1Department of Biology, College of Science, University of Jeddah, Jeddah 21959, Saudi Arabia
2Department of Biochemistry, College of Science, University of Jeddah, Jeddah 21959, Saudi Arabia
Corresponding Author Email: mwarsi@uj.edu.sa
Article Publishing History
Received: 16/07/2023
Accepted After Revision: 25/09/2023
The chickpea (Cicer arietinum L.) is the most significant legume crop and good source of protein. The biggest obstacle currently facing agriculture is salt stress which damage soil fertility and constantly shifting abiotic stressors result in low chickpea yields. Therefore, the purpose of this work was to identify and characterize the Rhizobium isolates and its effect on salt (NaCl) stress. Total 281 isolates were isolated from rhizospheric soil samples fromTaif agriculture filed of Kingdom of Saudi Arabia. All isolates were showing same phenotypically shape size and morphology on YEMA. Only 7.47 % (21) of isolates were showing the biochemical characterization of Rhizobium. Only 10 (47.61%) of the 21 isolates exhibited nodulation in the chickpea under controlled condition.
After the symbiosis establishment at 25 days the data clearly indicate that the dry weight of plant was increase at 50 and 75mM NaCl stress at four successive harvesting stages while at 100 mM decreases the accumulation of dry mass at the rate of 16.21%, 26.15%,10.97% and 13.04% in first, second, third and fourth harvesting stage respectively. With the salinity the root to shoot ratio increased at 0, 50 and 75mM at first, second and third harvesting. But decrease at 100mM NaCl at every harvesting stage. Nodule dry weight remains decrease under the salt stress conditions. Few isolates of Rhizobium exhibited good growth at high temperature and high pH. More research is needed to understand the Rhizobium isolates under abiotic stress conditions including salt stress to raise the better nitrogen fixers in the form of biofertilizer in harsh environment.
Abiotic stress, Rhizobium, salts stress, Cicer arietinum, Agriculture.
Alqarni A. S. M, Howladar S. M, Alghamdi A. S. M, Warsi M. K. Effects of salt stress on the growth and nodulation of the chickpea, Cicer arietinum. Biosc.Biotech.Res.Comm. 2023;16(3).
Alqarni A. S. M, Howladar S. M, Alghamdi A. S. M, Warsi M. K. Effects of salt stress on the growth and nodulation of the chickpea, Cicer arietinum. Biosc.Biotech.Res.Comm. 2023;16(3). Available from: <a href=”http://surl.li/lpttf“>http://surl.li/lpttf</a>
INTRODUCTION
The most important legume crop is the chickpea (Cicer arietinum L.). It is well known for its nutritional advantages and is a member of the Fabaceae (Leguminosae) family. Currently, salt stress is the main barrier to the agricultural sector, which has a significant impact on the development, survival, and metabolic activity of bacteria and plants that fix nitrogen (Shilev, S., 2020). In many nations, including Saudi Arabia, agriculture is at the forefront of economic development. However, one of the key barriers preventing the growth of the agricultural area or the rise in agricultural productivity for many crops is salinity, which affects the majority of the country (Sunita et al., 2020). One of the main causes of dryness and salinity is high temperatures. Due to the significant amount of soluble salts present in irrigation water and the high rate of evaporation brought on by Saudi Arabia’s high temperatures, ineffective drainage, or soil type. In light of the aforementioned information, the goal of our research is to identify salt-tolerant Rhizobium species in Saudi agricultural soil that promote chickpea growth under adverse and salt-stress conditions.
Numerous living creatures, including bacteria, fungi, nematodes, worms, and others, can be found in soil in their natural habitat. According to (Sunita et al., 2020) Rhizobia are a special class of bacteria that live as symbionts with legumes and fix atmospheric nitrogen. The most important legume crop is chickpea (Cicer arietinum L.). One of the main obstacles in the agricultural sector that has a significant impact on plant growth is salt stress. In desert habitats, the Rhizobium-legume symbiosis is particularly crucial in areas where the prevalence of saline soils is rising and posing a danger to plant productivity. Legumes, which are typically found in dry settings, may be better adapted than legumes grown in other habitats to fix more nitrogen dioxide in saline environments (Etesami, H. and Adl, S.M., 2020).
Toxic ion accumulations in various plant tissues, which disrupt some enzyme activity, can be blamed for the salt sensitivity. According to Lauter and Munns (1986), the chickpea (Cicer arietinum) is particularly sensitive to soil salt. Failure of the infection process resulting from salinity’s effect on rhizobia’s establishment may be the cause of unsuccessful symbiosis under salt stress. Legumes with salt stress have less nodulation because they block the symbiotic interactions (Chakraborty and Harris, 2022). Salinity levels that prevent the growth of each individual symbiont differ from those that prevent the symbiosis between legumes and rhizobia. Some legumes perform poorly in symbiotic relationships when exposed to salt, but this is not because salt limits rhizobial growth. In saline environments, rhizobial colonization and invasion of the rhizosphere, root-hair infection, and the development of efficient salt-tolerant nodules are necessary conditions (Ma et al., 2020).
Arid and semi-arid climates affect about one-third of the planet’s geographical area (Skujins, 1991). One-third of the world’s irrigated areas and around 15% of dry and semi-arid regions are affected by salinity, according to Pitman et al. (2002). Lack of nitrogen frequently restricts plant productivity on many semi-arid lands, particularly saline regions. Legumes that grow in arid environments may be treated with N fertilizers to help them tolerate salt better (El at al., 2020; Shevyakova 1984). The use of Nitrogen in agriculture land is likely to be further constrained by rising N fertilizer costs and the risk of growing soil salinity (Mohammad et al. 1989). As a result, biological nitrogen fixation has become more significant. Legumes, which are typically found in dry ecosystems, may significantly contribute to the global nitrogen economy, and their capacity to fix nitrogen could raise soil productivity ( Bhat et al., 2020).
Legumes may have evolved to adverse climatic conditions in part as a result of the host plant’s interaction with Rhizobium species. These modifications may make Rhizobium-legume relationships more capable of fixing N2 under stress situations than associations that have developed in other contexts (such as salt stress), according to Zehran (1999) it has long been known that legumes are either susceptible to salinity or only moderately resistant to it. Most legumes experience a growth reduction in response to moderate salt (Jamil et al., 2012). In many nations agriculture is at the forefront of economic development. Salinity is one of the key barriers which preventing the growth of the agricultural area or the rise in agricultural productivity for many crops. One of the main causes of dryness and salinity is high temperatures (Sindhu et al., 2020).
The high rate of evaporation brought on by Saudi Arabia’s high temperatures, the high concentration of soluble salts in irrigation fluids, ineffective drainage, or soil type are the causes of excessive salinity. We tryed to estaiblish as relation of salt-tolerant Rhizobium species with the chickpea seedlings which help to promote the growth of chickpeas in a controlled manner in light of the aforementioned facts in Saudi agricultural soil. Weimberg and Shanon (1988); Cordovilla et al., 1996) found that there is generally little association between salt concentration and the concentration of these chemicals (Chakraborty and Harris, 2022). The present study looked at how the well-established symbiosis between Cicer arietinum and Rzobium responded to salt stress during the vegetative phase. The purpose is to determine whether the adverse effect of salt stress influence the vegetative growth of Rhizobium and its association of nodulation.
MATERIAL AND METHODS
Collection of Soil Samples: The study site of research comprises five different Rhizospheric soil samples was collected from agriculture field of Taif, Saudi Arabia. Samples were kept in clean sterile bottles sealed and transferred to the Microbiology and Biochemistry laboratory at university of Jeddah and stored at 4° C.
Isolation of Rhizobium isolates: By vigorously vertexing, the soil samples were suspended in distilled water and prepared for serial dilutions up to 10-6 in pure distilled water. After the proper dilution, put the solution to the YEM Agar plate in a petri dish with the correct pH calibration (6.8 to 7) and cover for 72 hours at 32° C. Colony was obtained and was streaking on YEMA media. Sub-cultures of the cultures were developed and be used often. The biochemical characteristics of Rhizobium will be determined using Berge’s Manual of Bacteriology (Bergeys et al., 1939)
Purification of Isolates: Using a sterile inoculating loop, one well-separated rhizobial colony was chosen and added to 6 mL of sterilized yeast extract mannitol broth (Sindhu et al., 2020). The test tubes were then vortexed and swirled on a rotary shaker for 48 hours at room temperature. A loop of the culture suspensions from each test tube was removed after two days and streaked on sterile yeast extract mannitol agar (YEMA), where they were cultivated for three to four days at 28 °C. By repeatedly re-streaking, the colony’s purity and consistency were thoroughly examined.
Biochemical characterization of isolates: Rhizobium isolates were biochemically characterized using a variety of biochemical tests, including the Indole test, Methyl red test, Voges Proskauer test, Citrate utilization test, Catalase test. Tests for nitrate reduction, starch hydrolysis, gelatine hydrolysis, and oxidase also done to determine the biochemical characterization of Rhizobium isolates (Rafique et al., 2021).
Citrate utilization test: The only carbon source accessible to the bacteria in this medium is citrate, but the Rhizobium cannot grow on citrate, no change in colour takes place. A loop filled with Rhizobium culture was used to inoculate the slant. The stab and streak method were utilized, and the slant was then inspected after a 24-hour incubation period at 37℃.
Starch utilization test : The test was run to see if microorganisms might use starch as a source of carbon (Datta et al., 2015). Rhizobium was inoculated into starch agar media, which was then incubated and examined. Extracellular enzymes are produced when starch is present, showing that the organism could exploit starch as a carbon source. The capacity of bacteria to consume starch was evaluated using an iodine test. Iodine solution drops were applied to Petri-plate-grown cultures that had been cultured for 24 hours. No starch utilization was indicated by the formation of blue, and vice versa.
Gelatine test: This test was run to see if bacteria could produce the enzyme gelatinase and use gelatine as a media source. Gelatine degradation is a sign that the gelatinase enzyme is present. The actively growing cultures underwent nutritional gelatine medium inoculation and 48-hour growth. The cultures that produce gelatinase stay liquefied after being exposed to a low-temperature treatment at 40 °C for 30 min, but the cultures that do not produce gelatinase the media was remain solidify (Deka and Azad 2006).
Catalase test: This test was run to investigate whether the catalase enzyme was present in bacterial colonies. On glass slides, 24 hour-old Rhizobium colonies were collected, and one drop of 30% H2O2 was applied. The presence of the catalase enzyme was shown by the appearance of a gas bubble (Rafique et al., 2021).
Physiological Tolerance Test of Rhizobia: Physiology of the isolated rhizobia were done by the determination of the following parameters
Temperature, pH and Salt tolerance of the Isolates Antibiotic tolerance.
Temperature, pH and Salt tolerance of the Isolates: By allowing the isolates to grow on YEMA plates with various salt concentrations, the isolates’ salt tolerances were evaluated (Chakraborty and Harris, 2022). Different NaCl concentrations, including 0, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, 6.0, 6.5, 7.0, 7.5, and 8.0% NaCl, were used to make YEMA. Growing the isolates on YEMA modified with 0.1N HCl or NaOH at various pH values of 4, 4.5, 5, 5.5, 6, 8, 8.5, 9, 9.5, and 10 allowed researchers to establish the isolates’ pH tolerance (Maatallah et al., 2002). By inoculating and incubating the media at 5, 10, 15, 20, 35, 40, 45, and 50°C, YEMA medium was used to study the growth response of rhizobia isolates to various temperatures (Kulkarni and Nautiyal, 2000). The presence and lack of growth on the plates was noted after 4 days of incubation at 28 2°C.
Antibiotic sensitivity test: Antibiotic sensitivity tests were conducted using antimicrobial discs. The following antibiotic disc was employed: Nalidixic acid (Na), Cloxacillin (Cx), Chloramphenicol (C), Tetracycline (Tc), Streptomycin (S), Ciprofloxacin (Cf), Ampicillin (Am) and Penicillin (PEN) are some examples of antibacterial drugs. Every isolate was individually inoculated in YEM broth and incubated at 37 °C for an overnight period. A glass spreader was used to evenly distribute the culture broth of each isolate on the nutrient agar medium (NA). Different isolates’ inoculation plates were set with antimicrobial discs at about 2.5 cm, and they were then incubated at 37°C overnight. The zone of inhibition surrounding the discs was used to assess the sensitivity and resistance of Rhizobium isolates. All those isolates inhibit by antibiotic disc in the form zone consider as sensitive isolates while those perform the growth near to antibiotic disc is consider as resistance isolates (Warsi et al., 2017).
Surface sterilization and germination of chickpea seeds: Seed Preparation: Seeds are cleaned with tap water before being sterilized in 98% alcohol for 5 minutes, followed by 5 minutes of distilled water and another 2 minutes of sterile distilled water. For a short period of time, seeds were dried in laminar air flow (Sarwar et al., 2006; Yadav et al., 2010). After being surface sterilized, seeds germinated for 48 hours at 26 °C in moist autoclaved water. The immature plants were placed in freshly prepared nutrient solutions in autoclaved sand pots. Every 24 hours, the nutritional solution is replaced. Each seedling is injected with a suspension of 10–8 Rhizobium cells per millilitre. Plants that had been inoculated were placed in a control chamber with a 16/8-hour light/dark cycle operating at 25/17 C during the day and night.
In order to stress the plant with salt at different concentrations, we were to wait at least 20 to 25 days until the establishment of the symbiosis as per Banik et al., 2018 with bacteria. The development of chickpea seedlings under various salt stress conditions and its impact on nodulation in roots were studied. The seedlings were exposed to different concentrations of NaCl salt (0, 50, 75, and 100 mM NaCl). After inoculating chickpeas with Rhizobium isolates, we use methods from Elsheikh & Wood (1990) to assess the length of the root and shoot as well as the fresh and dry biomass. Salt stress (0, 50, 75, and 100 mM NaCl) was applied. There will be no salt concentration. Zero salt concentration will be considered as control plants. The harvesting was carried out at 4 different stages @ 4, 8, 12, & 16 days.
RESULTS AND DISCUSSION
Total 281 isolates were isolated from rhizospheric soil samples of given sites fromTaif agriculture filed of Kingdom of Saudi Arabia. In the first site of soil sample we got 57 isolates in which only 5.2% of isolates were showing the rhizobium biochemichal charecterstics while sampling site 2 were showing only 3.9% of rhizobium isolates among 51. On the other hand in the third soil samples the identified rhizobium isolates was only 8.9% among 56 isolates while in fourt and fifth soil samples it was 8.6% and 10.6% among 58 and 56 isolates respectively.All isolates were showing same phenotypically shape size and morphology on YEMA (Yeast extract Mannitol Agar) while on NA (Nutrient agar) were showing different morphology of bacterial isolates. Only 7.47 % (21) of isolates were showing the biochemical characterization of Rhizobium among all 281 isolates according to Bergey’s manual. In first soil sample only 3 isolates were showing Rhizobium on YEMA plates among 57 isolates. While in secound, third, fouth and five soil sample only 2, 5, 5 and 6 isolates were showing Rhizobium charecterstics among 51, 56, 58, and 59 isolates respectively.
Before the pot experiment we studied the seed germination of chick pea under different salt stress. Salinity has a negative impact on chickpea development and germination, as seen in the table 2. Particularly in stressful environments, the germination and seedling stages are crucial to plant survival and proper seedling establishment. The results of this investigation showed that rising salinity stress gradually inhibited chickpea seed germination and establishment. Seed germination was completely inhibited at a high salinity level of 100 mM NaCl.
According to numerous studies, increasing salinity levels decreased germination percentage and speed in the field for various legumes, including peas (Wolde and Adamu, 2018), chickpeas (Ashraf and Waheed, 1992), wheat (Majid et al., 2013), and other legumes (Esechie, 1995; Morais et al., 2012; Piwowarczyk et al, 2016). This fact demonstrates that the salinity increased slowly. This result demonstrates that salinity inhibits, and delays seed germination through a variety of mechanisms, including a reduction in water absorption, adjustments in the mobilisation of stored food, and disruptions in the structural organisation of proteins (Ibrahim, 2016).
The current study showed that saline levels dramatically affected plant leaf area, branches, height, plant biomass (fresh & dry), and plant mortality. The death of plants was observed at the high salinity concentration (100 mM NaCl) after four weeks of plant establishment. These findings agreed with earlier studies that had been published (Grozeva et al., 2019). The study’s findings clearly show that the toxicity of the salinity treatments manifests itself more visibly in dry weight. This result confirms earlier research that showed dry biomass production was more susceptible to growth inhibition by NaCl treatments (Rasool et al., 2013; Yousef et al., 2020). Additionally, the lack of water, ion toxicity, and nutrient imbalance brought on by the blocking of other nutrients, including N, P, K, Ca, and NO3, could all be contributing factors to the growth suppression (Hasegawa et al., 2000).
When cultivated in salt, other research has demonstrated comparable results in other legume plants, including sesbania (Mahmood et al., 2008), chickpea (Ashraf and Waheed, 1992), and pea plant (Hernandez et al., 1999). The outcome indicated that the number of leaves per plant was decreasing. Physiologically, salt stress has a detrimental impact on several processes, but the most notable effect is a reduction in cell division and expansion, which led to a decrease in leaf number. The results also showed that a high saline level reduced leaf area, which may have been caused by a decrease in cell division and cell expansion. These findings are consistent with another finding that indicated salt stress reduces the leaf surface expansion ratio, which leads to the stoppage of expansion (Kordrostami and Rabiei, 2019).
The purpose of this study was to look into how salt stress affected chickpea growth and nodulation. High salt stress hindered and postponed the germination and growth of chickpea plants, according to the study. The study found that whereas chickpeas are unaffected up to a salinity of 50 and can endure salinity at a level of 75 mM NaCl, this cultivar is extremely susceptible to 100 mM NaCl, and considerable salt-stress-related impairments were noted. However, compared to germination, plant development was more responsive to salt stress. The molecular, physiological, and metabolic alterations brought on by salinity stress must be the focus of future study. Additionally, comprehensive knowledge is necessary to comprehend this plant’s physiological responses to environmental factors in the field.
After the biochemical characterization of isolates we were tested the isolates for physical (Antibiotics, Ph, Temperature & salt) tolerance in which all the selected isolates were showing both sensitivity and resistance at various concentration (Figure 1 a). Streptomycin was effective against the isolates at doses of 10 µg/ml and were showing 52.08% resistance isolates and remain sensitive while 23.80%, 14.28% and 33.33% isolates were showing resistance to cloxacillin, ampicillin and penicillin respectively. 28.57% of isolates were showing resistance to ciprofloxacin at 5µg concentration of antibiotics disc. 33.33% and 38.09% isolates were resistance to Chloramphenicol and tetracycline respectively. The isolates varied in their resistance to and sensitivity to various antibiotics at various concentrations.
Only 10 (47.61%) of the 21 isolates exhibited nodulation in the chickpea under controlled condition. Chickpea plants were inoculated with Rhizobium isolates, grown in a control environment, exposed to various NaCl salt stress conditions (0, 50, 75, and 100 mM NaCl), and the growth parameters were examined up to four harvesting stages (4, 8, 12, and 16 days). The purpose of Wark was to determine whether salt stress affected chickpea development and nodulation. Data shows that high salt stress conditions affect plant growth and nodulation both during germination and nodulation.
The data clearly indicate that the dry weight of plant was increase at 50 and 75mM NaCl stress at three sucsessive harvesting stages while at 100 mM decrease the accumulation of dry mass at the rate of 16.21%, 26.15%,10.97% and 13.04% in first , secound, three and fourth harvesting stage respectively. With the salinity the root to shoot ratio increased at 0, 50 and 75mM at first, secound and third harvesting. But decrease at 100mM NaCl at every
harvesting stage. Although nodule dry weight remain decrease under the salt stress conditions except at the secound harvesting stage that is 8 day of treatment boost nodulation under the 50 & 75 mM NaCl salt stress conditions.
The growth rate didn’t noticeably slow down until the highest salt dosage was applied (Table 1). Only at the limited level of salt stress i.e is 50 & 75mM RSR increased. This reveals due to short-term response to salinity and increases RSR in the above samplings were hardly significant. Although on average nodule dry weight remain decrease under the salt stress conditions at high concentration. The above results justified by the other researcher those who work on effects of NaCl on the growth of legumes (Egamberdieva et al., 2016).
Table 1. Different collection of soil samples and number of isolated Rhizobacterial isolates
| Different rhizosphere soil Samples | ||||||
| Site 1 | Site 2 | Site3 | Site 4 | Site 5 | ||
| No of isolates (YEMA) | 57 (3)
5.2% |
51(2)
3.9 % |
56 (5)
8.9% |
58(5)
8.6 % |
59 (6)
10.6% |
T=281 (21)
7.47% |
| Total No of Isolates | 57 | 51 | 56 | 58 | 59 | |
Table 2. Biochemical characterization of Rhizobium isolates
| No of isolates | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | |
| Gram’s staining | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Morphology | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | |
| Biochemical Test | Indole test | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Methyl red test | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| Voges Proskauer | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| Catalase test | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| Nitrate reduction | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| Citrate utilization | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Starch Hydrolysis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| Gelatine Hydrolysis | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Oxidase test | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Carbohydrate utilization | Dextrose | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Mannitol | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| Lactose | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Sucrose | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |
Table 3. Effect of growth under salt stress (NaCl) at four different harvesting stages. PDW;
Plant dry weight, RSR; Root to Shoot Ratio and NDW; Nodule dry weight
| Antibiotics | Concentration/disc | Tolerant isolates (%) |
| Nalidixic acid (Na) | 30μg | 4 (19.04) |
| Cloxacillin (Cx) | 10μg | 5(23.80) |
| Chloramphenicol (C) | 30μg | 7 (33.33) |
| Tetracycline (Tc) | 30μg | 8 (38.09) |
| Streptomycin (S) | 10μg | 11 (52.08) |
| Ciprofloxacin (Cf) | 5μg | 6 (28.57) |
| Ampicillin (Am) | 10μg | 3 (14.28) |
| Penicillin (PEN) | 10μg | 7 (33.33) |
Table 4. Antibiotic tolerance of isolated Rhizobial isolates under different antibiotic concentrations.
Parentheses indicate percent tolerant isolates.
| Antibiotics | Concentration/disc | Tolerant isolates (%) |
| Nalidixic acid (Na) | 30μg | 4 (19.04) |
| Cloxacillin (Cx) | 10μg | 5(23.80) |
| Chloramphenicol (C) | 30μg | 7 (33.33) |
| Tetracycline (Tc) | 30μg | 8 (38.09) |
| Streptomycin (S) | 10μg | 11 (52.08) |
| Ciprofloxacin (Cf) | 5μg | 6 (28.57) |
| Ampicillin (Am) | 10μg | 3 (14.28) |
| Penicillin (PEN) | 10μg | 7 (33.33) |
Figure :1; a, b, c and d represent percent isolates of Rhizobium under the condition
of antibiotics, pH, Temperature and salt (NaCl) stress.
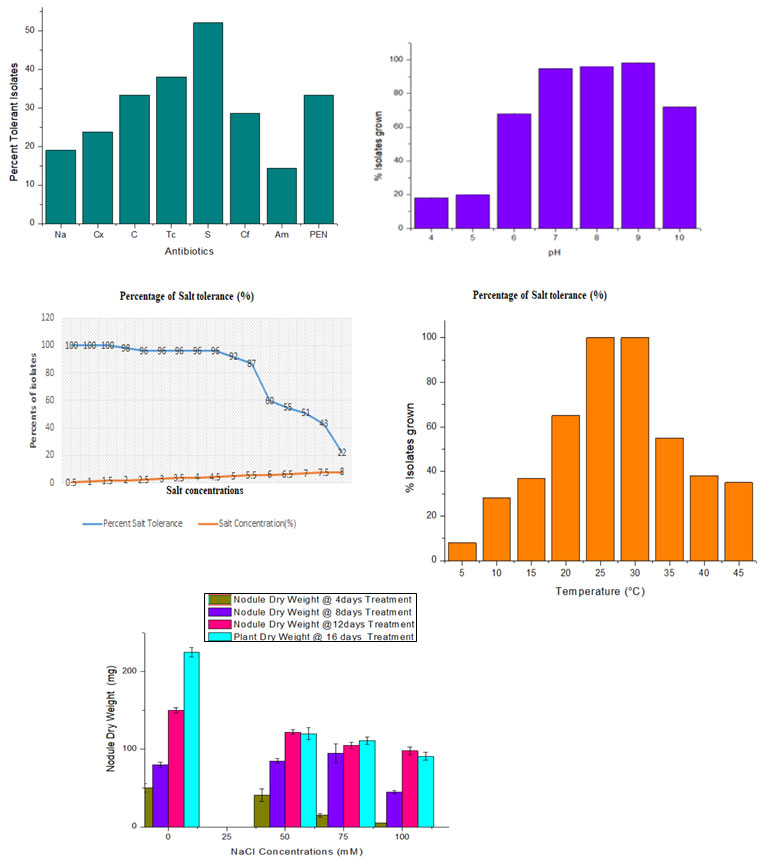
Figure: 2 a, b and c represents nodule dry weight, root to shoot ratio and plant dry weight
respectively under different salt (NaCl) stress conditions at four different harvesting
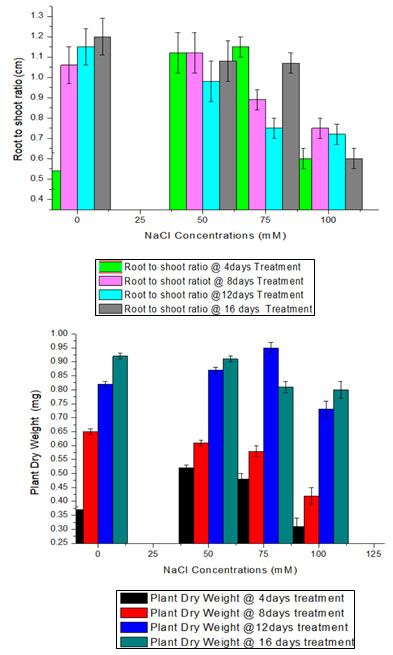
Figure: 3 (A-d) represents the nodulation in chickpea using isolated isolates (RTf-7, RTf-15, and RTf-17)
while the figure (K-N) showing an un inoculated check pea.

CONCLUSION
The current work focuses on the isolation and biochemical characterization of Rhizobium isolates from Saudi desert soil in the Taif region, as well as their impact on chickpea growth and nodulation under various salt stress conditions. The physiological tolerance of each Rhizobium isolate was examined (temperature, salt, pH, and antibiotic resistance). According to the study, rhizobial isolates are very susceptible to abiotic variables such as salt, pH, and temperature. Further, we investigate the impact of NaCl administered during the vegetative growth and nodulation of chickpea plants. We conclude that abiotic stress, such as salt stress, poses major hazards to plants.
ACKNOWLEDGEMENTS
We would like to acknowledge Department of Biology & Biochemistry College of Science, University of Jeddah, Saudi Arabia for providing us with conveniences and facility for this research work.
Conflict of Interests: The authors declare no potential conflict of interests.
REFERENCES
Ashraf, M. and Waheed, A., 1992. Screening chick-pea (Cicer arietinum L.) for salt tolerance. Der Tropenlandwirt-Journal of Agriculture in the Tropics and Subtropics, 93(1), pp.45-55.
Ashraf, M. and Waheed, A., 1992. Screening chick-pea (Cicer arietinum L.) for salt tolerance. Der Tropenlandwirt-Journal of Agriculture in the Tropics and Subtropics, 93(1), pp.45-55.
Banik, A., Pandya, P., Patel, B., Rathod, C. and Dangar, M., 2018. Characterization of halotolerant, pigmented, plant growth promoting bacteria of groundnut rhizosphere and its in-vitro evaluation of plant-microbe protocooperation to withstand salinity and metal stress. Science of the Total Environment, 630, pp.231-242.
Bergey, D.H., Breed, R.S., Murray, E.G.D. and Hitchens, A.P., 1939. Manual of determinative bacteriology. Manual of determinative bacteriology. Fifth Edn.
Bhat, M.A., Kumar, V., Bhat, M.A., Wani, I.A., Dar, F.L., Farooq, I., Bhatti, F., Koser, R., Rahman, S. and Jan, A.T., 2020. Mechanistic insights of the interaction of plant growth-promoting rhizobacteria (PGPR) with plant roots toward enhancing plant productivity by alleviating salinity stress. Frontiers in microbiology, 11, p.1952.
Chakraborty, S. and Harris, J.M., 2022. At the crossroads of salinity and rhizobium-legume symbiosis. Molecular Plant-Microbe Interactions, 35(7), pp.540-553.
Cordovilla, M.P., Ligero, F. and Lluch, C., 1996. Growth and nitrogen assimilation in nodules in response to nitrate levels in Vicia faba under salt stress. Journal of experimental botany, 47(2), pp.203-210.
Datta, A., Singh, R.K. and Tabassum, S., 2015. Isolation, characterization and growth of Rhizobium strains under optimum conditions for effective biofertilizer production. Int. J. Pharm. Sci. Rev. Res, 32(1), pp.199-208
Deka, A.K. and Azad, P., 2006. Isolation of Rhizobium strains: cultural and biochemical characteristics. Legume Research-An International Journal, 29(3), pp.209-212.
Egamberdieva, D., Jabborova, D. and Berg, G., 2016. Synergistic interactions between Bradyrhizobium japonicum and the endophyte Stenotrophomonas rhizophila and their effects on growth, and nodulation of soybean under salt stress. Plant and soil, 405, pp.35-45.
El Moukhtari, A., Cabassa-Hourton, C., Farissi, M. and Savouré, A., 2020. How does proline treatment promote salt stress tolerance during crop plant development? Frontiers in plant science, 11, p.1127
Elsheikh, E.A.E. and Wood, M., 1990. Effect of salinity on growth, nodulation and nitrogen yield of chickpea (Cicer arietinum L.). Journal of Experimental Botany, 41(10), pp.1263-1269.
Esechie, H.A., 1995. Partitioning of chloride ion in the germinating seed of two forage legumes under varied salinity and temperature regimes. Communications in soil science and plant analysis, 26(19-20), pp.3357-3370.
Etesami, H. and Adl, S.M., 2020. Can interaction between silicon and non–rhizobial bacteria help in improving nodulation and nitrogen fixation in salinity–stressed legumes? A review. Rhizosphere, 15, p.100229.
Hasegawa, P.M., Bressan, R.A., Zhu, J.K. and Bohnert, H.J., 2000. Plant cellular and molecular resp onses to high salinity. Annual review of plant biology, 51(1), pp.463-499.
Hernández, J.A. and Almansa, M.S., 2002. Short‐term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Physiologia Plantarum, 115(2), pp.251-257.
Hernandez, L., Pinyol, M., Hernández, S., Beà, S., Pulford, K., Rosenwald, A., Lamant, L., Falini, B., Ott, G., Mason, D.Y. and Delsol, G., 1999. TRK-fused gene (TFG) is a new partner of ALK in anaplastic large cell lymphoma producing two structurally different TFG-ALK translocations. Blood, The Journal of the American Society of Hematology, 94(9), pp.3265-3268.
Ibrahim, E.A., 2016. Seed priming to alleviate salinity stress in germinating seeds. Journal of plant physiology, 192, pp.38-46.
Jamil, M., Bashir, S.A.M.I.N.A., Anwar, S., Bibi, S., Bangash, A., Ullah, F. and Rha, E.S., 2012. Effect of salinity on physiological and biochemical characteristics of different varieties of rice. Pakistan Journal of Botany, 44(2), pp.7-13.
Kordrostami, M. and Rabiei, B., 2019. Salinity stress tolerance in plants: physiological, molecular, and biotechnological approaches. Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches, pp.101-12
Kulkarni, S. and Nautiyal, C.S., 2000. Effects of salt and pH stress on temperature-tolerant Rhizobium sp. NBRI330 nodulating Prosopis juliflora. Current Microbiology, 40, pp.221-226.
Ma, Y., Dias, M.C. and Freitas, H., 2020. Drought and salinity stress responses and microbe-induced tolerance in plants. Frontiers in Plant Science, 11, p.591911.
Maatallah, J., Sanjuan, J. and Lluch, C., 2002. Phenotypic characterization of rhizobia isolated from chickpea (Cicer arietinum) growing in Moroccan soils. Agronomie, 22(3), pp.321-329.
Mahmood, A., Athar, M., Qadri, R. and Mahmood, N., 2008. Effect of NaCl salinity on growth, nodulation and total nitrogen content in Sesbania sesban. Agriculturae Conspectus Scientificus, 73(3), pp.137-141.
Majid, A., Mohsen, S., Mandana, A., Saeid, J.H., Ezatollah, E. and Fariborz, S., 2013. The effects of different levels of salinity and indole-3-acetic acid (IAA) on early growth and germination of wheat seedling. Journal of Stress Physiology & Biochemistry, 9(4), pp.329-338.
Morais, M.C., Panuccio, M.R., Muscolo, A. and Freitas, H., 2012. Does salt stress increase the ability of the exotic legume Acacia longifolia to compete with native legumes in sand dune ecosystems?. Environmental and Experimental Botany, 82, pp.74-79.
Pitman, M.G. and Läuchli, A., 2002. Global impact of salinity and agricultural ecosystems. Salinity: environment-plants-molecules, pp.3-20.
Piwowarczyk, B., Tokarz, K. and Kamińska, I., 2016. Responses of grass pea seedlings to salinity stress in in vitro culture conditions. Plant Cell, Tissue and Organ Culture (PCTOC), 124, pp.227-240.
Rafique, M., Naveed, M., Mustafa, A., Akhtar, S., Munawar, M., Kaukab, S., Ali, H.M., Siddiqui, M.H. and Salem, M.Z., 2021. The combined effects of gibberellic acid and rhizobium on growth, yield and nutritional status in chickpea (Cicer arietinum L.). Agronomy, 11(1), p.105.
Rasool, S., Ahmad, A., Siddiqi, T.O. and Ahmad, P., 2013. Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta physiologiae plantarum, 35, pp.1039-1050.
Shilev, S., 2020. Plant-growth-promoting bacteria mitigating soil salinity stress in plants. Applied Sciences, 10(20), p.7326.
Sindhu S, Dahiya A, Gera R, Sindhu SS. Mitigation of abiotic stress in legume-nodulating rhizobia for sustainable crop production. Agricultural Research. 2020 Dec; 9:444-59.
Skujins, J., 1991. Nutrient Cycling in of Semiarid Arid: Soils Regions. In Semiarid Lands and Deserts (pp. 307-344). CRC Press.
Sunita, K., Mishra, I., Mishra, J., Prakash, J. and Arora, N.K., 2020. Secondary metabolites from halotolerant plant growth promoting rhizobacteria for ameliorating salinity stress in plants. Frontiers in Microbiology, 11, p.567768.
Warsi, M. K, Bee, N. and Hashim, M., isolation and molecular identification of pesticides tolerant bacteria in agriculture soil of rampur (up). International Journal of Biotechnology and Research, Vol. 7, Issue 1, Feb 2017, 25-40
Weimberg, R. and Shannon, M.C., 1988. Vigor and salt tolerance in 3 lines of tall wheatgrass. Physiologia Plantarum, 73(2), pp.232-237.
Wolde, G. and Adamu, C., 2018. Impact of salinity on seed germination and biomass yields of field pea (Pisum sativum L.). Asian J. Sci. Tech, 9, pp.7565-7569.
Wolde, G. and Adamu, C., 2018. Impact of salinity on seed germination and biomass yields of field pea (Pisum sativum L.). Asian J. Sci. Tech, 9, pp.7565-7569.
Yousef, F., Shafique, F., Ali, Q. and Malik, A., 2020. Effects of salt stress on the growth traits of chickpea (Cicer arietinum L.) and pea (Pisum sativum L.) seedlings. Biological and Clinical Sciences Research Journal, 2020(1).
Zahran, H.H., 1999. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiology and molecular biology reviews, 63(4), pp.968-989.


