1): 147-160.
Klyachko NL, Haney MJ and Zhao Y (2013). Macrophages offer a paradigm switch for CNS delivery of therapeutic proteins. Nanomedicine, 9:1403-1442.
Kohane DS, Holmes GL, Chau Y, Zurakowski D, Langer R and Cha BH (2002). Effectiveness of muscimol-containing microparticles against pilocarpine-induced focal seizures. Epilepsia,43: 1462-1468.
Krukemeyer MG, Krenn V, Huebner F, Wagner W and Resch R (2015). History and possible uses of nanomedicine based on nanoparticles and nanotechnological progress. Journal of Nanomedicine and Nanotechnology, 6(6): 1000336(1-7).
Kumar A and Singh A (2015). A review on Alzheimer’s disease pathophysiology and its management: an update. Current Pharmacology Reports, 67: 195-203.
Kumar A, Tan A, Wong J, Spagnoli JC, Lam J, Blevins BD, Natasha G, Thorne L, Ashkan K, Xie J and Liu H (2017). Nanotechnology for neuroscience: Promising approaches for diagnostics, therapeutics and brain activity mapping. Advanced Functional Materials, 19: 27-39.
Mahdavian AR, Ashjari M and Makoo AB (2007). Preparation of poly (styrene-methyl methacrylate) /SiO2 composite nanoparticles via emulsion polymerization. An investigation into the compatiblization. European Polymer Journal, 336-344.
Memisoglu E, Bochot A, Ozalp M, Sen M, Duchene D and Hincal A (2003). Direct formation of nanospheres from amphiphilic betacyclodextrin inclusion complexes. Pharmaceutical Research, 20: 117-125.
Mohanraj K, Sethuraman S and Krishnan UM (2013). Development of poly (butylene succinate) microspheres for delivery of levodopa in the treatment of Parkinson’s disease. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 101: 840-847.
Naksuriya O, Okonogi S, Schiffelers RM and Hennink WE (2014). Curcumin nanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials, 35: 3365-3383.
Naqvi S, Panghal A and Flora SJS (2020). Nanotechnology: A promising approach for delivery of neuroprotective drugs. Frontiers in Neuroscience, 14(494): 1-26.
Peng T, Britton GL, Kim H, Cattano D, Aronowski J and Grotta J (2013). Therapeutic time window and dose dependence of xenon delivered via echogenic liposomes for neuroprotection in stroke. CNS Neuroscience and Therapeutics, 19: 773-784.
Rappoport JZ (2008). Focusing on clathrin-mediated endocytosis. Biochemical Journal, 412: 415-423.
Reis CP, Neufeld RJ, Ribeiro AJ and Veiga F (2006). Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine, 2: 8-21.
Sahoo SK, Misra R and Parveen S (2017). Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine, 8: 73-124.
Saito Y and Wright EM (1983). Bicarbonate transport across the frog choroid plexus and its control by cyclic nucleotides. Journal of Physiology, 336: 635-648.
Schlageter KE, Molnar P, Lapin GD and Groothuis DR (1999). Microvessel organization and structure in experimental brain tumors: microvessel populations with distinctive structural and functional properties. Microvascular Research, 58: 312-328.
Shah L, Yadav S and Amiji M (2013). Nanotechnology for CNS delivery of biotherapeutic agents. Drug Delivery and Translational Research, 3: 336-351.
Siddiqi KS, Husen A, Sohrab SS and Yassin MO (2018). Recent status of nanomaterial fabrication and their potential applications in neurological disease management. Nanoscale Research Letters, 13(231):1-17.
Sohail I, Bhatti IA, Ashar A, Sarim FM, Mohsin M, Naveed R, Yasir M, Iqbal M and Nazir A (2020). Polyamidoamine (PAMAM) dendrimers synthesis, characterization and adsorptive removal of nickel ions from aqueous solution. Journal of Materials Research and Technology, 9: 498-506.
Taylor M, Moore S, Mourtas S, Niarakis A, Re F and Zona C (2011). Effect of curcumin-associated and lipid ligand-functionalized nanoliposomes on aggregation of the Alzheimer’s Ab peptide. Nanomedicine: Nanotechnology, Biology and Medicine, 7:541-550.
Teleanu DM, Negut I, Grumezescu V, Grumezescu AM and Teleanu RI (2019). Nanomaterials for drug delivery to the central nervous system. Nanomaterials, 9(371):1-18.
TorchilinVP (2007). Micellar nanocarriers: pharmaceutical perspectives. Pharmaceutical Research, 24(1): 1-16.
Tripathy S and Das MK (2013). Dendrimers and their applications as novel drug delivery carriers. Journal of Applied Pharmaceutical Science, 3: 142-149.
Valavanidis A and Vlachogianni T (2016). Engineered nanomaterials for pharmaceutical and biomedical products new trends, benefits and opportunities. Pharmaceutical Bioprocessing, 4(1): 13-24.
Vinogradov SV, Batrakova EV and Kabanov AV (2004). Nanogels for oligonucleotide delivery to the brain. Bioconjugate Chemistry, 15: 50-60.
Yasuda K, Cline C and Vogel P (2013). Drug transporters on arachnoid barrier cells contribute to the blood-cerebrospinal fluid barrier. Drug Metabolism and Disposition, 41(4): 923-931.
Yoo HS, Oh JE, Lee KH and Park TG (1999). Biodegradable nanoparticles containing PLGA conjugate for sustained release. Pharmaceutical Research, 16: 1114-1118.
Young TJ, Mawson S, Johnston KP, Henriksen IB, Pace GW and Mishra AK (2000). Rapid expansion from supercritical aqueous solution to produce submicron suspensions of water-insoluble drugs. Biotechnology Progress, 16(3): 402-407.