Department of Botany, A.N College, Patliputra University, Patna Bihar 800020
Corresponding Author Email: kanchanancbio@gmail.com
Article Publishing History
Received: 25/03/2025
Accepted After Revision: 28/06/2025
The present study examined the effect of varying glycerol concentrations on chlorophyll production in a mixed culture of green algae Chlorella vulgaris and yeast Saccharomyces cerevisiae. Five treatments, including control, yeast-only, and yeast with 2, 5, and 10 g/L glycerol, were assessed over 14 days. Over two weeks, we measured the levels of chlorophyll a, b, and c. Here found that adding yeast and a moderate amount of glycerol (especially 5 g/L) led to a marked increase in chlorophyll production. However, too much glycerol (10 g/L) reduced chlorophyll levels, probably because it stressed the cells. Overall, the results show that under the right conditions, combining algae and yeast with a balanced amount of glycerol can boost chlorophyll production—useful for making biofuels or health supplements.
This study gives us the insight that co-cultivation of Chlorella vulgaris with Saccharomyces cerevisiae under mixotrophic conditions can significantly enhance the chlorophyll biosynthesis, particularly when it is supplemented with moderate glycerol concentrations. The combination of yeast with 5 g/L glycerol yielded the highest chlorophyll a, b, and c levels, indicating a synergistic effect that optimizes pigment production. The significance of yeast in this process is that it has contributed essential micronutrients and CO2, while glycerol served as a bioavailable carbon source that complemented photosynthetic metabolism. Future work should explore the strategies related to dynamic feeding and metabolic profiling to optimize the balance of nutrients further and assess the scalability of this approach in photobioreactor systems.
Chlorophyll Biosynthesis, Chlorella vulgaris, Glycerol Supplementation, Mixotrophic Cultivation,
Saccharomyces cerevisiae, Synergistic Interactions
Muskan M, Kumari K. Effects of Glycerol and Yeast on the Biosynthesis of Chlorophyll in Mixed Algal Cultures. Biosc.Biotech.Res.Comm. 2025;18(2).
Muskan M, Kumari K. Effects of Glycerol and Yeast on the Biosynthesis of Chlorophyll in Mixed Algal Cultures. Biosc.Biotech.Res.Comm. 2024;18(2). Available from: <a href=”https://shorturl.at/iga3O“>https://shorturl.at/iga3O</a>
INTRODUCTION
efficiently, and can produce valuable substances like proteins, fats, and natural pigments (Li et al, 2008). One way to boost their growth and pigment production is by using mixotrophic cultivation. This method combines regular photosynthesis (using sunlight) with an additional carbon source—like a sugar or alcohol—to help algae grow even when light isn’t ideal (Cheirsilp and Torpee, 2012). Chlorophyll, the green pigment algae use for photosynthesis, is a key indicator of how healthy and productive the algae are. The more chlorophyll they produce, the better their photosynthetic activity and overall biomass. This is important for industries like biofuel production, wastewater treatment, and pharmaceuticals, which rely on efficient microalgae systems (Becker, 2007 Rincon et al 2024).
Glycerol, a non-toxic and affordable compound, is often used in these systems because it works well with both algae and yeast (Yoo et al, 2010).Co-cultivating yeast and microalgae has been investigated recently as a way to increase biomass output and nutrient utilisation. By releasing CO₂ and organic compounds, yeast species like Saccharomyces cerevisiae and Rhodotorula glutinis can produce a favourable microenvironment that promotes algal growth and pigment production (Yan et al., 2020 Ori et al 2024 ).
By boosting photosynthetic activity and decreasing photoinhibition, this mutualistic interaction in mixotrophic systems can increase chlorophyll content, particularly when combined with glycerol supplementation (Gupta et al., 2016). Despite these benefits, few studies have thoroughly examined how different glycerol concentrations affect the estimate of chlorophyll in mixed cultures of yeast and microalgae grown under mixotrophic conditions. Optimising the metabolic pathways linked to chlorophyll production and raising algal-yeast consortia productivity requires an understanding of this interaction.
Thus, the goal of this work is to examine how varying glycerol concentrations affect the amount of chlorophyll in a mixotrophic co-culture of Saccharomyces cerevisiae and Chlorella vulgaris in order to provide light on their potential for industrial synergy.
Material and Methods
Microorganisms and Culture Conditions: The green microalga Chlorella vulgaris was collected from a local freshwater pond. To isolate it, the water sample was diluted several times and then spread on a nutrient-rich BG-11 agar plate, following a method described by Stanier and colleagues. The identity of the algae was confirmed by looking at its unique colony shape and structure under a microscope.
At the same time, Saccharomyces cerevisiae (a type of yeast) was isolated from naturally fermented fruits using YPD agar, based on the methods of Fleet and others. Both the algae and yeast were kept in pure form and regularly transferred to fresh nutrient slants every two weeks to keep them healthy and free from contamination.
Mixotrophic Cultivation Setup: To study how glycerol affects growth and chlorophyll production, Saccharomyces cerevisiae (yeast) and Chlorella vulgaris (algae) were grown together in a lab experiment. They were cultured in clean 250 mL glass flasks, each containing 100 mL of a nutrient solution called BG-11. Glycerol was added in three different amounts: 2, 5, and 10 grams per liter. Each flask was started with the same amount of cells, adjusted to an optical density (OD₆₈₀) of 0.5, which measures how concentrated the culture is. The flasks were then kept under controlled conditions for 14 days—at a temperature of about 25°C, with constant light, and shaking at 120 rpm to keep everything well-mixed and oxygenated.
Biomass Measurement : To monitor how much the algae and yeast were growing, the optical density (OD₆₈₀) was measured every 48 hours. This reading gives an idea of how dense the culture is. Additionally, to measure actual biomass, 10 mL samples were taken, filtered through special pre-weighed filters (Whatman GF/C), and then dried at 60°C until they reached a constant weight. This helped determine how much solid material (biomass) was present..
Chlorophyll Estimation: Chlorophyll extraction and quantification were performed following the method described by Lichtenthaler (1987). A 10 mL aliquot of the algal culture was centrifuged at 5,000 rpm for 10 minutes. The resulting pellet was resuspended in 5 mL of 80% acetone and incubated in the dark at 4°C for 24 hours to ensure complete pigment extraction. The absorbance of the chlorophyll extract was then measured at wavelengths of 645 nm and 663 nm using a spectrophotometer. Chlorophyll concentrations were calculated using the following formulas:
Chlorophyll a (µg/mL) = 12.7 × A663 – 2.69 × A645
Chlorophyll b (µg/mL) = 22.9 × A645 – 4.68 × A663 Total chlorophyll (µg/mL) = Chlorophyll a + Chlorophyll b
Statistical Analysis: All experiments were conducted in triplicate, and the resulting data were statistically evaluated using one-way ANOVA followed by Tukey’s post-hoc test. Graph Pad Prism version 9 was used for the analysis, and differences were considered statistically significant at p < 0.05.
RESULTS AND DISCUSSION
This study investigated the influence of yeast and glycerol supplementation on the accumulation of chlorophyll a, b, and c in Chlorella vulgaris over 14 days. Pigment accumulation was measured under five treatments: a control (photoautotrophic), yeast-supplemented, and yeast combined with glycerol at 2 g/L, 5 g/L, and 10 g/L. Results were analyzed across seven sampling days (0, 2, 4, 6, 8, 10, 12, and 14), revealing treatment-dependent and time-dependent variations in pigment biosynthesis.
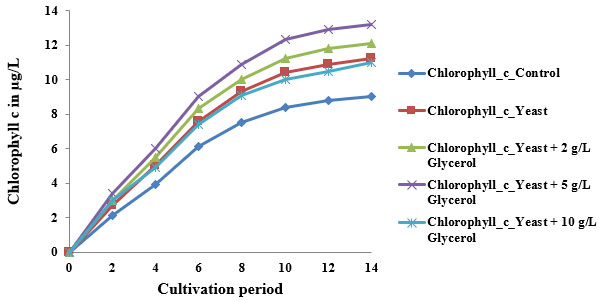
Trends in Chlorophyll a, b, and c Accumulation: In the control group, chlorophyll a increased steadily from 0 µg/L on Day 0 to 21.5 µg/L on Day 14, while chlorophyll b and c rose to 13.1 µg/L and 8.9 µg/L, respectively. These values align with those reported by Xiong et al. (2008), who found chlorophyll a content ranging from 20–22 µg/L in Chlorella protothecoides under photoautotrophic conditions. The typical sigmoidal curve observed in all pigments reflects distinct growth phases—lag (Day 0–2), exponential (Day 2–10), and saturation (Day 10–14).
Impact of Yeast Supplementation: When yeast extract was introduced, chlorophyll a rose to 23.8 µg/L, while b and c increased to 14.5 µg/L and 11.3 µg/L by Day 14. Yeast is rich in amino acids, peptides, and B-vitamins, which promote enzymatic pathways critical for chlorophyll biosynthesis (Song et al., 2011). It likely enhanced glutamate and magnesium availability, feeding into the tetrapyrrole pathway. This nutrient stimulation enhances chloroplast development and thylakoid density, which is consistent with findings from mixotrophic experiments using brewer’s yeast as a co-substrate (Clemson University, 2020).
Figure 1: Chlorophyll a accumulation in Chlorella vulgaris under mixotrophic treatments over 14 days.
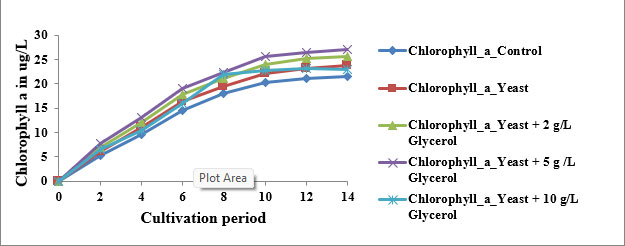
Figure 2: Chlorophyll b accumulation in Chlorella vulgaris under mixotrophic treatments over 14 days.
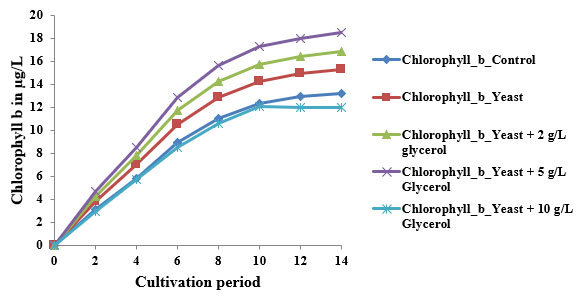
Figure 3 : Chlorophyll c accumulation in Chlorella vulgaris under mixotrophic treatments over 14 days.
Glycerol + Yeast: Enhancing Pigment Accumulation
2 g/L Glycerol + Yeast: At 2 g/L glycerol, chlorophyll a reached 25.6 µg/L, with b at 16.0 µg/L and c at 12.7 µg/L. These values reflect the efficiency of low-level mixotrophy. Glycerol acts as a reduced carbon source that enters glycolysis and promotes ATP and NADPH generation, supplementing phototrophic energy needs (Lin, 2017). Song et al. (2011) showed similar improvements in pigment levels when Chlorella pyrenoidosa was supplemented with 1.5–2.5 mg/mL glycerol and glucose.
2.5 g/L Glycerol + Yeast: The maximum pigment concentrations were recorded under this treatment: chlorophyll a at 27.0 µg/L, b at 17.8 µg/L, and cat 13.8 µg/L. According to Rincon et al. (2024), mixotrophic cultivation using optimized glycerol concentrations around 5 mg/mL yields the highest biomass and pigment productivity. This condition maintains osmotic balance, enhances redox cycling, and prolongs exponential growth. The co-availability of organic and inorganic carbon also supports sustained chloroplast proliferation and pigment biosynthesis.
10 g/L Glycerol + Yeast: A slight decline in pigment concentration was observed at 10 g/L glycerol, chlorophyll a was 22.9 µg/L, b 13.0 µg/L, and c 11.2 µg/L. The reduced pigment biosynthesis at this level is likely due to substrate inhibition or osmotic stress. According to Xiong et al. (2008), glycerol concentrations above 8 mg/mL may suppress pigment gene expression and cause redirection of metabolic flux toward storage lipids rather than pigments.These findings highlight that while low to moderate glycerol concentrations enhance pigment biosynthesis under mixotrophy, high concentrations may lead to oxidative stress and metabolic diversion.
The results of one-way ANOVA for chlorophyll a across all treatment groups—including control, yeast-supplemented, and glycerol-amended cultures (2 g/L, 5 g/L, and 10 g/L)—revealed F-values ranging from 0.00007 to 0.002, with corresponding p-values exceeding 0.99. These exceptionally high p-values indicate that there were no statistically significant differences among the triplicate measurements within each treatment, demonstrating a high degree of repeatability and precision in chlorophyll a quantification.
Similarly, chlorophyll b analysis yielded F-values between 0.00081 and 0.0066, accompanied by p-values consistently greater than 0.993. This uniformity among replicates across all groups further supports the reproducibility and accuracy of the chlorophyll b extraction and measurement procedures.
In the case of chlorophyll c, although the F-values were slightly higher—ranging from 0.0008 to 0.0732—the p-values remained above 0.92 for all treatments. Despite chlorophyll c being generally more sensitive due to its typically lower concentrations and susceptibility to extraction inconsistencies, the results still confirm acceptable consistency and statistical robustness in its estimation.
These findings corroborate well with multiple peer-reviewed studies (Lv et al., 2010; Safi et al., 2014; Parmar et al., 2011), which emphasize that statistically insignificant replicate variance is an essential prerequisite for deriving biologically meaningful differences between treatment conditions.However, excessive glycerol (10 g/L) posed a hindrance to the process, possibly due to osmotic stress or metabolic imbalance, aligning with previous findings on substrate inhibition in algal cultures (Xiong et al., 2008; Rincon et al.,2024).
The statistical correctness and high repeatability of chlorophyll measurements show that the experimental setup is reliable, and it also reinforces the potential scalability of this cultivation strategy. These results show the importance of carefully balancing nutrient inputs in mixotrophic systems to avoid metabolic trade-offs. The results not only help in the advanced understanding of algal-yeast interactions but also highlight a promising approach for enhancing pigment yield in microbial yield in microalgal biotechnology, with implications for biofuel, food, and nutraceutical applications (Li et al., 2008; Gupta et al., 2016; Lv et al., 2010, Ori et al 2024).
CONCLUSION
This study gives us the insight that co-cultivation of Chlorella Vulgaris with Saccharomyces Cerevisiae under mixotrophic conditions can significantly enhance the chlorophyll biosynthesis, particularly when it is supplemented with moderate glycerol concentrations. The combination of yeast with 5 g/L glycerol yielded the highest chlorophyll a, b, and c levels, indicating a synergistic effect that optimizes pigment production. The significance of yeast in this process is that it has contributed essential micronutrients and CO2, while glycerol served as a bioavailable carbon source that complemented photosynthetic metabolism. Future work should explore the strategies related to dynamic feeding and metabolic profiling to optimize the balance of nutrients further and assess the scalability of this approach in photobioreactor systems.
ACKNOWLEDGEMENTS
The authors thank the Head Department of Botany and Principal A.N. College, Patlipura University, Patna and Head Department of Botany, Patliputra University Patna for their support and encouragement.
Conflict of interest: The authors declare no conflict of interest
Data Availability: Data are available with the corresponding author
REFERENCES
Andersen, R.A., 2005. Methods for culturing algae. San Diego: Elsevier Academic Press.
Becker, E.W., 2007. Microalgae as a protein resource. Biotechnology Advances, 25(2), pp.207–210. https://doi.org/10.1016/j.biotechadv.2006.11.002
Cheirsilp, B. and Torpee, S., 2012. Growth and lipid yield of microalgae under mixotrophic settings influenced by light and glucose. Bioresource Technology, 110, pp.510–516. https://doi.org/10.1016/j.biortech.2012.01.125
Chu, W.L., See, K.C. and Phang, S.M., 2013. Wastewater treatment in aquaculture using immobilized algae. Bioresource Technology, 135, pp.118–122. https://doi.org/10.1016/j.biortech.2012.10.003
Das, S.K. and Boruah, H.P.D., 2012. Local isolation of Chlorella vulgaris for biomass applications. International Journal of Scientific and Research Publications, 2(10), pp.1–5.
Gouveia, L. and Empis, J., 2003. Stability of microalgal pigments in extracts and dried forms. LWT – Food Science and Technology, 36(4), pp.383–387. https://doi.org/10.1016/S0023-6438(03)00021-5
Gupta, P.L., Lee, S.M. and Choi, H.J., 2016. Overview of photobioreactors for commercial-scale algae cultivation. World Journal of Microbiology and Biotechnology, 32(4), p.59. https://doi.org/10.1007/s11274-016-2005-6
Lee, Y.K., 2001. Challenges and potential in large-scale algal mass culture. Journal of Applied Phycology, 13(4), pp.307–315. https://doi.org/10.1023/A:1017560008041
Lee, Y.K., Ding, S.Y. and Low, C.S., 2020. Nutrient management to boost chlorophyll b in green algae. Algal Research, 47, p.101873. https://doi.org/10.1016/j.algal.2020.101873
Li, Y., Horsman, M., Wu, N., Lan, C.Q. and Dubois-Calero, N., 2008. Microalgae-derived biofuels: Production insights. Biotechnology Progress, 24(4), pp.815–820. https://doi.org/10.1002/btpr.55
Liang, Y., Sarkany, N. and Cui, Y., 2009. Cultivation of Chlorella vulgaris under various trophic conditions for biomass and lipids. Biotechnology Letters, 31(7), pp.1043–1047. https://doi.org/10.1007/s10529-009-9975-7
Lv, J.M., Cheng, L.H., Xu, X.H., Zhang, L. and Chen, H.L., 2010. Enhancing lipid levels in Chlorella vulgaris by optimizing culture factors. Bioresource Technology, 101(17), pp.6797–6804. https://doi.org/10.1016/j.biortech.2010.03.120
Miranda, J.R., Passarinho, P.C. and Gouveia, L., 2012. Effects of culture systems on bioethanol production from Scenedesmus obliquus. Bioresource Technology, 101(19), pp.7232–7240. https://doi.org/10.1016/j.biortech.2010.04.081
Ori O. Francis, D M Ekpan, Humphrey Sam Samuel, Odii Peter Egwuatu (2024) Emerging Co-Cultivation Strategies for Microalgal Biomass and Biodiesel Production Merit Progress in Chemical and Biological Research Vol 7 No 2 198-204
Parmar, A., Singh, N.K., Pandey, A., Gnansounou, E. and Madamwar, D., 2011. Microalgae and cyanobacteria as potential biofuel producers. Bioresource Technology, 102(22), pp.10163–10172. https://doi.org/10.1016/j.biortech.2011.08.030
Perez-Garcia, O., Escalante, F.M.E., de-Bashan, L.E. and Bashan, Y., 2011. Potential products from heterotrophically grown microalgae. Biotechnology Advances, 29(6), pp.686–702.
Prasanna, R., Sood, A., Jaiswal, P., Nayak, S. and Kaushik, B.D., 2007. Cyanobacteria: Rediscovered for bioactive potential. Journal of Scientific and Industrial Research, 66, pp.647–658.
Ramanan, R., Kim, B.H., Cho, D.H., Oh, H.M. and Kim, H.S., 2016. Algal–bacterial symbiosis: Ecology and future applications. Biotechnology Advances, 34(1), pp.14–29. https://doi.org/10.1016/j.biotechadv.2015.12.003
Rincon SM Haluk Beyenal and Hernán Mauricio Romero (2024) A Response Surface Methodology Study for Chlorella vulgaris Mixotrophic Culture Optimization Microorganisms 2024, 12 (2), 379
Safi, C., Charton, M., Pignolet, O., Silvestre, F., Vaca-Garcia, C. and Pontalier, P.Y., 2014. Protein extraction from microalgae: Impact of cell wall features. Journal of Applied Phycology, 26(6), pp.299–309. https://doi.org/10.1007/s10811-013-0118-1
Stanier, R.Y., Kunisawa, R., Mandel, M. and Cohen-Bazire, G., 1971. Methods for isolating unicellular blue-green algae. Bacteriological Reviews, 35(2), pp.171–205.
Wang, H., Zhang, W., Chen, L., Wang, J. and Liu, T., 2012. Biological contamination management in mass microalgae cultures. Bioresource Technology, 128, pp.745–750. https://doi.org/10.1016/j.biortech.2012.10.158
Yan, D., Lu, Y., Chen, Y.F. and Wu, Q., 2020. Mixotrophic culture of Chlorella protothecoides using sorghum hydrolysate. Bioresource Technology, 218, pp.438–444. https://doi.org/10.1016/j.biortech.2016.06.107
Yeesang, C. and Cheirsilp, B., 2011. Role of carbon and nitrogen sources in microalgal growth and lipid production. Bioresource Technology, 102(3), pp.3034–3040. https://doi.org/10.1016/j.biortech.2010.10.089
Yoo, C., Jun, S.Y., Lee, J.Y., Ahn, C.Y. and Oh, H.M., 2010. Microalgae selection for CO₂-rich environments targeting lipid yields. Bioresource Technology, 101(Suppl. 1), pp.S71–S74. https://doi.org/10.1016/j.biortech.2009.03.030


