1Department of Biochemistry, AIIMS Bhopal, India
2SOS in Biochemistry, Jiwaji University, Gwalior, India
Corresponding author email: shivjayant7aug@gmail.com
Article Publishing History
Received: 15/10/2019
Accepted After Revision: 25/12/2019
Diabetes is associated with imbalance of antioxidant defense mechanism, causes alteration in various biomolecules, including DNA and increases the level of serum glucose, superoxide anion, and hydrogen peroxide. It is known to induce toxic effect on pancreas, liver, brain and biochemical parameters.The excessive reactive oxygen species (ROS) damage lipid and DNA. Reactive oxygen species are responsible for histological changes in architecture of liver, brain tissue. Alloxan induced diabetes produces oxidative stress induced damage in rat tissues. Diabetic rats treated with oral administration of aqueous suspension of Ocimum sanctum and Allium sativum daily for 14 days showed decrease in the level of glucose, superoxide anion, hydrogen peroxide, tail lengh of DNA damage and regeneration of liver, brain tissues cells. Results showed that diabetic group treated with aqueous suspension of Ocimum sanctum and Allium sativum reduced the level of oxidative stress, cytotoxicity and also serum blood glucose levels.
Diabetes, DNA damage, Histopathology, Ocimum sanctum, Allium sativum
Jayant S. K, Srivastava N. Effects of Ocimum sanctum and Allium sativum Extracts Against Diabetes and Determination of DNA Damage And Cytotoxicity in Alloxan Induced Diabetic Rats. Biosc.Biotech.Res.Comm. 2019;12(4).
Jayant S. K, Srivastava N. Effects of Ocimum sanctum and Allium sativum Extracts Against Diabetes and Determination of DNA Damage And Cytotoxicity in Alloxan Induced Diabetic Rats. Biosc.Biotech.Res.Comm. 2019;12(4). Available from: https://bit.ly/2oQaCYK
Copyright © Jayant and Srivastava This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Diabetes mellitus is a chronic metabolic disease. It is one of the most challenging health problems in the 21stcentury (Narayan et al., 2017). The prevalence of diabetes is increasing globally and the number of diabetics is expected to increase to 366 million by 2030 (Zimmet et al., 2017). Diabetes is associated with increased oxidative stress that results in damage of several cellular biomolecules such lipid, carbohydrates and protein (Tan et al., 2018). Oxygen-free radicals induce a variety of lesions in DNA, including oxidized bases, abasic sites, DNA strand breaks and formation of cross-links between DNA and proteins (Klages-Mundt et al., 2017). Bigagli et al., (2019) reported that the products generated by oxidative DNA damage are significantly elevated in diabetes mellitus (DM) and the pattern of modification was the same as one expected from the attack of the hydroxyl radical (OH•) upon DNA. Moreover, it has been shown that hydroxyl radical is produced by the Fenton reaction in the presence of transition metal ions is responsible for DNA damage (Liguori et al., 2018).
Oxidative stress is the imbalance between the production and scavenging of ROS and biological system’s ability to readily detoxify the reactive intermediates or to repair the resulting damage (Andersson et al., 2018). Disturbances in the normal redoxstatus of tissues can cause toxic effects through the production of peroxides and free radicals that damage all components of the cell, including proteins, lipids, and DNA (Roy et al., 2017). In humans, oxidative stress is involved in many diseases including atherosclerosis, Parkinson’s disease, heart failure, myocardial infarction, Alzheimer’s disease, schizophrenia, bipolar disorder, fragile X syndrome, etc.(Manna et al., 2018). Several drugs, xenobiotics and environmental pollutants are known to cause this imbalance between formation and removal of free radicals (Aguilar et al., 2016). Biological antioxidants including vitamins can prevent this uncontrolled formation of free radicals and activated oxygen species or inhibit their reaction with biological structures (Tan et al., 2018). The destruction of most free radicals and ROS rely on the oxidation of endogenous antioxidants and reducing molecules (Ighodaro et al., 2018). It is observed that in case of Alloxan induced diabetes, DNA damage comet tail length increases (Zhong et al., 2018).
The present study was aimed to evaluate the possible cytoprotective effect of the Ocimum sanctum and Allium sativum plant extracts and determine the antidiabetic propertes of O. sanctum and A. sativum plant extracts in the treatment of type I diabetic rats, compared to normal control and untreated diabetic rats.
MATERIAL AND METHODS
Experimental Animals
Male albino rats of Wistar strain (weight 120 ± 20 g) were used in the proposed study. Animals were obtained from the animal house facility of the Defense Research and Development Establishment, Gwalior, India, and were maintained under controlled conditions of temperature (25 ± 20C), relative humidity of (50 ± 15%), and normal photoperiod (light-dark cycle of 12 h). The rats were maintained in the animal room of School of Studies in Biochemistry on standard pellet diet and tap water ad lib. Animals were housed throughout the experiment in polypropylene cages containing paddy husk as bedding and allowed to acclimatize to the environment of animal room for 7 days before the start of experiment.
Experimental Design
Thirty six rats were randomly divided into six groups of six rats each. Animals were divided into six groups and were given following treatments:
Group 1 : Control (normal blood glucose level).
Group 2 : Treated control group (treated with aqueous suspension of O. sanctum 2.5 mg/kg body weight).
Group 3 : Treated control group (treated with aqueous suspension of A. sativum 0.25 mg/kg body weight).
Group 4 : Diabetic (I.V. injection of alloxan 70 mg/kg body weight).
Group 5 : Treated diabetic group (treated with aqueous suspension of O. sanctum 2.5 mg/kg body weight).
Group 6 : Treated diabetic group (treated with aqueous suspension of A. sativum 0.25 mg/kg body weight).
Induction of experimental diabetes and plant extract treatment
Type I diabetes was induced by giving single intravenous injection of alloxan monohydrate 70 mg/kg body weight, dissolved in 0.9% solution of sodium chloride (Misra et al., 2012). The animals were checked for blood glucose level after 48 h and rats with blood sugar level above 200 mg/dl were used for the experiment.Ocimum sanctum (Tulsi) leaves were sourced from the Botanical Garden of Jiwaji University, Gwalior, and were cleaned and aqueous extract was prepared. A total of 2.5 mg/kg body weight of this extract was given orally to the rats of group 2 and 5 with the help of cannula, daily for two weeks.
Allium sativum (Garlic) seeds were purchased from the local herbal market, cleaned, and aqueous extract was prepared and administered art the rate of 0.25 mg/kg body weight orally to the rats of group 3 and 6 with the help of cannula, daily for two weeks.Rats were humanely killed after the last treatment by cervical dislocation. The different tissues were excised off, washed with 0.9% NaCl and used for different estimations. Animals were handledand treated ethically and were sacrificed humanely as per rules and instructions of Ethical Committee of Animal Care of Jiwaji University, Gwalior, India, in accordance with the Indian National law on animal care and use.
Superoxide Anion
Superoxide anion release was measured by superoxide dismutase inhabitable reduction of ferricytochrome-c (Cohen et al., 1980). The tissue homogenate was incubated in PBS-EDTA buffer (EDTA 5 mM, pH 7.4) with phorbol-12, 13-myristate (PMA) at 370C for 15 min. The total assay volume was 1 ml, the final concentrations of ferricytochrome-c and PMA were 50 µmol/L, and 100 nmol/L, respectively. The change in absorbance was measured spectrophotometrically at 550 nm for 10 min with a double beam Shimadzu UV-1800A spectrophotometer at room temperature. The amount of superoxide anion secreted into the medium was calculated on the basis of the molar extinction coefficient of reduced cytochrome c as 2.1×104 M−1 cm−1 and values are expressed as n mole O2.−/ min.
Hydrogen peroxide
Hydrogen peroxide in tissue was measured by the method of Pick et al., (1986). For assay of H2O2, 100 µl of tissue homogenate prepared in Tris-HCl buffer (0.02M, pH 7.5), 100 µl of assay solution (containing 0.2 ml phenol red, 0.2 g/l and 0.2 ml of horse radish peroxidase, 20 U/ml in potassium phosphate buffer, 0.05 M, pH 7.0 and 9.6 ml of 0.9% NaCl), was taken and reaction was started by the addition of 10 µl of 1.0 N NaOH. Absorbance was recorded at 600 nm in a microplate using ELISA reader. Hydrogen peroxide standard curve was plotted by taking different concentrations of H2O2, ranging from 20 to 100 µmol in a total volume of 100 µl and processed in the same way. Results are expressed as µmol H2O2 formed ml-1 preparation.
Single cell gel electrophoresis (SCGE) or Comet assay
Single strand breaks were measured by alkaline comet assay as described by Sasaki et al., (1997). Fresh tissues were collected and homogenates (25% w/v) were prepared in homogenizing buffer (0.075 M NaCl containing 0.024 M EDTA, pH 7.5) with a single stroke.To obtain nuclei, homogenates were centrifuged at 700 g for 10 min and the resulting pellets were gently resuspended in 4.0 ml of chilled homogenizing buffer.Seventy five ml of normal melting agarose (1% prepared in 0.1 M sodium phosphate buffer, pH 7.2 containing 0.9% NaCl) was quickly layered on an end frosted slide, covered gently with another slide, and allowed to solidify.The upper slide was gently removed and the precoated slide was coated with 100 ml of mixture containing equal volumes of sample (nuclei preparation) and low melting agarose (2% in phosphate buffer saline). Slides were then immersed in the lysis buffer (containing 0.25 M NaCl, 100 mM EDTA, 10 mMTrizma base, 1% sarcosine, pH 10.0 adjusted with 10 N NaOH; 5% DMSO and 1% Triton X-100 was added just before use) for 1 h at 4°C in the dark. After lysis, the slides were rinsed with chilled distilled water, transferred on a horizontal electrophoresis platform and immersed in electrophoresis buffer (300 mM sodium hydroxide and 1 mM EDTA, pH > 13.0) for 20 min for unwinding of DNA.Electrophoresis was performed for 20 min at constant voltage (1 V/cm and 300 mA). After electrophoresis, the slides were washed thrice with neutralizing buffer (0.4 M Tris-HCl, pH 7.4) for 5 min each.Slides were dehydrated in absolute methanol for 10 min and left at room temperature to dry. The whole procedure was performed in dim light to minimize artefactual DNA damage. Just before visualization, each slide was stained with 50 µl of ethidium bromide (20 µg/ ml), rinsed with water, and covered with a cover slip. A total of 50 cells were scored per tissue per animal (25 from each replicate slide). Analyses were performed on the basis of the type of comet visualized on the slide. The nuclei were counted and divided into five types as stage 0, 1, 2, 3 and 4.The DNA damage index was calculated as # 0 + # 1 + # 2 + # 3 + # 4/ # of cell scored. Two independent experiments were conducted in each treatment. The animal (and not the cell) was used as the experimental unit. Slides were viewed under fluorescence microscope (Leica 4000B Digital Microscope). Analyses were performed at 100X magnification, with a Leica Optiphase microscope equipped with an excitation filter of 515-560 nm and barrier filter of 590 nm.
Histopathology: For histopathological analyses, tissues were collected at the time of sacrifice, freed from fat bodies, washed with normal saline and fixed in Bouin’s fluid for 12–24 h. After fixation, tissues were washed overnight under running tap water to remove excess fixative, and embedded in paraffin blocks. Paraffin blocks were cut at 4 µm for liver and pancreas and at 12 µm for brain with the help of semi-automated microtome (Leica EG 1106 Microtome). Six slides per tissue were prepared and stained with hematoxylin and eosin (McManus et al., 1960). Stained tissue sections were mounted in DPX, covered with cover slip and viewed under light microscope at 10X magnification (Leica Optiphase microscope).
Statistical analysis
Results are expressed as mean ± S.E. of different sets of observation taken on different days.All the statistical analyses were performed using one-way analysis of variance (ANOVA) with post hocDunnett’s multiple comparison test applied across treatment groups for each tissue.Significance level was based on p< 0.05.
Results
Blood glucose
The blood glucose level of all the rats was tested by taking the blood from the tail vein and using electronic glucometer. Administration of alloxan (70 mg/kg, i.v) led to 4-fold elevation of fasting blood glucose levels, which was maintained for a period of 2 weeks (Table 1).It was observed that oral administration of aqueous extract of Ocimum sanctum and Allium sativum, significantly decreased the blood glucose levels in diabetic as compared to the blood glucose level of control rats. The results of the present study showed that oral administration ofextract of O. sanctum and Allium sativum daily for 14 days, to the diabetic rats caused 23.5%, and 22% decrease on 7th day and 43.72%, and 41.2% decrease inthe blood glucose level on day 14th of the start of treatmet when compared with respective untreated diabetic rats.The results clearly showed the hypoglycemic potential of O. sanctum and A. sativum extracts.
Table 1: Effect of oral treatment of extracts of Ocimum sanctum and Allium sativum for 7, 14 days on levels of level of blood glucose in control and alloxan induced diabetic rats.
| S No. | Level of glucose in experimental animals Groups 0 day 7th day 14th day |
|||
| 1. | Control | 103.67±2.4 | 109.00±2.65 | 111.67±3.53 |
| 2. | Control + Ocimum sanctum | 109.67±1.76# | 106.33±1.86# | 100.67±2.6# |
| 3. | Control + Allium sativum | 110.33±2.6# | 107.67±1.76# | 102.67±2.96# |
| 4. | Diabetic | 413.00±8.14*** | 419.00±6.66*** | 422.33±6.36*** |
| 5. | Diabetic + Ocimum sanctum | 421.00±7.21*** | 320.67±3.48*** | 237.67±6.17*** |
| 6. | Diabetic + Allium sativum | 418.67±4.63*** | 326.67±3.28** | 248.33±2.91*** |
Glucose concentration is expressed as mg/dl. Values are given as mean ± S.E. of six set of observation. Values are significance at p>0.05 #, p<0.05 *, p<0.01 **, p<0.001 ***. Diabetic rats were compared with control rats, Ocimum sanctum and Allium sativum treated diabetic rats as compared with diabetic control rats.
Superoxide anion
Superoxide anion (O2.−) production in diabetic rat tissues was monitored by the superoxide dismutase inhabitable cytochrome-c reduction assay. Diabetic rats showed 106.6% and 151.7% increase in O2.−production in the liver and the brain when compared with control (Table 2). Diabetic rats given oral administration of O. sanctum and A. sativum extracts daily for 14 days, showed 36.5%, and 33.4% decrease in the rate of production of O2.− in the liver and 41.2%, 33.1% in the brain superoxide anion production when compared with diabetic rats. Feeding of extracts of O. sanctum and A. satiuam to the control rats 14 days,showed 16.3%, and 8.1% decrease in the rate of production of O2.− radicals in the liver while 16.6% and 8.4% decrease in superoxide anion production was observed in the brain when compared tissues of untreated control rats.
Table 2: Effect of oral treatment of extracts of Ocimum sanctum and Allium sativum for 14 days on levels of superoxide anion in the tissues of control and alloxan induced diabetic rats.
| S No. | Groups | Brain | Liver |
| 1. | Control | 4.76±0.28 | 9.68±0.42 |
| 2. | Control + Ocimum sanctum | 3.97±0.16# | 8.10±0.27* |
| 3. | Control + Allium sativum | 4.36±0.21# | 8.89±0.42* |
| 4. | Diabetic | 11.98±0.35*** | 20.00±0.55*** |
| 5. | Diabetic + Ocimum sanctum | 7.05±0.20*** | 12.70±0.16*** |
| 6. | Diabetic + Allium sativum | 8.02±0.21*** | 13.33±0.27*** |
Superoxide anion concentration is expressed as n mole. Results are expressed as mean ± S.E. of six set of observation. Significance is based on p>0.05#, p<0.05*, p<0.01**, p<0.001 Diabetic rats were compared with control rats, Ocimum sanctum and Allium sativum treated diabetic rats as compared with diabetic control rats.
Hydrogen peroxide
Levels of hydrogen peroxide also increased in the tissues of diabetic rats. The results showed that there was significantly high concentration of H2O2 in the liver and the brain of diabetic rat tissues. An increase of 128.9% and 84.1% in the level of H2O2 in the liver and the brain of diabetic rats was observed when compared with control. Oral administration of extract of O. sanctum and A. sativum for 14 days to diabetic and control rats showed reduction in the level of H2O2.The treated diabetic rats showed 33.7% and 29.8% decrease in the liver and 35.2% and 30% decrease in the brain levels of H2O2 as compared with diabetic rat tissues, respectively. Control rat tissues treated with O. sanctum and A. sativum extracts for 14 days, showed 8.2%, 4.9% decrease in the liver and 7.8%, 4.2% decrease in the brain H2O2levels when compared with unteated control rat tissues, respectively. The same O. sanctum and A. sativum treatment of diabetes caused 51.8%, 60.7% increase in the liver and 19.2%, 28.8% increase in the brain H2O2 levels when compared with control rat tisuues (Table 3).
Table 3: Effect of oral treatment of extracts of Ocimum sanctum and Allium sativum for 14 days on the levels of hydrogen peroxide in the tissues of control and alloxan induced diabetic rats.
| S No. | Groups | Brain | Liver |
| 1. | Control | 52.42±0.40 | 50.45±0.26 |
| 2. | Control + Ocimum sanctum | 47.88±0.40* | 46.54±1.68# |
| 3. | Control + Allium sativum | 49.84±0.15* | 48.34±0.15* |
| 4. | Diabetic | 120.00±0.26*** | 92.88±1.58*** |
| 5. | Diabetic + Ocimum sanctum | 79.55±1.05*** | 60.15±0.84*** |
| 6. | Diabetic + Allium sativum | 84.24±1.18*** | 64.99±0.79*** |
Hydrogen peroxide concentration is expressed as µ mole/ml. Results are expressed as mean ± S.E. of six set of observation. Significance is based on p>0.05#, p<0.05*, p<0.01**, p<0.001*** Diabetic rats were compared with control rats, Ocimum sanctum and Allium sativum treated diabetic rats as compared with diabetic control rats.
Diabetes induced DNA damage
Results of the present study clearly showed that intravenous injection of alloxan caused significantly marked DNA damage in all the rat tissues examined, as evidenced by the increase in the number of nuclei of Type I, II, III and IV. In the tissues of control rats, almost all the nuclei were of Type 0, the typical condensed type, round nuclei indicating intact DNA. The result showed that a single I. V. dose of alloxan caused 1304.5% and 1471.4% increase in DNA damage index in liver and brain tissue of rats, when compared with control rats’ tissue and measured diabetic rats’ tissue after 14 days of alloxan exposure (Table 4). Results showed that oral administration of O. sanctum and A. sativum plants extract daily for 14 days, to the diabetic rats caused 74.8%, 68.6% decrease in DNA damage index in liver and 75.2%, 69.1% decrease in DNA damage index in brain when compared with diabetic untreated group. O. sanctum and A. sativum treatmemt for 14 days to the control rats showed 13.6%, 9.1% decrease DNA damage index in liver and 33.3%, 9.5% decrease in DNA damage index in brain when compared with control group tissue (Figure 1).
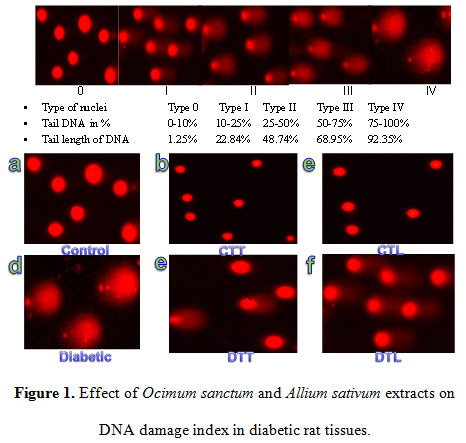 |
Figure 1: Figure 1. Effect of Ocimum sanctum and Allium sativum extracts on DNA damage index in diabetic rat tissues. |
Histopathological Studies in Diabetes
The light microscopic changes in the tissues of alloxan exposed animals were compared with respective controls. The liver of control and control treated oral O. sanctum and A. sativum plants extract for two weeks rats showed normal histopathological structure of the central vein and surrounding hepatocytes (Figure2a, 2c and 2d). Alloxan exposed rats showed severe dilation and congestion of the central vein with degeneration in the surrounding hepatocytes in the liver.Inflammatory cell infiltration in portal area, surrounding the congested portal vein with degeneration in the hepatocytes, was observed in alloxan treated rats liver. Diffuse kupffer cells proliferation between the degenerated hepatocytes was also observed in some treated tissues.The hepatic blood vessels showed congestion, fibrosis and infiltration of red blood cells (Figure2b) When the diabetic rats were given O. sanctum and A. sativum oral treatment for two weeks and its showed regenerating liver tissue (Figure2e and 2f) which could be comparable to that of non-diabetic control rats (Figure2).
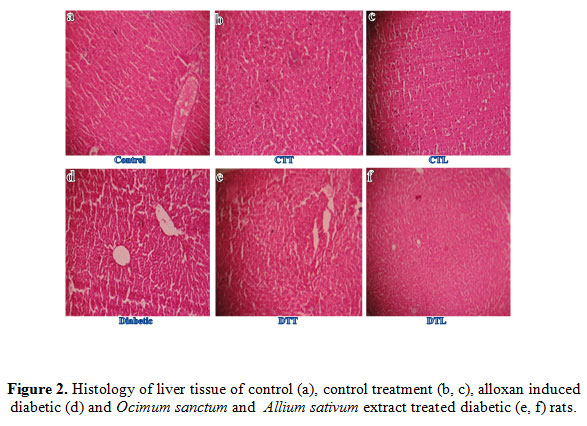 |
Figure 2: Figure 2. Histology of liver tissue of control (a), control treatment (b, c), alloxan induced diabetic (d) and Ocimum sanctum and Allium sativum extract treated diabetic (e, f) rats. |
The light microscopic changes in brain of experimental animals were compared to those of controls. Alloxan exposure caused degenerative morphological changes in brain and these changes were clear in brain of rats receiving single dose of alloxan. Mild ischemia was seen in brain of rats and Changes in nuclear shape and chromatin condensation were seen in the brain of rats. Alloxan exposure caused marked changes in brain including affected granular cell neurons in cerebrum, disruption of cerebral cortex with neuronal loss and gliosis (Figure3b). These diabetic rats were given O. sanctum and A. sativum oral treatment for two weeks and its showed regenerating brain tissue (Figure3e and 3f) which could be comparable to that of non-diabetic control rats. These histological observations showed the protective role of polyphenolic extract on brain in alloxan induced diabetic rats. Result of brain control and control treatments O. sanctum and A. sativum tissue rats showed normal nuclear shape, chromatin condensation of brain rats (Figure3a, 3c and 3d) and no histopathological alterations were observed in these animals (Figure3).
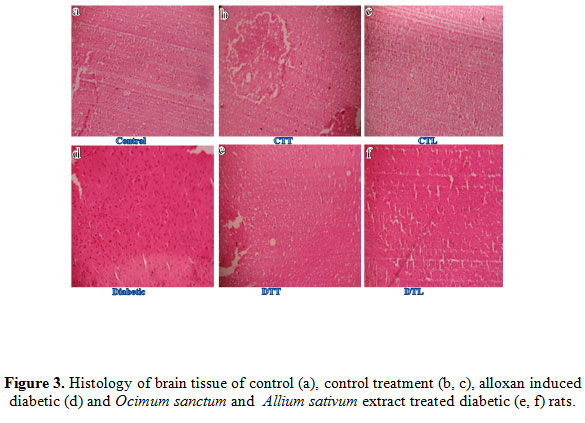 |
Figure 3: Histology of brain tissue of control (a), control treatment (b, c), alloxan induced diabetic (d) and Ocimum sanctum and Allium sativum extract treated diabetic (e, f) rats. |
DISCUSSION
ROS play an important role in the deterioration of diabetes, causing high chemical reactivity and capable of damaging lipids, proteins and DNA (Nita et al., 2016). Further, it can be stated that the free radical scavenging property reduces the DNA damage in alloxan induced diabetic rats. Higher levels of MDA and 4-HNE, resulted from lipid peroxidation process were reported in the alloxan induced rats, which could be due to the poor glycemic control and high production of free radicals. MDA and 4-HNE are known to interact with DNA (Liguori et al., 2018). Such interactions can cause DNA damage, which can lead to cytotoxicity and genotoxicity (Lui et al., 2016). Recently, significant increase in the level of 8-oxodeoxyguanosine, a marker for oxidative damage have been reported in the diabetic rat tissues (Ortiz et al., 2016). As cellular enzymes efficiently repair DNA damage, its measurement gives a snapshot view of the level of oxidative stress, in contrast to measurement of oxidation of other biomolecules which are not repaired and have a slow turnover such as lipids or proteins. DNA oxidation may therefore be of considerable value in following the progress of the disease and its metabolism (Nasif et al., 2016). Comet assay was performed to detect the oxidative DNA damage and alkylated bases in diabetes.
Oxidative stress depicts the existence of products called free radicals and ROS which are formed under normal physiological conditions but become deleterious when not being quenched by the antioxidant systems (Fang et al., 2002). There are convincing experimental and clinical evidences that the generation of reactive oxygen species is increased in both types of diabetes and that the onset of diabetes is closely associated with oxidative stress (Johansen et al., 2005, Rosen et al., 2001). Free radicals are formed disproportionately in diabetes by glucose autoxidation, polyol pathway and non-enzymatic glycation of proteins (Obrosova et al., 2002). Abnormally high levels of free radicals and simultaneous decline of antioxidant defence systems can lead to the damage of cellular organelles and enzymes, increased lipid peroxidation and development of complications of diabetes mellitus (Maritim et al., 2003).
It is well established that oxidative stress is produced under diabetic conditions through multiple sources causing an increase of hydroxyl radicals (Turko et al., 2001). A hydroxyl radical in turn produces a multiplicity of modifications in DNA. Oxidative attack by OH radical on the deoxyribose moiety of DNA is lead to the release of free bases from DNA, generating strand breaks with various sugar modifications, nucleotide modifications, particularly in sequences with high guanosine content (Hegde et al., 2008) and simple a basic (AP) sites. In fact, one of the major types of damage generated by Reactive Oxygen Species (ROS) is AP site, a site where a DNA base is lost. The oxidative DNA damage occurs in their peripheral blood lymphocytes (Sardas et al., 2001) and the DNA damage in tissue, lymphocytes and leucocytes can be used as a marker of oxidative stress in diabetes (Pitozzi et al, 2003). Additionally, it has been demonstrated that DNA damage was significantly higher in the poorly controlled diabetic patients compared to well control subject, regardless of sex (Dinçer et al., 2002). Van Loon et al., (1992) showed significantly increased basal levels of DNA damage in whole blood.
ROS induces several types of lesions in DNA, including single or double-strand breaks, alkali-labile sites, and various species of oxidized purines and pyrimidines, which are easily detected by alkaline comet assay (Nikitaki et al., 2015). A large variety of oxidized bases have been identified in nuclear DNA but 8-oxo-7,8-dihydroguanosine (8-oxoGua) is one of the most abundant and readily formed. The 8-oxoGua in DNA mispair with adenine during replication. Thus presence of 8-oxoGua in DNA may lead to transversion mutations. It has been suggested that this kind of lesion play an important role in the initiation, promotion and progression of tumors. The high levels of oxidized bases in patients infected with the human immunodeficiency virus (HIV) are reported which might influence the progression of the infection into acquired immunodeficiency syndrome (Olinski et al., 2002). High levels of 8-oxo-Gua have also been found in lesions of the aorta wall in atherosclerosis patients (Olinski et al., 2002). DNA oxidative damage has also been linked to other diseases, notably Alzheimer’s disease, Huntington’s disease and Parkinson’s disease (Cooke et al., 2005).
CONCLUSION
Alloxan induced diabetes mellitus is associated with elevated level of oxidative DNA damage, increase in the level of blood glucose, superoxide anion, hydrogen peroxidesusceptibility to cytotoxicity and the decrease efficacy of DNA repair. Diabetic rats treated with oral administration of O. sanctum and A. sativum aqueous extracts decrease in the level of blood sugar, superoxide anion, hydrogen peroxide, length of DNA tail, decrease cytotoxicity and increase efficacy of DNA repair. Further investigation of the mechanism of action of this herbal plant leave extracts against diabetes.
ACKNOWLEDGMENTS
Mr. SKJ has received stipend from the Department of Higher Education, Government of M.P., Bhopal, India. The financial support from the Department of Science and Technology, New Delhi, India, in the form of FIST grant to the School is thankfully acknowledged.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCE
Aguilar TAF, Navarro BCH and Perez JAM (2016). Endogenous Antioxidants: A Review of their Role in Oxidative Stress. https://www.intechopen.com/books/a-master-regulator-of-oxidative-stress-the-transcription-factor-nrf2/endogenous-antioxidants-a-review-of-their-role-in-oxidative-stress.
Andersson KE (2018). Oxidative stress and its possible relation to lower urinary tract functional pathology. BJU Int; 121(4):527-533.
Bigagli E and Lodovici M (2019). Circulating Oxidative Stress Biomarkers in Clinical Studies on Type 2 Diabetes and Its Complications. Oxidative Medicine and Cellular Longevity; 2019:1-17.
Cohen HJ, Chovaniec ME, Davies WA (1980). Activation of the guinea pig granulocyte NAD(P)H-dependent suproxide generating enzyme: Localization in a plasma membrane enriched particle and kinetics of activation. Blood; 55: 355-363.
Cooke MS, Evans MD, Dove R, Rozalski R, Gackowski D, Siomek A (2005). DNA repair is responsible for the presence of oxidatively damaged DNA lesions in urine. Mutat Res; 574: 58-66.
Dinçer Y, Akçay T, Alademir Z,Ilkova H (2002). Assessment of DNA base oxidation and glutathione level in patients with type 2 diabetes. Mutat Res; 505: 75-81.
Fang YZ, Yang S, Wu G (2002). Free radicals, antioxidants, and nutrition. Nutrition;18:872–9.
Hegde ML, Karza TK, Mitra S (2008). Early steps in the DNA base excision/single strand interruption repair pathway in mammalian cells. Cell Res; 18: 27-47.
Ighodaro OM and Akinloye OA (2018). First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria Journal of Medicine; 54(4): 287-293.
Johansen JS, Harris AK, Rychly DJ (2005). Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardio vasc Diabetol; 4: 5.
Klages-Mundt NL and Li L (2017). Formation and Repair of DNA-Protein Crosslink Damage. Sci China Life Sci; 60(10):1065-1076.
Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D,1 and Abete P (2018). Oxidative stress, aging, and diseases. Clin Interv Aging; 13: 757–772.
Liguori I, Russo G,Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, and Abete P (2018).Oxidative stress, aging, and diseases. Clin Interv Aging; 13: 757–772.
Liu YK, Deng XX and Yang HL (2016).Cytotoxicity and genotoxicity in liver cells induced by cobalt nanoparticles and ions. Bone Joint Res; 5(10): 461–469.
Manna I (2018). Effects of yoga training on body composition and oxidant-antioxidant status among healthy male. Int J Yoga; 11: 105-110.
Maritim AC, Sanders RA, Watkins JB (2003). 3rd Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol; 17:24–38.
McManus JFA, Mowry RW (1960). Staining methods: histologic and histochemical, paul B Hoeber Inc; USA.
Misra M and Aiman U (2012). Alloxan: An unpredictable drug for diabetes induction? Indian J Pharmacol; 44(4): 538–539.
Narayan KM (2017). Public health challenge for 21st century: convergence of demography, economics, environment and biology: nalanda distinguished lacture. Natl Med J India; 30:219-23.
Nasif WA, Mukhtar MH, Eldein MMN, and Ashgar SS (2016). Oxidative DNA damage and oxidized low density lipoprotein in Type II diabetes mellitus among patients with Helicobacter pylori infection. Diabetol Metab Syndr; 8: 1-34.
Nikitaki Z, Hellweg CE, Georgakilas AG, and Ravanat J (2015).Stress-induced DNA damage biomarkers: applications and limitations. Front Chem; 3: 35.
Nita M and Andrzej Grzybowski A (2016). The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid Med Cell Longev. 2016; 2016: 3164734.
Obrosova IG, Vanteysen C, Fathallah L, Cao X, Greene DA, Stevens MJ (2002). An aldose reductase inhibitor reverses early diabetes-induced changes in peripheral nerve function. Federation of American Societies for Experimental Biology (FASEB) Journa; 16: 123–25.
Olinski R, Gackowski D, Foksinski M, Rozalski R, Roszkowski K, Jaruga P (2002). Oxidative DNA damage: assessment of the role in carcinogenesis, atherosclerosis, and acquired immunodeficiency syndrome. Free Radic Biol Med; 33: 192-200.
Ortiz MS, Forti KM, Edu B. Martinez S, Muñoz,LG, Husain K, and Muniz WH (2016). Effects of Antioxidant N-acetylcysteine Against Paraquat-Induced Oxidative Stress in Vital Tissues of Mice. Int J Sci Basic Appl Res; 26(1): 26–46.
Pick E (1986). Microassay for superoxide and hydrogen peroxide production and nitrobluetetrszolium reduction using an enzyme immunoassay microplate reader. Met Enzymol; 132, 407- 21.
Pitozzi V, Giovannelli L,Bardini G, Rotella CM,Dolara P (2003).Oxidative DNA damage in peripheral blood cells in type 2 diabetes mellitus: higher vulnerability of polymorphonuclear leukocytes. Mutat Res; 529: 129-33.
Rosen P, Nawroth PP, King G (2001). The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev; 17:189–12.
Roy J, Galano J, Durand T, Guennec JL and Lee JC (2017). Physiological role of reactive oxygen species as promoters of natural defences. FASEB J; 31: 3729–3745
Sardas S, Yilmaz MO, Ztok U, Takir NC, Karakaya AK (2001). Assessment of DNA strands breakage by comet assay in diabetic patients and the role of antioxidant supplementation. Mutat Res; 490: 123-29.
Sasaki YF, Izumiyama F, Nishidate E, Matsusaka N, Tsuda S (1997). Detection of rodent liver carcinogen genotoxicity by alkaline single cell gel electrophoresis (Comet assay) in multiple mouse organs (liver, lung, spleen, kidney and bone marrow). Mutat Res; 391: 201-14.
Tan BL, Norhaizan MEand Liew WP (2018). Nutrients and Oxidative Stress: Friend or Foe? Oxidative Medicine and Cellular Longevity; 2018:1-24.
Tan BL, Norhaizan ME, Liew W and Rahman HS (2018). Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front Pharmacol; 9: 1162.
Turko IV, Marcondes S, Murad F (2001). Diabetes-associated nitration of tyrosine and inactivation of succinyl-CoA:3-xoacid CoA transferase. Am J Physiol Heart CircPhysiol; 281(6): 2289-2294.
Van Loon AAWM, Groenendijk RH, Timmerman AJ, van der Schans GP, Lohman PHM, Baan RA (1992). Quantitative detection of DNA damage in cells after exposure to ionising radiation by means of an improved immunochemical assay. Mutat Res; 274: 19-27.
Zhong A, Chang M, Yu T, Gau R, Riley DJ, Chen Y and Chen P (2018). Aberrant DNA damage response and DNA repair pathway in high glucose conditions. J Can Res Updates. 2018; 7(3): 64–74.
Zimmet PZ (2017). Diabetes and its drivers: the largest epidemic in human history? Zimmet Clinical Diabetes and Endocrinology (2017) 3:1-8.


