1Department of Nutrition and Food Science, Faculty of Home Economics, Menofia University, Egypt.
2Department of Physiology, Faculty of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia.
Corresponding author email: oashaikhomar@uqu.edu.sa
Article Publishing History
Received: 10/07/2021
Accepted After Revision: 28/09/2021
The aim of this study was to investigate the effects of daily oral feeding 8% and 15% of powdered leaves of Murraya koenigii leaves (MKL) (commonly knoun as curry) and Brassica juncea seeds (BJS) (commonly knoun as mustard) for 45 days on serum glucose concentration, serum lipids, liver and kidney functions in diabetic rats. A total of 36 adult male albino rats (Sprague Dawley strain) weighting 159±2.4g each were used in this investigation. Non- diabetic control (-) (6 rats) were fed basal diet ,while diabetic control (+) main group (30) rats divided into five groups after injected with alloxan (150mg/kg), at the end of the experiment, weight gain was calculated. Liver of each rat were removed rapidly then weighted separately.
Blood samples were used for estimation of fasting serum glucose, ALT, AST, ALP, triglycerides, total cholesterol, high density lipoprotein (HDLc), low density lipoprotein (LDLc), very low density lipoprotein (VLDLc). Data showed that serum AST and ALT levels declined significantly (p< 0.05) in all treated groups fed on 7% and 15% curry and mustard compared with diabetic positive control. Moreover, both spices resulted in reduction of serum total cholesterol and LDLc + VLDLc a companied with an increase in the HDLc and significantly lowering of serum glucose levels. Thus, these plants can be best utilized by promoting them as preferable food for diabetic patients.
Diabetes Mellitus, M. Koenigii, Curry, Brassica Juncea, Mustard, Triglycerides, Cholesterol, Glucose.
Header E. A, Shaikhomar O. A. Effects of Murraya koenigii Leaves and Brassica juncea Seeds on Hyperglycemic Rats. Biosc.Biotech.Res.Comm. 2021;14(3)
Header E. A, Shaikhomar O. A. Effects of Murraya koenigii Leaves and Brassica juncea Seeds on Hyperglycemic Rats. Biosc.Biotech.Res.Comm. 2021;14(3). Available from: <a href=”https://bit.ly/3o7gwRx“>https://bit.ly/3o7gwRx</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
The World Health Organization (WHO) projected that 80% of the population relies on traditional medicine, which was elucidated by the 19.4 billion USD global revenue for herbal remedies in 2010 (Ujowundu et al., 2010). Moreover, the demand for traditional medicinal plants is increasing; for instance, the market for medicinal plants is expanding at an annual rate of 20% in India. Likewise, in China, 30% to 50% of the total medicinal consumption and around 90% of the German population uses natural remedies for certain health issues (Kang et al., 2018; Phumthum and Balslev, 2018; Raghu, 2020).
Therefore, medicinal plants are used in both developing and industrialized countries. Curry leave (Murraya koenigii (L.) Spreng) is an aromatic, tropical, and sub-tropical plant with several culinary, nutraceutical, medicinal, therapeutic values (Wojdyło et al., 2007; Reddy et al., 2018).
Though curry leave is an ancient crop native to India, its nutritive and medicinal values are not enough known yet (Raghu, 2020). The medicinal properties of M. koenigii have been recorded to several chemical constituents of different carbazole alkaloids and other metabolites, like terpenoids, flavonoids, phenolics, carbohydrates, carotenoids, vitamins, and nicotinic acid from different parts of the M. koenigii tree (Balakrishnan et al., 2020).
In recent years, limited studies have been conducted for evaluating the pharmacological and medicinal efficacy of M. koenigii in promoting health benefits and curing disease. M. koenigii has numerous disease remedial activities, for instance, different parts of the plant, such as the leaves, roots, and bark, can be prepared as tonics for inducing digestion and flatulence or as antiemetics (Mandal et al., 2010; Adebajo et al., 2006). The leaves and roots are also useful in managing blood disorders (Sim and Teh., 2011; Dar et al., 2017; Zang et al., 2017; Balakrishnan et al., 2018; Balakrishnan et al., 2020).
Balakrishnan et al., (2020) review described the pharmacological activities of the major components of M. koenigii against different pathological conditions. Moreover, M. koenigii showed significantly decreased glycemic levels and protected the animals against the development of diabetic neuropathy (Tembhurne and Sakarkar., 2010). In addition, M. koenigii showed a significant decrease in blood glucose, HbA1C, and altered lipid profile. M. koenigii was reported to extend a protective effect in liver impairments in chronic alcoholism and was proved effective in maintaining the enzymatic oxidant status (Shah et al., 2015; Husna et al., 2018, Suman et al., 2019). When Gul et al. (2012) tested M. koenigii, they found it was inhibited α glycosidase.
These Alpha-glucosidase inhibitors are widely used in the treatment of patients with type 2 diabetes. In most developing countries, medicinal plants play a helpful role in managing diabetes mellitus due to their cost effectiveness. Diabetes mellitus, a metabolic disorder, is becoming a serious threat to human health. During the past few years, many phytochemicals responsible for anti-diabetic effects have been isolated from plants. Alkaloids present in the leaves of M. koenigii have been explored and reported to have inhibitory effects on the aldose reductase enzyme, glucose utilization, and other enzyme systems for extending anti-diabetic effects (Patel et al., 2012).
Recently, it has been reported that the M. koenigii significantly reduced the glycosylated hemoglobin in the treated group compared with the Control group (Suman et al., 2019). In addition, M. koenigii exhibited a profound antioxidant effect by reducing the malondialdehyde (MDA) level, increasing the GSH level, and significantly decreasing the homeostatic model assessment (HOMA)-insulin resistance index. Overall, it is evident that M. koenigii possesses antidiabetic activity and has antioxidant effects in rats (Husna et al., 2018; Bhatt et al., 2020).
The aqueous seed extract of the Brassica juncea medicinally valued plant clearly envisaged the hypoglycemic effect. This might be due to the time taken for the intestinal absorption of the aqueous seed extract of B. juncea (Ahad et al., 2010; Mohammad et al., 2010). The hypoglycemic effect of the seed extract of B. juncea was attributed to stimulation of glycogen synthesis leading to an increase in hepatic glycogen content and suppression of glycogen phosphorylase and other gluconeogenic enzymes (Khan et al., 1995 & Xu et al., 2011).
Previous reports have demonstrated that the leaves, roots, and bark of the plant are rich sources of carbazole alkaloids, which produce potent biological activities and pharmacological effects. The present study provides insight into the major components of M. koenigii (leaves and seed) and their pharmacological activities in the management of serum glucose concentration, serum lipids, and liver functions in alloxan-induced diabetic rats.
MATERIAL AND METHODS
The studied samples of Murraya koenigii (MKL) (curry leaves powder) and Brassica juncea (BJS) (mustard seeds) were obtained from the local market of Al-Taif region, Makkah province, KSA.
Animals: A total of 36 adult male albino rats (Sprague Dawley strain) were used in the investigation. Animals were obtained from Laboratory Animal Centre, Department of Biochemistry, Faculty of Medicine, Umm Al-Qura University, Makkah, KSA. Each rat was housed in a special cage under controlled condition.
All rats were fed for 7 days on the control diet before the beginning of the experiment. Rats were weighed after 7 days separately then were weighed once a week for 6 weeks. The diet was presented to rats in special covered cups to avoid food loss, water was provided. At the end of the experiment rat were killed and organs weight was recorded.
Induction of diabetic rate and experimental design: Rats were divided into two main groups the first groups (6 rats) fed on basal diet as a negative control (-). For the second group (30 diabetic rats), diabetes mellitus was induced in overnight fasted rats by a single intraperitoneal Streptocytocin (STZ) injection (65 mg/kg b.w.) (Ravi et al., 2004). After 3 days, fasting blood glucose levels were measured and the animals showing blood glucose level ≥225 mg/dL were used for the study (Ewart et al., 1975).
Rats having fasting serum glucose 190 mg/dl were considered diabetic (NDDG, 1994). Diabetic rats were divided into 5 groups, 6 rats each, and fed experimental diets for 45 days as follows: Group 1: Diabetic standard group; positive group (+). Group 2: Fed on basal diet + 7% MKL powder. Group 3: Fed on basal diet + 15% MKL powder. Group 4: Fed on basal diet + 7% MKS powder. Group 5: Fed on basal diet + 15% MKS powder.
Diets: The basal diet consists of casein (12 %), corn oil (10 %), choline chloride (0.2 %), cellulose (5%), vitamin mixture (1 %) (Bunce and Bloomer, 1972), salt mixture (4 %) (Hegested et al., 1941) and corn starch (up to 100 %).
Blood sampling: At the end of the experiment, rats were fasted overnight and anesthetized with chloroform. Blood samples were collected in clean dry centrifuge tubes from hepatic portal vein. Blood was centrifuged for 10 minutes at 3000 rpm to separate serum, which was kept in tubes at– 18°C until analysis. Organs were taken, washed with saline solution (10% Nacl) and dried with filter paper, then weighed and kept in freezer until analysis.
Biochemical analysis: Serum blood glucose was determined according to the method of (Trinder, 1969). Serum aspartate and alanine amino transferees (AST, ALT) and alkaline phosphatase (ALP) were determined by using enzymatic colorimetric method after (Reitman and Frankel, 1957; and Haussement, 1977), respectively.Serum total cholesterol, triglyceride (TG) and high-density lipoprotein cholesterol (HDLc) were determined by using enzymatic colorimetric method (NIHP, 1987; Young and Pestaner, 1975; Fendewaid, 1972; & Grodon and Amer, 1977), respectively.
The determination of low-density lipoprotein cholesterol (LDLc) and very low-density lipoprotein cholesterol (VLDLc) were carried out according to the method of (Lee and Nieman, 1996) as follows: VLDLc = TG /5 and LDLc =Total cholesterol – HDLc – VLDLc. Atherogenic indices were calculated as HDLc /T. cholesterol % and LDLc / HDLc (Castelli and levitar, 1977).
Histopathological examination of some internal organs: Specimens from liver were collected from rats of all experimental groups at the end of the experimental period, fixed in 10% neutral buffered formalin (pH=7.0), dehydrated in ethyl alcohol, then cleared in xylol and embedded in paraffin; 4-6 microns thickness sections prepared and stained with heamtoxylin and eosin for examining both for and glandular parts of the stomach (Bancroft and Gamble, 2008).
Statistical Analysis: Statistical analyses were performed by using computer program, statistical package for social science version 24 for windows. Data were expressed as mean standard deviation (SD). Paired-sample t-test was used to compare the parameters between controls positive group and diabetic rats groups. A P-value less than 0.05 was considered statistically significant.
Results AND DISCUSSION
Table 1. Fasting Serum Glucose (mg/dl) for Diabetic Rats Fed on Curry
Leaves and Mustard Seeds for 45 Days.
| Groups
Variables |
Control (-) | Control (+) | MKL | MKS | ||
| 7% | 15% | 7% | 15% | |||
| glucose | 101±2.1*** | 205.3±8.8 | 116.1±4.2* | 108.1±1.9** | 120±5.1* | 117.5±2.1** |
| Data are expressed as Mean±SD of six experiments. A P-value less than 0.05 was considered statistically significant. Parameter of positive group were compared to negative group, and treated groups. *(P≤ 0.05) significant change; **(P≤ 0.01) high significant change. ***(P≤ 0.01) very high significant change. | ||||||
Table 2. Fasting Serum AST, ALT and ALP (IU/L) for Diabetic
Rats Fed on Curry Leaves and Mustard Seeds for 45 Days
| Groups
Variables |
Control (-) | Control (+) | MKL | MKS | ||
| 7% | 15% | 7% | 15% | |||
| AST | 22.8±1.5*** | 48.9±2.1 | 34.1±2.4* | 31.3±2.2* | 40.2±1.4* | 33.6±1.8* |
| ALT | 27.1±3.5** | 49.1±2.1 | 29.3±3.2** | 27.2±1.1** | 37.8±1.4 | 31.6±1.1* |
| ALP | 148.3±22.7*** | 393.6±51.3 | 201±43.5** | 208±12.3** | 301 ±15.1 | 249.2±5.1* |
| Data are expressed as Mean±SD of six experiments. A P-value less than 0.05 was considered statistically significant. Parameter of positive group were compared to negative group, and treated groups. *(P≤ 0.05) significant change; **(P≤ 0.01) high significant change. ***(P≤ 0.01) very high significant change. | ||||||
Table 3. Fasting Serum Lipid Fraction (mg/dl) for Diabetic Rats
Fed on Curry Leaves and Mustard Seeds for 45 Days.
| Groups
Variables |
Control (-) | Control (+) | MKL | MKS | ||
| 7% | 15% | 7% | 15% | |||
| Triglyceride | 45.7±12.2** | 92.3±6.7 | 50.3±5.9** | 43.3±1.9** | 51.7±9.3** | 47.7±8.8** |
| Cholesterol | 79.4±3.4** | 135.7±15.1 | 121.6±12.2 | 90.7±16.1* | 103.2±12.6* | 89.3±2.3** |
| HDL-C | 46.3±6.4* | 24.7±1.4 | 29.3±2.3 | 31.3±2.2* | 29.2±4.8* | 36.7±1.8* |
| VLDL-C | 9.14±2.4* | 18.46±1.4 | 10.1±1.2* | 8.7±0.4** | 10.34±1.9* | 9.54±1.8* |
| LDL-C | 23.96±9.5*** | 92.54±9.8 | 82.2±5.4 | 50.7±10.4** | 63.7±11.8* | 43.1±4.1** |
| Data are expressed as Mean±SD of six experiments. A P-value less than 0.05 was considered statistically significant. Parameter of positive group were compared to negative group, and treated groups. *(P≤ 0.05) significant change; **(P≤ 0.01) high significant change. ***(P≤ 0.01) very high significant change. | ||||||
Table 4. Atherogenic Indices for Diabetic Rats Fed on
Curry Leaves and Mustard Seeds for 45 Days.
| Groups
Variables |
Control (-) | Control (+) | MKL | MKS | ||
| 7% | 15% | 7% | 15% | |||
| LDL/HDL
Ratio |
0.51±0.021*** | 3.75±0.47 | 2.8±0.29 | 1.61±0.004* | 2.18±0.028 | 1.17±0.06** |
| HDL /T.C %
Ratio |
58.3±3.7** | 18.2±2.8 | 24.1±3.1 | 34.5±2.9* | 28.29±3.5* | 41.1±4.7** |
| Data are expressed as Mean±SD of six experiments. A P-value less than 0.05 was considered statistically significant. Parameter of positive group were compared to negative group, and treated groups. *(P≤ 0.05) significant change; **(P≤ 0.01) high significant change. ***(P≤ 0.01) very high significant change. | ||||||
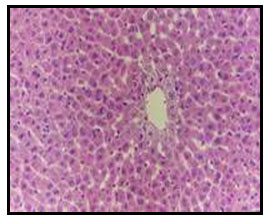
Histopathological Results: Examined liver of control, untreated rat revealed the normal histology of hepatic lobule, which consists of central vein and around it arranged highly specialized cells (hepatocytes) (Fig. 1). Concerning liver of diabetic rat, it showed vacuolar degeneration of hepatocytes as well as focal hepatic haemorrhage (Fig. 2). Examined liver sections of diabetic rat treated with 5% curry showed vacuolations of hepatocytes especially around the central vein (Fig. 3).
However, apparent normal hepatocytes associated with slight activation of kupffer cells (Fig. 4) were noticed in liver of diabetic rat treated with 10% curry. Examined liver of diabetic rat treated with 5% mustard showed portal infiltration with few leucocytic cells (Fig. 5). Moreover, no histopathological changes were observed in examined liver of diabetic rat treated with 10% mustard (Fig. 6).
Figure 1: Liver of control untreated rat showing the normal
histology of hepatic lobule (H and E X 200).
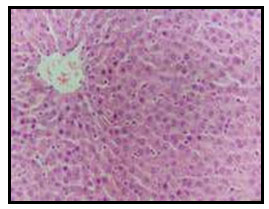
Figure 2: Liver of diabetic rat showing vacuolar degeneration of hepatocytes
as well as focal hepatic haemorrhage (H and E X 200).
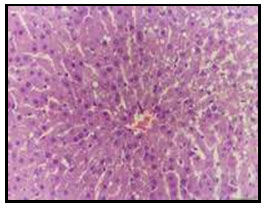
Figure 3: Liver of diabetic rat treated with 7% curry showing vacuolation
of hepatocytes around the central vein (H and E X 200).
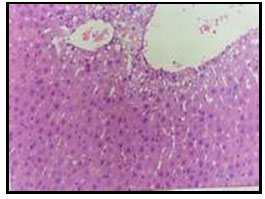
Figure 4: Liver of diabetic rat treated with 15% curry showing apparent normal
hepatocytes associated with kupffer cells activation (H and E X 200).
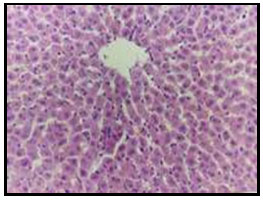
Figure 5: Liver of diabetic rat treated with 7% mustard showing
portal infiltration with few leucocytic cells (H and E X200).
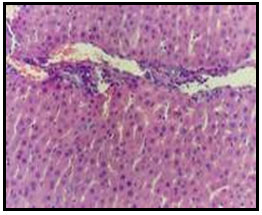
Figure 6: Liver of diabetic rat treated with 15% mustard showing
no histopathological alterations (H and E X 200).
The current study was performed to evaluate the hypoglycemic effect of Murraya koenigii leaves and B. juncea seeds in alloxan induced diabetic rats. Diabetes is considered one of the major causes of morbidity and mortality affecting the elder and middle-aged population (Guariguata et al., 2014). The long-term use of present oral hypoglycemic tablets or insulin is associated with the development of resistance and various side effects.
The traditional herbal options may help in fulfilling these unmet needs (Fatima et al., 2012). There are various herbs having proven antidiabetic effect such as Memordica charantia, Eugenia jambolana, Trigonella foenum graecum, Embilca officinalis, Azadirachta indica, Phaseolus vulgaris, and Gymnema sylvestere and Murraya koenigii (Fatima et al., 2012; Husna et al., 2018; Balakrishnan et al., 2020; Bhatt et al., 2020).
Murraya koenigii is a well-known curry leave tree. Its leaves and seeds are used as a spice in food recipe in India. Its related antidiabetic activity is attributed to alpha glucosidase activity of carbazole alkaloids contribute to its hyperglycemic activity through antioxidant effect and preservation of β-cell function. Its Alpha glucosidase inhibitory activity prevents digestion of carbohydrates and thereby reduces glucose absorption (Kesari et al., 2007; Lawal et al., 2008; Mangesh et al., 2018).
Alloxan causes partial destruction of pancreatic β-cells, which leads to reduced levels of insulin and consequently resulting into hyperglycemia (Szkudelski, 2001; Lenzen, 2008). In our results, fasting serum glucose (mg/dl) for diabetic rats fed on curry leaves and mustard seeds for 45 days showed a significant decline. The hypoglycemic activity of curry leaves and mustard seeds could be due to the presence of carbazole alkaloids, which possess alpha-glucosidase inhibitory property (Duraisamy et al., 2012).
Alpha-glucosidases are enzymes in the digestive tract that hydrolyze carbohydrates into glucose. One strategy that has been developed to treat type-2 diabetes is inhibition of the activity of alpha-glucosidases using synthetic drugs or natural drug candidates for the treatment of type-2 diabetes mellitus. Other possible mechanism of action of curry-leave-treated group could be potentiating insulin secretion from β cells of islets, which leads to reduced blood glucose levels (Vinuthan et al., 2004; Samuel et al., 2020).
Moreover, both spices resulted in reduction of serum total cholesterol and LDLc + VLDLc accompanied with an increase in the HDLc (Virdi et al., 2003). Administration of the extracts significantly decreased cholesterol level to near normalcy and therefore may reduce the risk of diabetes-associated cardiovascular diseases.In the present study, the B. juncea seed extract augmented the serum insulin levels suggesting an improved state of availability of serum insulin to control blood sugar.
In addition, the present study showed that insulin serum augmenting effect was recorded highest at the dose of 7% suggesting that the serum insulin effect of the seed extract is dose dependent. This might be due to the inability of the β cells to recoup from the alloxan effect in these (Iftikhar et al., 2020).
Our data showed that serum AST and ALT levels declined significantly (p< 0.05) in all treated groups fed on 7% and 15% curry and mustard compared with diabetic positive control. Damage to the structural integrity of the liver is reflected by an increase in the activity of this enzyme in the serum, probably because of leakage from altered cell membrane structure (Akanji et al., 1993; Rahman et al., 2001; Iftikhar et al., 2020).
Therefore, increase ALP in serum of the untreated diabetic rats confirms damage to the plasma membrane. The combination treatment attenuated the elevated activity of ALP enzyme in diabetic rats as compared with the normal controls. Our results illustrated that B. juncea seeds consumption significantly lowered the risk of atherosclerosis by bringing fall in concentration of plasma total cholesterol, LDL cholesterol as well as an improvement in HDL-cholesterol levels that is in full agreement with other studies described by (Khan et al., 1996; Rusdi et al., 2021).
This lipid lowering property of B. juncea may be due to its emulsification properties that were contained in its water-soluble portion of proteins as reported by (Cui, 1997). Reduced plasma cholesterol concentration is also affected by improved function of LDL receptor, which accelerates LDL uptake from plasma (Ness et al., 1996). These findings are in favour of former studies, showing that plant has cholesterol reducing capacity (O’Brien and Reiser, 1979).
Histopathological examination of liver of control, untreated rat revealed the normal histology of hepatic lobule, however liver of diabetic rat showed vacuolar degeneration of hepatocytes as well as focal hepatic haemorrhage especially around the central vein. Examined liver of diabetic rat treated with 5% mustard showed portal infiltration with few leucocytic cells thus indicating that the extract of leaves and seeds exhibits inhibitory effect against hepatotoxicity. These results are in conformity with the previous findings (Bhatt et al., 2020; Hend et al., 2021).
CONCLUSION
From the current study, it is concluded that M. koenigii leaves and B. juncea seeds were found to show antihyperglycemic and hypolipedimic activity. Hence, this compound could be used as an oral hypoglycemic agent in diabetes. However further studies need to be done to confirm this activity in animal models as well as human trials.
The consumption of the leave and seeds may be potentially beneficial against atherogenesis hence protective against cardiovascular disease as it possesses quality of lowering the plasma cholesterol, triglycerides, LDL-C and improving HDLC. Vacuolation of hepatocytes and portal infiltration in liver treated with the leave and seed extract respectively further indicates a need to evaluate the isolated phytochemicals from of the plant for the benefit of mankind. It can be achieved by using scientific experimental animal models and clinical trials to get the information about their action mechanism on the molecular level.
Conflict of Interest: Authors declares no conflicts of interests to disclose.
Ethical Clearance Statement: The Current Research Work Was Ethically Approved by the Institutional Review Board (IRB) of Animals were obtained from Laboratory Animal Centre, Department of Biochemistry, Faculty of Medicine, Umm Al-Qura University, Makkah, KSA.
REFERENCES
Adebajo A.C., O.F. Ayoola, E.O. Iwalewa, A.A. Akindahunsi, N.O.A. Omisore, C.O. Adewunmi and T.K. Adenowo (2006). Anti-trichomonal, biochemical and toxicological activities of methanolic extract and some carbazole alkaloids isolated from the leaves of Murraya koenigii growing in Nigeria. Phytomedicine, 13: 246–254.
Ahad HA., C.S. Kumar and P.S.M Nanda (2010). Udaya Bhanu T, Ravindra BV, Mohan VG. Traditional Indian Herbs Used for Diabetes. JITPS, 1(2): 69–78.
Akanji M.A., O.A. Olagoke and O.B. Oloyede (1993). Effect of chronic consumption of metabisulphite on the integrity of the rat kidney cellular system. Toxicology. 81: 173-9.
Bhatt S., B. Singh, M. Gupta (2020). Antioxidant and prebiotic potential of Murraya koenigii and Brassica oleracea var. botrytis leaves as food ingredient, Journal of Agriculture and Food Research, (9)2, 1-6. https://doi.org/10.1016/j.jafr.2020.100069.
Balakrishnan R., K. Tamilselvam, A. Sulthana, T. Mohankumar, D. Manimaran and N. Elangovan (2018). Isolongifolene Attenuates Oxidative Stress and Behavioral Impairment in Rotenone-Induced Rat Model of Parkinson’s Disease. Int. J. Nutr. Pharmacol. Neurol. Dis., 8: 53–58.
Bancroft J.D., and M. Gamble (2008). Theory and Practice of Histological Techniques, 6th. ed. Pub. by Clurechill Livingston, Elsevier, USA.
Bunce G.E. and J.E. Bloomer (1972). The composition of vitamin mixture. J.of Nutr., 102: 863-869.
Castelli T. and Y. Levitar (1977). Athrogenic Index. Curr Presc., P39.
Ewart R.B.L.; S. Kornfeld and D.M. Kipnis (1975). Effect of Lectins on Hormone Release from Isolated Rat islets of Langerhans. Diabetes, 24: 705-714.
Cui W., (1997). Mustard: chemistry and potential as a nutraceutical ingredient. Canadian Chemical News.
Dar R.A., M. Shahnawaz, P.H. Qazi and H. Qazi (2017). General Overview of Medicinal Plants: A review. J. Phytopharm, 6: 349–351.
Duraisamy G, Manokaran Kalaiselvi, Chandrasekar Uma, (2012). In vitro α-amylase and α-glucosidase inhibitory effects of ethanolic extract of Evolvulusalsinoids (L). International Research Journal of Pharmacy 3(3):226-229.
Fatima A., P. Agrawal and P.P. Singh (2012). Herbal Option for Diabetes: An Overview. Asian Pacific J Trop Dis., 2: S536-44
Fendewaid W.T., (1972). Determination of HDL. Clin. Chem., 18: 499.
Grodon T. and M. Amer (1977). Determination of HDL. J. of Med., 62: 707.
Guariguata L, D.R. Whiting, I. Hambleton, J. Beagley, U. Linnenkamp and J.E. Shaw (2014). Global Estimates of Diabetes Prevalence for 2013 and Projections for 2035. Diabetes Res Clin Pract., 103: 137-49.
Gul M. Z., V. Attuluri, I. A. Qureshi and I. A. Ghazi (2012). Antioxidant and α-Glucosidase Inhibitory Activities of Murraya koenigii Leaf Extracts. Pharmacognosy Journal, 4 (32): 65 – 72. https://doi.org/10.5530/pj.2012.32.12.
Haussement, T.U. (1977). Determination of Alkaline Phosphatase. Clin. Chem. Acta., 35: 271-273.
Hegested D., R. Mills, and E. Perkins (1941). Salt Mixture. J. of Biol. Chem., 438-459.
Hend M., A. Eman and H.B. Ibtihal (2021). Anticandidal Efficacy of Brassica juncea Seeds Extract: Characterization, In Vitro and In Vivo Studies. Advances in traditional Medicine. 21: 97-110.
Husna F., F.D. Suyatna, W. Arozal and E.H. Poerwaningsih (2018). Anti-Diabetic Potential of Murraya koenigii (L) and its Antioxidant Capacity in Nicotinamide-Streptozotocin Induced Diabetic Rats. Drug Res. (Stuttg), 68: 631–636.
Iftikhar A., Bilal Aslam, Maryam Iftikhar, Wafa Majeed, Mehwish Batool, Bushra Zahoor, Naseem Amna, Hareem Gohar, Iqra Latif (2020). Effect of Caesalpinia bonduc Polyphenol Extract on Alloxan-Induced Diabetic Rats in Attenuating Hyperglycemia by Upregulating Insulin Secretion and Inhibiting JNK Signaling Pathway, Oxidative Medicine and Cellular Longevity, 1-14. ID 9020219. https://doi.org/10.1155/2020/9020219
Kang W. and Y. Wang (2018). China Digital Governance Development Review Over the Past Two Decades. Int. J. Public Adm. Digit. Age, 5: 92–106.
Kesari A.N., S. Kesari, S.K. Singh, R.K. Gupta and G. Watal (2007). Studies on the Glycemic and Lipidemic Effect of Murraya koenigii in Experimental Animals. J Ethnopharmacol, 112: 305-11.
Khan B.A., A. Abraham, and S. Leelamma (1995). Hypoglycemic Action of Murraya koenigii (Curry Leaf) and Brassica juncea (mustard): Mechanism of Action. Indian J Biochem Biophys. 32: 106–108.
Khan B.A., A. Abraham and S. Leelamma (1996). Biochemical Response in Rats to the Addition of Curry Leaf (Murraya koenigii) and Mustard Seeds (Brassica juncea) to the Diet. Plant Foods Hum Nutr., 49(4): 295-9.
Lawal HA, M.K. Atiku, D.G. Khelpai, and N.N. Wannang (2008). Hypoglycaemic and Hypolipidaemic Effect of Aqueous Leaf Extract of Murraya koenigii in Normal and Alloxan-Diabetic Rats. Niger J Physiol Sci., 23: 37-40.
Lee R. and D. Nieman (1996). Nutritional Assessment. 2nd Ed. Mosby, Missouri, USA.
Lenzen S. (2008). The Mechanisms of Alloxan- and Streptozotocin-Induced Diabetes. Diabetologia, 51: 216-26.
Mandal S., A. Nayak, M. Kar, S.K. Banerjee, A. Das, S.N. Upadhyay, R.K. Singh, A. Banerji and J. Banerji (2010). Antidiarrhoeal Activity of Carbazole Alkaloids from Murraya koenigii spreng (Rutaceae) Seeds. Fitoterapia., 81: 72–74.
Mangesh A B., Somnath Devidas Bhinge, Dheeraj S Randive, Ganesh H Wadkar, Sachin S Todkar (2018). Screening of in-vitro hypoglycemic activity of Murraya koenigii and Catharanthus roseus. Ars Pharm; 59(3): 145-151.
DOI: 2340-9894-ars-59-03-145
Mohammad Ismail M.Y., N.M. Assem and M. Zakriya (2010). Role of Spices in Diabetes Mellitus. RJPBCS, 1(3): 30–34.
Ness G.C., Z. Zhao and D. Lopez (1996). Inhibitor of Cholesterol Biosynthesis Increase Hepatic Low Density Lipoprotein Receptor Protein Degradation. Arch. Biochem Biophys., 325: 242-248.
NIHP (1987). Detection, Evaluation and Treatment of High Cholesterol in Adults, National Institute of Health Publication No. 88-292.
O’Brien B.C. and R. Reiser (1979). Comparative Effects of Purified and Human-Type Diets on Cholesterol Metabolism in the Rat. J. Nutr. 109: 98-104.
Patel D.K., R. Kumar, D. Laloo and S. Hemalatha (2012). Natural Medicines from Plant Source Used for Therapy of Diabetes Mellitus: An Overview of its Pharmacological Aspects. Asian Pac. J. Trop. Dis., 2: 139–150.
Phumthum M. and H. Balslev (2018). Thai Ethnomedicinal Plants Used for Diabetes Treatment. OBM Integr. Complement. Med., 3: 1–25.
Raghu B. R., and J. Hortl, (2020). Diversity and Distribution of Curry Leaf in India. Sci., 15(1): 1-8.
Rahman M.F., M.K. Siddiqui and K. Jamil (2001). Effects of Vepacide (Azadirachta indica) on Aspartate and Alanine Aminotransferase Profiles in a Subchronic Study with Rats. Hum. Exp. Toxicol., 20: 243-9.
Suman R. K, Ipseeta Ray Mohanty, Manjusha K. Borde, Y. A. Deshmukh, Anurag Pathak, Arun Kumar Adhikari, H. K. Singh (2019). Evaluation of antidiabetic efficacy of Murraya koenigii on Streptozotocin induced diabetes in experimental rats. International Journal of Basic & Clinical Pharmacology, 8(8): 1906-1910.
DOI: http://dx.doi.org/10.18203/2319-2003.ijbcp20193200
Ravi K., B. Ramachandran and S. Subramanian (2004). Protective Effect of Eugenia jambolana Seed Kernel on Tissue Antioxidants in Streptozotocin-Induced Diabetic Rats. Biol. Pharm. Bull., 27: 1212-1217.
Reitman S. and S. Frankel (1957). Determination of Alanine Aminotranspherase and Aspartate Aminotranspherase. Amer. J. of Clin. Path., 28: 56.
Balakrishnan R, Dhanraj Vijayraja, Song-Hee Jo, Palanivel Ganesan, In Su-Kim, and Dong-Kug Choi (2020). Medicinal Profile, Phytochemistry, and Pharmacological Activities of Murraya koenigii and Its Primary Bioactive Compounds, Antioxidants, 9, (2)101: 1-28. https://doi.org/10.3390/antiox9020101.
Rusdi B., Yuliawati, K.M., Khairinisa, M.A. (2021). Comparison on the prebiotic polysaccharides and oligosaccharides from plant studies in Indonesia and outside of Indonesia (Article). Journal of Engineering Science and Technology, 16 (3): 2260-2272.
Samuel Tilahun Assefa, Eun-Young Yang, Soo-Young Chae, Mihye Song, Jundae Lee, Myeong-Cheoul Cho and Seonghoe Jang (2020). Alpha Glucosidase Inhibitory Activities of Plants with Focus on Common Vegetables. Plants, 9(1): 2. doi.org/10.3390/plants9010002
Sim K.M. and H.M. Teh (2011). A New Carbazole Alkaloid from the Leaves of Malayan Murraya koenigii. J. Asian Nat. Prod. Res., 13: 972–975.
Shah P., S. P. Singh, and A. Kumar, (2015). Combined Effect of Hydroethanolic Extracts of Murraya koenigii and Phyllanthus niruri Leaves on Paracetamol and Ethanol-induced Toxicity in HepG2 Cell Line. Current Science, 109(7): 1320 – 1326. DOI: 10.18520/v109/i7/1320-1344
Szkudelski T. (2001). The Mechanism of Alloxan and Streptozotocin Action in B Cells of the Rat Pancreas. Physiol Res., 50: 537-46.
Tembhurne S.V. and D.M. Sakarkar (2010). Influence of Murraya koenigii on experimental model of diabetes and progression of neuropathic pain. Res Pharm Sci.; 5(1): 41–47. PMID: 21589767.
Trinder, P. (1969). Determination of Blood Glucose. Bicon Diagnostics, Germany, Ann. Clin. Biochem., 6: 24–29.
Ujowundu C.O., O.E. Okafor, N.C. Agha, L.A. Nwaogu, K.O. Igwe and C.U. Igwe (2010). Phytochemical and Chemical Composition of Combretum zenkeri Leaves. J. Med. Plants Res., 4: 965–968.
Vinuthan M.K., V. Girish Kumar, J.P. Ravindra, Jayaprakash and K. Narayana (2004). Effect of Extracts of Murraya koenigii Leaves on the Levels of Blood Glucose and Plasma Insulin in Alloxan-Induced Diabetic Rats. Indian J Physiol Pharmacol, 48: 348-52. PMID: 15648408.
Virdi J., S. Sivakami, S. Shahani, A.C. Suthar, M.M. Banavalikar and M.K. Biyani (2003). Antihyperglycemic Effects of Three Extracts from Momordica charantia. J. Ethnopharmacol, 88: 107-11.
Wojdyło A., J. Oszmiański and R. Czemerys (2007). Antioxidant Activity and Phenolic Compounds in 32 Selected Herbs. Food Chem., 105: 140–149.
Young, D. and L. Pestaner (1975). Determination of Triglycerides. Bincon Diagnostics, Germany, Ann. Clin. Bio. Chem., 21:25.
Zang Y.D., C.J. Li, X.Y. Song, J. Ma, J.Z. Yang, N.H. Chen and D.M. Zhang (2017). Total Synthesis and Neuroprotective Effect of O-Methylmurrayamine A and 7-m J. Asian Nat. Prod. Res., 19: 623–629.
Xu K, Morgan KT, Todd Gehris A, Elston TC, Gomez SM (2011). A Whole-Body Model for Glycogen Regulation Reveals a Critical Role for Substrate Cycling in Maintaining Blood Glucose Homeostasis. PLOS Computational Biology 7(12): e1002272. https://doi.org/10.1371/journal.pcbi.1002272


