Department of Biotechnology, University Institute of Engineering and Technology,
Kurukshetra University, Kurukshetra–136119, Haryana, India
Corresponding author email: sunkhatak@rediffmail.com
Article Publishing History
Received: 30/10/2020
Accepted After Revision: 04/12/2020
Plants owe eminence in healing and curing dreadful diseases from vedic civilization.The present investigation includes Aegles marmelos (Baelpatra) and Terminalia chebula (Harad) as potential plant entities to be used against Staphylocoocus aureus. Methicillin Resistant S. aureus (MRSA) is major cause of nosocomial infections and are very difficult to cure because these strains are resistant to almost all clinically available antibiotics. Although present research did not employ MRSA strain being pathogenic but activity against susceptible strains offers propensity of these sacred plants to be exploited for bacterial conjunctivitis cure in India. The fruit pulp excluding seed and outer covering of Terminalia chebula and Aegle marmelos were used as plant part to prepare methanolic extracts using standard methods. The methanolic extracts were diluted two fold starting from a higher concentration of 250mg/ml to 0.97mg/ml and were tested against Staphylococcus aureus and P.aeruginosa using agar well diffusion assay.
In addition methanolic extract of leaf as plant part from both plants were also tested starting from a higher concentration of 10mg/ml to 1.25mg/ml using two fold dilution. DMSO was used as control.Antimicrobial analysis confirmed that the plant contains bioactive compounds that exhibit measurable antimicrobial activity against selected bacteria.The zone size of 24mm using Baelpatra and 26mm using Terminalia at a higher concentration of 250mg/ml reveal the significance of present work to be exploited against resistant strains of staphylococcus.Although the plants possess a number of pharmacological activities due to the presence of bioactive compounds, very little work has been done on this potential medicinal applications of fruit extracts of plant against the diseases particularly on multidrug resistant bacterial pathogens. Researchers need to exploit these medicinal plants as good candidates to overcome developing resistant of antibiotics to infectious disease which are caused by these microorganisms.
T. chebula, Antimicrobial, Zone of Inhibition, Conjunctivitis, MRSA, Aegle marmelos.
Khatak S, Wadhwa N, Jain P. Efficacy of Methanolic Extract of Fruit Pulp and Leaf of Terminalia chebula and Aegles marmelos Against Staphylococcus aureus. Biosc.Biotech.Res.Comm. 2020;13(4).
Khatak S, Wadhwa N, Jain P. Efficacy of Methanolic Extract of Fruit Pulp and Leaf of Terminalia chebula and Aegles marmelos Against Staphylococcus aureus. Biosc.Biotech.Res.Comm. 2020;13(4). Available from: https://bit.ly/3mYj00R
Copyright © Khatak et al., This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Aegle marmelos belongs to family Rutaceae and is one of the most important medicinal plants since times of Sage Charaka (1500B.C.). It is a native plant of India commonly called “Bael” and “Temple Garden Plant”. The leaves are used to worship Lord Shiva. The plant is a slow growing 25-30 feet tall tree. Flowers are greenish white in color with peculiar fragrance. The tree is subtropical plant growing well in dry forests, hilly and plain areas. It is found in India Ceylon, Sri Lanka, Pakistan, Malaysia, Nepal, Cambodia, Thailand and in almost all states of India. The plant has been attributed to have enormous ethno-medicinal applications in medical health care. Vedic literature has reported the plant to treat jaundice, constipation, bronchitis, snake-bite, abdominal discomfort, Spermatorrhoea, Leucoderma, eye disorders, and ulcers.
Its Fruit is a rich source of nutrition as the pulp consists of water, sugar, protein, fibers, fat, calcium, phosphorus, potassium, iron minerals, and vitamins A, B, and C. The leaves cause infertility or abortive action in women. The plant possesses antioxidant activity and aids in fighting against gastrointestinal and cardiovascular disorders. The plant has been cited to be antidiabetic, antimicrobial, and anti-inflammatory in literature (Sekar et al., 2011). Aegle marmelos is known to be enriched bioreserve of more than hundred phytoconstituents extracted from different plant parts namely terpenoids, steroids, phenols, flavonoid alkaloids, cardiac glycosides and saponins which in turn have been reported to be well known biological agents against chronic disorders and as boosters of immunity.
The leaves have been considered as a rich repository of phytochemicals in comparison to other conventional fruits and shows promising results for curing eye, ear, and skin infections. The dried fruit pulp has anti diarrheal activity and shows activity against several pathogens associated with dysentery. The aqueous extract of unripe fruit is recommended as a potent agent in fighting against Rota virus and Giardia. Methanolic extracts from leaves have been reported to have antidiabetic activity. Terminalia chebula commonly known as “Harad” belongs to family Combretaceae found in the forests of Northern India, Uttar Pradesh, Bengal and is very common in existence in Southern part of India. The plant is a medium to large sized tree distributed throughout tropical and subtropical Asia including China (Rao et al., 2011). The plant is widely consumed in China and is referred as “Tibet Olive”, the fruit peel revealed the presence of 29 compounds and was confirmed through Ultra High Performance Liquid Chromatography(Li et al, 2019).
Tribal people in Tamil Nadu, Karnataka routinely use Harad to cure several ailments like fever, cough, diarrhea, gastroenteritis, skin disease, urinary tract infection (Dash, 1991). The antimicrobial activity of this plant has been reported against several bacterial strains using fruit pulp (Dutta et al., 1998; Malckzadeh et al, 2001). The plant part (fruit pulp) has been tested against H. pylori, X. campestris, and S. typhi. The plant fruit pulp is also reported to be effective against a number of Dermatophytes and yeasts. The fruit of the plant possess complex antimicrobial compounds to cure diseases like digestive and cardiovascular ailments along with pathogenic bacteria (Bag et al., 2009). Our present research compared the potential of fruit pulp to evaluate antimicrobial activity against bacteria.
Bacterial conjunctivitis is less epidemic with 135 in 10,000 cases of incidence reported. It can be contacted directly from infected individuals or due to proliferation of native conjunctival flora. Contaminated fingers and occulo-genital spread are most common ways of spreading bacterial infections. The most common pathogen for bacterial conjunctivitis is Staphylococcal species followed by Streptococcus pneumoniae and H. influenzae. Children are more susceptible to H. influenzae, S. pneumoniae and Moraxella catarrhalis (Ronnerstain et al., 1985; Bag et al., 2009).
Conjunctiva is a thin translucent membrane, lining the anterior part of sclera and inside of the eyelids which in turn has two parts-Bulbar and Palpebral. Bulbar part starts at the edge of the cornea and covers the visible part of sclera; the palpebral part lines the inside of the eyelids. Infection of conjunctiva is known as conjunctivitis and characterized by dilation of the conjunctival vessels which in turn results in edema of the conjunctiva typically associated with discharge. Majority of bacterial conjunctivitis cases (50-75%) have been reported in children. It is more frequent from December to April (Horven, 1993; Morrow and Abbott, 1998; Epling and Smucny, 2005; Hovding, 2008). Conjunctivitis can be infectious and non-infectious. Infectious type is owed to virus and bacteria whereas non-infectious is allergic, toxic and cicatricial.
Further classification includes acute, hyperacute and chronic depending upon mode of onset and severity. The red eye disease should be differentially diagnosed from other ocular disease which has similar symptoms. Purulent and muco-purulent discharge is due to bacterial infection while watery discharge is due to viral conjunctivitis. In comparison, itching is due to allergic conjunctivitis. The course of infection ends in 7 to 10 days and if the problem persists, one should refer to ophthalmologist (Smith et al., 2009). A 2005 study showed that the economic impact of bacterial conjunctivitis is significant and ranges between $377 million to $857 million in America (Ta et al., 2020).
Decreased vision and purulent discharge are generally observed in hyperacute bacterial conjunctivitis accompanied with eyelid swelling, eye-pain, palpitation, and preauricular adenopathy. Chronic conjunctivitis is referred to prolonged infection of 4 weeks with S. aureus, M. lacunata and enteric bacteria being most common cause (Yannof and Duker, 2004). At least 60% cases of suspected acute bacterial conjunctivitis are self-limiting within 1-2 weeks of initialization. Topical antibiotics seem to be more effective in patients who have acute bacterial infection. All broad-spectrum antibiotics eye drops seem in general to be effective in treating bacterial conjunctivitis. Alternate to antibiotic therapy fortified Vancomycin or ophthalmologist is only option for suspected cases (Shanmugnathan, 2005; Freidlin et al., 2007).
MATERIAL AND METHODS
Plant and culture collection: The fruits of T. Chebula and A. marmelos are used in the present study for antimicrobial activity and were procured from Kurukshetra university campus itself and identified from Botany Department of Kurukshetra University, Kurukshetra, Haryana, India. The human pathogenic microorganisms were procured from Microbial Type Culture Collection (MTCC) Institute of Microbial Technology (IMTECH), Chandigarh; which included Gram-negative bacteria: P. aeruginosa (MTCC- 2295) and Gram-positive bacteria: S. aureus (MTCC -3160).
Preparation of Terminalia chebula fruit pulp extract: The fruit pulp was extracted by removing seed and outer covering of Terminalia chebula and were thoroughly washed with water, allowed for oven drying at 50-60º C for 3-4 hours and grounded into fine powder. The 20 gm of this powder was soaked in 100 ml of methanol, and incubated for 72 hr. at room temperature. The extracts were filtered with Whatman filter paper. The extra solvent from the filtrate were evaporated by using water bath at 45-50 °C. The residual powder after solvent extraction was dissolved in DMSO and stored at 4° C.
Preparation of Aegle marmelos fruit pulp extract: The fruits of Aegle marmelos were thoroughly washed with water then allowed for oven drying at 50-60 ºC for 3-4 hours and grounded into fine powder. The 20 gm of this powder was soaked in 100 ml of methanol and incubated for 72 hours at room temperature. The extracts were filtered with Whatman filter paper. The extra solvent from the filtrate were evaporated by using water bath at 45-50° C. The residual powder after solvent extraction was dissolved in DMSO and stored at 4° C.(Bag et al., 2009)(Ganpathy et al., 2016)
Antimicrobial activity of plant extracts by Agar Well Diffusion Assay: The antimicrobial activities of plant extracts were evaluated by agar well diffusion assay (Pereze et al., 1990). The microbial inoculums were inoculated aseptically spread uniformly on surface of pre solidified Mueller Hinton Agar (MHA) plates with the help of sterile glass spreader or sterile cotton swabs. A well of about 6.0 mm diameter was aseptically punctured using a sterile cork borer. The cut agar was carefully removed by the use of sterile forceps. DMSO was used as a negative control. The Petri Plates were kept in laminar for 30 minutes for pre-diffusion to occur then Petri Plates were incubated overnight at 37 °C for 24 hours. The antimicrobial spectrum of extract was determined in terms of zone sizes (inhibition zone diameters) around each well. Zones were measured by high media zone scale.
RESULTS AND DISCUSSION
The increasing rate of mortality among developing countries can be assigned to infectious diseases which are aggravated due to rapid resistance developed against pathogenic bacteria including most pathogenic S. aureus. The incidence of S. aureus disease infection and complications has increased abruptly in recent years because of increased frequency of invasive procedures which led to resistance of S. aureus strains to the available antibiotics. Various researchers have reported the efficacy of several medicinal plants against S. aureus. The antibacterial activity of ten medicinal plant extracts on antibiotic resistant bacteria which includes P. granatum(pomegranate), A. millifolium (yarrow), C. aromaticus (clove), M. officinalis (lemonbalm), O. basilucum (basil), P. guajava (guava), R. officinalis (rosemary) and S. officinalis (Sage) alongwith T. vulgaris (Thyme) and Syzygium joabolanum (Jambolan).Highest activity was observed using plant extracts of C. aromaticus (clove) while no activity was resulted in A. millifolium and S. officinalis. Syzygium resulted in MIC against S. aureus at 300 ppm (Gislene et al., 2016).
Chandra et al. (2013) evaluated antimicrobial activity of medicinal plants against human pathogenic bacteria.Leaf extract of two medicinal plants Lagerstroemia indica and Annona reticulata was extracted using aqueous and methanolic solvent systems against K. pneumoniae, S. aureus, S. typhi, P. vulgaris and P. aeruginosa.Methanolic extracts were found to be better solvent system than aqueous extracts. L. indica resulted in a zone of 12mm and 8mm while A. reticulata resulted in zones of 11mm and 10mm using methanol and aqueous extracts respectively. Mohammed et al. (2018) carried out agar well diffusion assay to evaluate antimicrobial activity and used refluxed methanolic extracts and macerated methanolic extracts of B. vulgaris, C. augustifolia, C. cassia, C. monspeliensis, N. sativa,P. granatum, R. tripartata, W. frutescens and Zingiber officinalis against gram positive and gram negative strains.
The zones of inhibition ranged from 6.0 to 23.0mm while MIC ranged from 0.1 to 12.8 mg/ml. Berberis vulgaris, Cistus monspeliensis and P. granatum resulted in highest activity against S. aureus resulting in zones of 12.0 and 23.0,17.0 and 16.0, 20.0 and 20.0 mm in refluxed methanolic extract and macerated methanolic extracts respectively. In another study, water and organic solvent extracts (methanol, ethanol, petroleum ether and chloroform) of five medicinal plants against seven bacetrial pathogens. A. calamus, T. bellerica, N. arbortritis, C. borivilianum and E. cardamomum leaf and fruit extracts were tested against E. coli, S. aureus, P. aeruginosa, S. typhii, S. pyogenes, P. mirabilis and A. baumani (Khatri et al., 2016; Mohammed et al., 2018).
The methanolic extracts of T. bellerica resulted in a zone of 22mm which was highest while C. borivilianum resulted in a zone of 21mm against both S. typhi and S. aureus using chloroform and ethanolic extracts respectively. Ethanolic extracts of selected medicinal plants were tested against S. aureus, S.marcescens, S. saprophyticus, S. pneumoniae, S. pyogenes, A. baumannii, E. faecalis and P. mirabilis using broth microdilution method. Maximum inhibition concentration was showed by ethanolic extracts of Sasamum indicum of 100 ppm against S. aureus, S. pneumoniae, S. pyogenes, A. baumannii, E. faecalis and P. mirabilis. MIC values ranged from 25-100ppm (Shahla et al., 2014). Rachuonyo and his coworkers (2016) reported antibacetrial activity of methanolic leaf extracts of A. secundiflora, B. frutescens, T. minuta and V. lasiopus against S. aureus. Tagetes minuta was found to be most active even at low concentration with a MIC value of 8.9 mg/ml and MBC value of 10 mg/mL while Vernoia lasiopus resulted in a MIC value of 12.2mg/ml and MBC value of 14.2mg/ml. They also reported the presence of flavonoids, alkaloids,tannins and saponins in all extracts (Rachuonyo et al. 2016).
Bishnu and his coassociates (2015) evaluated antimicrobial activity of 16 traditionally available medicinal plants of Nepal against 13 clinical, 2 reference bacterial species using Microbroth Dilution Method.The research reported that Cynodon dactylon ethanolic extracts resulted in moderate activity against MRSA and 13 bacterial strains while chloroform extracts found to be best against S.aureus giving a MIC value of 31µg/ml. Usmaan Ali Khan et al. (2013) reported antibacterial activity of Bergenia ciliata, Jasminium officinale, Santalum album using agar well diffusion assay against B. subtilis, P.vulgaris, E. coli, P. aeruginosa and S. aureus by using hot and cold aqueous extracts. The roots of B. ciliata cold water extracts showed highest activity against pathogens resulting in a zone of 16mm against S. aureus while highest 19mm against B. subtilis (Bishnu et al. 2015). In another study, the macerated methanolic plant extracts of Dacryedulis showing significant activity against all S.aureus isolates with MIC value 64-256 ug/ml.
Ocimum gratissimum showed inhibitory activity on 9 out of 11 isolates of S. aureus while Commelina erecta and Spilanthes filicaulis revealed similar results on 6 out of of 11 clinical isolates. The study also elucidated the corelation between phytochemicals present in these plants which are the key route to significant antimicrobial activity. The presence of saponins alongwith alkaloids, anthocyanins, anthraquinones, flavonoids, phenols, tannins and triterpenes were correlated to inhibit the S. aureus strain even at low concentrations (Leonard Sama Fonkeng et al., 2015). In another study, the ethanolic extracts of Punica granatum, Syzygium aromaticum, Zingiber officinalis and Thymus vulgaris were found to be effective against S. aureus. At a concentration of 10mg/ml Cuminum cyminum was effective against S. aureus only while other plants show inhibitory activity against both S. aureus and P. aeruginosa with a MIC value of 2.5-5.0mg/ml and MBC value of 5.0 and 10mg/ml (Leonard Sama Fonkeng et al., 2015).
Terminalia chebula-Harad and Aegle marmelos-Baelpatra propensity against S. aureus: Methanolic extracts were prepared using fruit pulp were found to possess significant antimicrobial activity against Gram Positive S. aureus and Gram Negative P. aeruginosa bacteria compared to standard taken as DMSO. The extracts of fruit pulp have been assessed for antimicrobial activity against P. aeruginosa and S. aureus at ten different concentrations (250 mg/ml to 0.97 mg/ml) as shown in Table 1. Starting from a higher concentration of 250 mg/ml two fold dilutions were made using DMSO resulting in concentration of 150, 125, 62.5, 31.25, 15.625 mg/ml and so on till a final concentration of 0.97 mg/ml (Table 1, 2 and 3).
Table 1. Inhibition zone diameters (in mm) of methanolic fruit pulp extract of T. chebula.
| Conc. (mg/ml) | 250 | 150 | 125 | 62.5 | 31.25 | 15.625 | 7.81 | 3.90 | 1.95 | 0.97 | DMSO |
| Zone of Inhibition against S. aureus | 26 | 24 | 24 | 21 | 19 | 16 | 12 | – | – | – | – |
| Zone of Inhibition against P. aeruginosa | 20 | 19 | 18 | 17 | 17 | 15 | 13 | – | – | – | – |
Table 2. Inhibition zone diameters (in mm) of methanolic fruit pulp extract of A. marmelos.
| Conc. (mg/ml) | 250 | 150 | 125 | 62.5 | 31.25 | 15.625 | 7.81 | 3.90 | 1.95 | 0.97 | DMSO |
| S. aureus | 24 | 24 | 21 | 18 | 14 | – | – | – | – | – | – |
| P. aeruginosa | 14 | 12 | 12 | 10 | 8 | 6 | – | – | – | – |
Table 3. Inhibition zone diameters (in mm) of methanolic extracts of leaf of T. chebula and A. marmelos against S. aureus and P. aeruginosa
| Sr. no. | Concentration of extracts(mg/ml) | Leaf(T) S. aureus | Leaf(T) P. aeruginosa | Leaf(A)
S. aureus |
Leaf(A) P. aeruginosa |
| 1. | 10 | 17 | 19 | 14 | 10 |
| 2. | 5 | 16 | 17 | 10 | 6 |
| 3. | 2.5 | 14 | 15 | 7 | 6 |
| 4. | 1.25 | 13 | 15 | 7 | – |
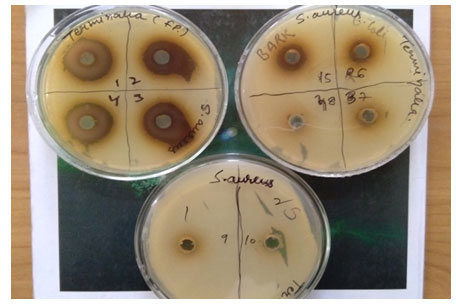
The volume of all different dilutions was kept same as 100 µl and media plates were incubated and afterwards the standard procedure of agar well diffusion assay was carried out. The agar well diffusion method was used to evaluate the antimicrobial activity by measuring the inhibition zones against the test microorganism. A zone of 26 mm was observed at higher concentration of 250 mg/ml. The zone size reduced to 24 mm on two fold dilutions of 250 mg/ and remains constant at two concentrations of 150 and 125 mg/ml (given in Fig-1 and Fig-2).
Figure 1: Terminalia fruit pulp showing zones of inhibition against S. aureus.
Figure 2: Baelpatra fruit pulp showing zones of inhibition against S. aureus
On further dilution the zone size of 21 mm was observed at 62.5 mg/ml which showed positive correlation with decrease in concentration. A zone of 19 mm at 31.25 mg/ml and zone of 16 mm at 15.625 mg/ml further validated that increasing plant extracts have positive effects on zone size or say inhibition rate against S. aureus. While a zone of 12 mm was reported to be final zone at 7.81 mg/ml because on further dilutions no zone of inhibition was observed. It can be assumed that lower concentrations were unable to inhibit S. aureus. But results obtained showed potential approach to exploit fruit pulp of Harad plant to be used against different strains of S.aureus to explicit its resistance developed so far. Ghosh et al. (2011) reported similar findings in methanolic leaf extracts of Terminalia chebula exhibited higher antimicrobial potential amongst all plant parts used followed by fruit extracts showing effective antimicrobial activity in five different medicinal plants viz; T. bellerica, T. chebula, E. officinalis, Punica granatum and Lawsonia inermis. Samy and Ignachimuthu, 2000 reported similar results in C.auriculata exhibiting significant antimicrobial activity against E. coli and S. aureus at a concentration of 6mg/ml which correlate to present investigation where a minimum zone of inhibition of 12mm was observed at 7.81 mg/ml (Samy and Ignachimuthu, 2000).
The results reported were in corroboration to the earlier reults where methanolic extracts have been found to be more effective as compared to other solvents (Ghosh et al., 2011). Fruit pulp extract showed a minimum inhibition zone of 13mm in methanolic extracts when taken as MIC assay. While on testing against P. aeruginosa agar well diffusion assay resulted in a zone of 20mm at 250mg/ml which showed constant decrease in zone size with further dilution to 0.97mg/ml. A zone of 19mm at (150), 18mm at (125),17mm at (62.5 and 31.25), 15mm at (15.625) and a zone of 13mm at 7.81mg/ml were observed and were in well accordance to decreasing concentration or dilutions.
Similar to previous zones against S. aureus no further zones were resulted at lower concentrations than 7.81mg/ml. Kannan et al. (2009) reported the potential of using dry fruit extracts even at 1 mg/ml to possess broad spectrum activity showing the ethanolic extracts of Terminalia chebula using fruit extracts to be effective against S. typhi ,S. epidermidis, S. aureus, B. subtilis and P. aeruginosa. In comparison to fruit pulp the leaves of the plants were also exploited to test efficacy in inhibiting both S. aureus and P. aeruginosa. The leaves were washed dried and methanolic extracts were prepared using standard preparation methods.
However, the investigation researched the activity at lower concentration than that of fruit pulp. As in both cases the fruit pulp was limited to generate zone of inhibition approximately near 10 mg/ml so the lower concentrations were prepared starting from 10 mg/ml and serially diluted two fold resulting in concentration of 5 mg/ml, 2.5 mg/ml and 1.25 mg/ml (Table-2). A zone of 17 mm was observed at 10 mg/ml using methanolic leaf extract of Terminalia followed by zone of 16 mm at 5 mg/ml, 14 mm at 2.5 and a minimum size zone of 11 mm at 1.25 mg/ml. So, the leaf as plant part is more effective than fruit pulp against S. aureus (Kannan et al., 2009).
Similar findings were observed in Baelpatra where the zones were although of smaller size but can be considered resulting in zone of 14 mm at 10 mg/ml followed by 10 mm at 5 mg/ml, 7 mm at 2.5 mg/ml and a similar zone of 7 mm at 1.25 mg/ml against S. aureus. While inhibition zones against P. aeruginosa were more prominent using Terminalia leaf extracts. A zone of 19 mm was observed at 10 mg/ml followed by 17 mm at 5 mg/ml ,15 mm at 2.5 mg/ml and 15 mm at least concentration of 1.25 mg/ml. The results depict better efficacy of leaf extracts against P. aeruginosa than fruit pulp.
The fruit pulp of Terminalia is more effective against S. aureus. In comparison Baelpatra resulted in zones of 10 mm at 10 mg/ml followed by a zone of 6 mm at 5 and 2.5 mg/ml while no zone was observed at 1.25 mg/ml. Although traditional wisdom indicates the importance of using these trees in historic times and reveal their potential in using against S. aureus a multi strain bacterium which developed resistant in present scenario. Similar agar well diffusion assay was performed for A. marmelos fruit pulp extract starting from 250 mg/ml as initial concentration using methanol against S. aureus. Two fold dilutions were made using DMSO and same is used as control. A similar zone of 24 mm was observed at 250 mg/ml followed by 24 mm at 150 mg/ml, 21 mm at 125 mg/ml.
The zone size decreased to 18 mm at 62.5 mg/ml and further goes on decreasing to 14 mm at 31.25 mg/ml. As compared to Terminalia (Harad) which resulted in inhibition zones at further two concentrations of 15.625 and 7.81 mg/ml Baelpatra fruit pulp extract showed no zone of inhibition on these concentrations while as compared to S. aureus agar well diffusion assay performed against P. aeruginosa resulted in inhibition zones at initial five concentrations. A zone of 14 mm was observed at 250 mg/ml which in turn is followed by a zone of 12 mm at 150 mg/ml and 125 mg/ml, 10 mm at 62.5 mg/ml followed by 8 mm at 31.25 mg/ml. The least size zone of 6 mm at 15.625 mg/ml Table-2.
The zone size obtained were in corroboration of Poonkothai and Saravanan (2008) who reported antimicrobial activity of different plant parts of Baelpatra such as leaf, bark and fruit extracts using methanolic, chloroform and aqueous extracts using disc diffusion assay against seven pathogens which includes S.aureus, B. subtilis, P. mirabilis, E. coli, K. pneumoniae, S. paratyphi A, Salmonella paratyphi B. Methanolic extract reported to be more effective in inhibiting the pathogens tested in comparison to chloroform which was more effective solvent than aqueous extracts, but the zone of inhibition observed were less effective as compared to commercial antibiotic while discussing in concern to MRSA the zone of inhibition obtained using methanol as solvent resulted in zone size of 14 mm (leaf), 7 mm (bark), 11 mm (fruits)while chloroform extracts resulted in zones of smaller sizes like 6 mm, 2 mm and 5 mm using leaf, bark and fruit respectively. The aqueous extracts produced a zone of 2 mm using leaf while a zone of 10 mm using fruits as plant part while bark did not result in any zone of inhibition. The differential zones of inhibition observed were attributed to differential polarity and non-polarity of constituents extracted using different solvents (Suresh et al., 2009).
Leaves and flowers of Aegle marmelos were extracted using methanol as solvent system at three different concentrations 50, 100, 200 ppm against E. coli, P. aeruginosa, P. mirabilis, S. typhi, S. aureus using disc diffusion method. E. coli was found to be more susceptible due to tannin alkaloids giving a significant zones against E. coli (17 mm), S.typhi (17 mm), S.aureus(15 mm),P. aeruginosa (13 mm)at all concentration but the highest concentration of 200 ppm did not revealed marked differences using leaves as plant parts. Flowers extract prepared using methanol as solvent system resulted in highest activity against S.aureus giving a zone of 18 mm at 200 ppm similar to P. mirabilis (18 mm), 16 mm against P. aeruginosa and 15 mm against E. coli and S.typhi and the flavonoids were found to be main constituents in flowers responsible for this activity.
Sridhar et al., 2014 reported antibacterial and anti- helmintic and cardiotonic activities using aqueous and ethanolic extracts of dried fruits of Aegle marmelos using cup diffusion method. Ethanolic extracts resulted in zones of 18 mm while aqueous extract gave a zone of 19 mm against E. coli. MIC assay revealed a zone of 6.25 µg/ml against E. coli. Karumaran et al., (2016) observed highest zone of inhibition of 20mm using acetone and hexane extracts at 10 mg /ml concentration against P. aeruginosa and B. subtilis. Lowest zone of 5 mm was observed against K. pneumoniae at same concentration in acetone extracts (Sridhar et al., 2014)..
Acetone extracts showed a zone of 16 mm against S. aureus, 20 mm against P. aeruginosa and B. subtilis and 11 mm against E. coli and gave a MIC value of 10.5 mg/ml for both acetone and ethanol extracts. Rajan et al., (2011) reported that fruit pulp is used as a remedy for gastrointestinal infections of human. Antioxidant potential of fruit pulp extract and showed the presence of steroids, terpenoids, saponins, tannins, lignin, and flavonoids. The plant is a perennial tree found wild in sub Himalayan tract, central and south India. Fruits have greatest medicinal values. Mujeeb et al., (2014) screened the phytoconstituents using aqueous and methanolic leaf extracts which revealed the presence of alkaloids, flavonoids and phenols observed highest inhibitory activity of aqueous extracts against S.epidermidis, while methanolic extracts showed more potent activity against S.aureus at 40mg/ml. IC values revealed presence of aldehydes, flavonoids, fatty acids, methyl esters, terpenoids, phenolics, steroids and aromatic compounds along with alcohols (Mujeeb et al., 2014).
CONCLUSION
Phytochemicals present in these plants are key route to significant antimicrobial activity. The presence of saponins alongwith alkaloids, anthocyanins, anthraquinones, flavonoids, phenols, tannins and triterpenes were correlated to inhibit the S. aureus strain even at low concentrations. The T. chebula extract showed inhibition zones at 7.81 mg/ml whereas A. marmelos extract didn’t show significant zones after 31.25mg/ml which shows that T.chebula is densely packed with phyto-constituents and can be referred to be more potent for curing Conjunctivitis.The bio active substances from these plants can be employed in the formulation of antimicrobial agents for the treatment of various bacterial infections. The results of present investigation indicate that antibacterial activity varies with the plant part and solvent extract concentration. Further, the research needs to be done for finding potential solutions for Multi Drug Resistant pathogens.
ACKNOWLEDGEMENTS
We are thankful to Department of Biotechnology, University Institute of Engineering and Technology, Kurukshetra, Haryana, to provide the pathogenic culture to carry out this work.
Conflict of Interests: There was no conflict among the interests of the workers.
REFERENCES
Bag, A., Bhattacharyya, S.K., Pal, B.N.K. and Chattopadhyay, R.R., (2009). Evaluation of antibacterial properties of Chebulic myrobalan (fruit of Terminalia chebula Retz.) extracts against methicillin resistant Staphylococcus aureus and trimethoprim-sulphamethoxazole resistant uropathogenic Escherichia coli. African journal of plant science, 3(2), pp.025-029.
Behera, P., Raj, V.J., Prasad, A.B. and Basavaraju, R., (2014). A Review on Phytochemical and Pharmacological Values of Fruit Pulp of Aegle Marmelos Global Journal of Research on Medicinal Plants and Indigenous Medicine, 3(9), p.339.
Bereksi, M.S., Hassaïne, H., Bekhechi, C. and Abdelouahid, D.E., (2018). Evaluation of antibacterial activity of some medicinal plants extracts commonly used in Algerian traditional medicine against some pathogenic bacteria. Pharmacognosy Journal, 10(3), pp.507-512 .
Chandra, M., (2013). Antimicrobial activity of medicinal plants against human pathogenic bacteria. International journal of biotechnology and bioengineering research, 4(7), pp.653-658.
Cronau, H., Kankanala, R.R. and Mauger, T., (2010). Diagnosis and management of red eye in primary care. American family physician, 81(2), pp.137-144.
Dash, B. (1991). Marteria Medica of Ayurveda. New Delhi: B. Jain Publishers, New Delhi, 170-174 p.
Evans, J.S., Pattison, E. and Morris, P., (1986). Antimicrobial agents from plant cell culture. secondary metabolites in plant cell culture. Morris P, Scraggs A, Stanfford A, Fowler M. Cambridge University, London, 12.
Fonkeng, L.S., Mouokeu, R.S., Tume, C., Njateng, G.S.S., Kamcthueng, M.O., Ndonkou, N.J. and Kuiate, J.R., (2015). Anti-Staphylococcus aureus activity of methanol extracts of 12 plants used in Cameroonian folk medicine. Bmc research notes, 8(1), p.710
Freidlin, J., Acharya, N., Lietman, T.M., Cevallos, V., Whitcher, J.P. and Margolis, T.P., (2007). Spectrum of eye disease caused by methicillin-resistant Staphylococcus aureus. American journal of ophthalmology, 144(2), pp.313-315.
Ghosh, A., Das, B.K., Roy, A., Mandal, B. and Chandra, G., (2008). Antibacterial activity of some medicinal plant extracts. Journal of Natural Medicines, 62(2), pp.259-262.
Hørven, I., (1993). Acute conjunctivitis: a comparison of fusidic acid viscous eye drops and chloramphenicol. Acta ophthalmologica, 71(2), pp.165-168.
Høvding, G., (2008). Acute bacterial conjunctivitis. Acta ophthalmologica, 86(1), pp.5-17.
Kannan, P., Ramadevi, S.R. and Hopper, W., (2009). Antibacterial activity of Terminalia chebula fruit extract. African Journal of Microbiology Research, 3(4), pp.180-184.
Karumaran, S., Nethaji, S. and Rajakumar, R., (2016). Antimicrobial and antioxidant activity of leaf extracts of Aegle marmelos. Adv. Appl. Sci. Res, 7(3), pp.205-208.
Khan, Usman Ali, Hazir Rahman, Zeeshan Niaz, Muhammad Qasim, Jafar Khan, Tayyaba, and Bushra Rehman., (2013) Antibacterial activity of some medicinal plants against selected human pathogenic bacteria. European Journal of Microbiology and Immunology 3, no. 4: 272-274.
Khatri, P., Jamdagni, P. and Rana, J S. (2016). Antimicrobial potential of important medicinal plants of India. International Journal of Microbial Resource Technology. 3. 301-308.
Malekzadeh, F., Ehsanifar, H., Shahamat, M., Levin, M. and Colwell, R.R., (2001). Antibacterial activity of black myrobalan (Terminalia chebula Retz) against Helicobacter pylori. International journal of antimicrobial agents, 18(1), pp.85-88.
Marasini, B.P., Baral, P., Aryal, P., Ghimire, K.R., Neupane, S., Dahal, N., Singh, A., Ghimire, L. and Shrestha, K., (2015). Evaluation of antibacterial activity of some traditionally used medicinal plants against human pathogenic bacteria. BioMed research international, Article ID 265425, 6 pages.
Morrow, G.L. and Abbott, R.L., (1998). Conjunctivitis. American family physician, 57(4), p.735.
Mostafa, A.A. and Abdulaziz, A., (2018). Al-Askar, Khalid S. Almaary, Turki M. Dawoud, Essam N. Sholkamy, Marwah M. Bakri, Antimicrobial Activity of Some Plant Extracts Against Bacterial Strains Causing Food Poisoning Diseases. Saudi Journal of Biological Sciences, 25, pp.361-366.
Mujeeb, F., Bajpai, P. and Pathak, N., (2014). Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegles marmelos. BioMed research international, Article ID 497606, 11 pages.
Muthukumar, B., Abubacker, M.N. and Gunasekaran, K., (2014). In vitro Antibacterial Activities of Wedelia caledulacea Less (Asteraceae) Leaf Extract on Pathogenic Bacterial Strains. Drug Invention Today, 6(2), pp.120-122.
Nascimento, G.G., Locatelli, J., Freitas, P.C. and Silva, G.L., (2000). Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Brazilian journal of microbiology, 31(4), pp.247-256.
Perez, C., (1990). Antibiotic assay by agar-well diffusion method. Acta Biol Med Exp, 15, pp.113-115.
Poonkothai, M. and Saravanan, M., (2008). Antibacterial activity of Aegle marmelos against leaf, bark and fruit extracts. Ancient science of life, 27(3), p.15.
Rachuonyo, H.O., Ogola, P.E., Arika, W.M., Kiboi, N.G. and Wambani, J.R., (2016). Antimicrobial potency of methanolic leaf extracts from selected medicinal plants against Staphylococcus aureus. J Med Microb Diagn, 5(219), pp.2161-0703
Rapuano, C.J., Feder, R.S., Jones, M.R.,(2008). Prepared by the American Academy of Ophthalmology Cornea/External Disease Panel Cornea/External Disease Panel Members.
Raza, M., Fozia, R.A., Wahab, A., Iqbal, H., Ullah, H., Ahmad, S., Ahmad, I. and Shah, S.M., (2012). Comparative antibacterial study of Convolvulus arvensis collected from different areas of Khyber Pakhtunkhwa, Pakistan. Int Res J Pharm, 3(10), pp.220-222.
Rönnerstam, R., Persson, K., Hansson, H., et al (1985). Prevalence of chlamydial eye infection in patients attending an eye clinic, a VD clinic, and in healthy persons. British journal of ophthalmology, 69(5), pp.385-388.
Rubenstein, J.B. and Jick, S.L., (2004). Disorders of the conjunctiva and limbus. Ophthalmology. 2nd ed. Philadelphia, Pa: Mosby Elsevier.
Sahraei, S., Mohkami, Z., Golshani, F., Javadian, F., Saeidi, S. and Baigi, G.S., (2014). Antibacterial activity of five medicinal plant extracts against some human bacteria. E Euro J Exp Bio, 4, pp.194-196.
Samy, R.P. and Ignacimuthu, S., (2000). Antibacterial activity of some folklore medicinal plants used by tribals in Western Ghats of India. Journal of Ethnopharmacology, 69(1), pp.63-71.
Sekar, D.K., Kumar, G., Karthik, L. and Rao, K.B., (2011). A review on pharmacological and phytochemical properties of Aegle marmelos (L.) Corr. Serr. (Rutaceae). Asian Journal of Plant Science and Research, 1(2), pp.8-17.
Shanmuganathan, V.A., Armstrong, M., Buller, A. and Tullo, A.B., (2005). External ocular infections due to methicillin-resistant Staphylococcus aureus (MRSA). Eye, 19(3), pp.284-291.
Smith, A.F. and Waycaster, C., (2009). Estimate of the direct and indirect annual cost of bacterial conjunctivitis in the United States. BMC Ophthalmology, 9(1), p.13.
Sridhar, N., Raghavendra, M., Prasad, M.N.V., Kiran, B.S. and Kanthal, L.K., (2014). Screening the Fruits of Aegle marmelos for Antibacterial, Anthelmintic and Cardiotonic Properties. International Journal of Pharma Research and Review, 3, pp.48-55.
Stenson, S., Newman, R. and Fedukowicz, H., (1982). Laboratory studies in acute conjunctivitis. Archives of Ophthalmology, 100(8), pp.1275-1277.
Suresh, K., Senthilkumar, P.K. and Karthikeyan, B., (2009). Antimicrobial activity of Aegle marmelos against clinical pathogens. Journal of phytology1(5), pp. 323–327.
Veena, C., (2015). Bacteriological study of conjunctivitis. Int. J. Contemp. Med. Res. ISSN, 3, pp.2393-2915.
Devi, M., Devi, S., Sharma, V., Rana, N., Bhatia, R.K. and Bhatt, A.K., (2020). Green synthesis of silver nanoparticles using methanolic fruit extract of Aegle marmelos and their antimicrobial potential against human bacterial pathogens. Journal of traditional and complementary medicine, 10(2), pp.158-165.
Chaubey, A. and Dubey, A.K., (2020). Chemistry and Antioxidant Potential of Phytoconstituents from Aegle marmelos Fruit-Shell. Current Drug Metabolism.
Nigam, M., Mishra, A.P., Adhikari‐Devkota, A., Dirar, A.I., Hassan, M.M., Adhikari, A., Belwal, T. and Devkota, H.P., (2020). Fruits of Terminalia chebula Retz.: A review on traditional uses, bioactive chemical constituents and pharmacological activities. Phytotherapy Research.
Sun, G.L. and Wang, D., (2020). Gallic acid from Terminalia chebula inhibited the growth of esophageal carcinoma cells by suppressing the Hippo signal pathway. Iranian Journal of Basic Medical Sciences, 23(11), pp.1401-1408.
Ta, C.N., Raizman, M.B., Gross, R.D., Joshi, S., Mallick, S., Wang, Y. and Segal, B., (2020). A prospective, randomized trial of povidone-iodine 0.6% and dexamethasone 0.1% ophthalmic suspension for acute bacterial conjunctivitis. American journal of ophthalmology, 215, pp.56-65.
DeCory, H.H., Sanfilippo, C.M., Proskin, H.M. and Blondeau, J.M., 2020. Characterization of baseline polybacterial versus monobacterial infections in three randomized controlled bacterial conjunctivitis trials and microbial outcomes with besifloxacin ophthalmic suspension 0.6%. Plos one, 15(8), p.e0237603.
Pingali, U., Sukumaran, D. and Nutalapati, C., (2020). Effect of an aqueous extract of Terminalia chebula on endothelial dysfunction, systemic inflammation, and lipid profile in type 2 diabetes mellitus: A randomized double‐blind, placebo‐controlled clinical study. Phytotherapy Research.
Li, K., Han, X., Li, R., Xu, Z., Pan, T., Liu, J., Li, B., Wang, S., Diao, Y. and Liu, X., (2019). Composition, antivirulence activity, and active property distribution of the fruit of Terminalia chebula Retz. Journal of food science, 84(7), pp.1721-1729.
Aritonang, H.F., Koleangan, H. and Wuntu, A.D., (2019). Synthesis of silver nanoparticles using aqueous extract of medicinal plants’(Impatiens balsamina and Lantana camara) fresh leaves and analysis of antimicrobial activity. International journal of microbiology.
Ganapathy, S. and Karpagam, S., (2016). In vitro antibacterial and phytochemical potential of Aegle marmelos against multiple drug resistant (MDR) Escherichia coli. Journal of Pharmacognosy and Phytochemistry, 5(1), p.253.
Krishna, S. and Priyadarshini, A.P.,(2020). Phytochemical investigation, antioxidant and antibacterial activities, and GC-MS analysis of ethanol extract of Retz. Dried seeds Terminalia chebula. Advance Pharmaceutical Journal 2020; 5(3), pp.92-102.
Owk, A.K. and Lagudu, M.N., 2020. Aegle marmelos (Rutaceae): Evaluation of Root Phytochemical Constituents for Antimicrobial Activity. In Medicinal Plants: Biodiversity, Sustainable Utilization and Conservation, pp. 573-58.
Aung, H.T., Zar, T., Sein, M.M., Komori, Y., Vidari, G. and Takaya, Y., (2020). Constituents of Aegle marmelos from Myanmar. Journal of Asian Natural Products Research, pp.1-7.