1Department of Biological Sciences, Faculty of Science, P.O. Box 80203, King Abdulaziz University, Jeddah, 21589, Saudi Arabia,
2King Fahad Medical Research Center, P.O. Box 80216, King Abdulaziz University, Jeddah, 21589, Saudi Arabia.
Corresponding author email: sonoor@kau.edu.sa
Article Publishing History
Received: 10/07/2020
Accepted After Revision: 17/09/2020
Follicular fluid (FF) is one of the important sources of micro-organisms that may affect IVF outcomes. IVF cultures were found to contaminate the fungi. The source of the contaminating fungus is possibly from the FF, and the culture medium does not regularly contain any antifungal agents, which is the cause of widespread fungal contamination in the culture of IVF. A lot of people are therefore opting for IVF to get a child. In vitro fertilization, assisted reproductive technologies (ART) includes extracorporeal fertilization using specific surgical procedures to support pregnant people. It is typically carried out when certain, less costly forms of reproduction struggle. Infertility is a global public health concern and accepted by Saudi society as a big issue. Many triggers can contribute to female infertility, such as ovulatory disorders, endometriosis, endocrine disorders, genetic factors, tubal factors, and pelvic inflammatory disease.
Additionally, many factors in lifestyle, such as age, weight, obesity, smoking, environmental and other toxins, can affect overall health and lead to infertility. The embryo culture medium also contains antibacterial agents, but some species of bacteria may be resistant to these antibiotics and do not routinely produce any antifungal agents. The FF was not always sterile but contained a range of microorganisms that affected IVF results, and a broader sample of patients needed to be studied to further confirm our theory. Furthermore, identification of FF microbes in women with repeated failed IVF cycles will provide an opportunity to start antimicrobial therapy before the next pregnancy. The study aimed to provide a summary of the microorganisms and their effects on in vitro fertilization of human follicular fluid.
IVF, Human, Follicular Fluid, Microorganisms, In Vitro, Fertilization, Outcomes
Noor S. O. Effects of Follicular Fluid Contamination by Microorganisms During In vitro Fertilization. Biosc.Biotech.Res.Comm. 2020;13(3).
Noor S. O. Effects of Follicular Fluid Contamination by Microorganisms During In vitro Fertilization. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/31OAYtK
Copyright © Noor et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Infertility is a global public health concern and is considered a major clinical problem today (Kumar & Singh, 2015). The prevalence of infertility measured by a systematic study of 277 health surveys from 1990 to 2010, in 190 countries. They found that this issue occurred in 48.5 million couples worldwide, 1.9% of women were unable to have their first child, which was identified as primary infertility, and 10.5% of women were unable to have another child after five years, which was represented as secondary infertility. This problem has been the most popular for some regions such as South Asia, North Africa, the Middle East, and Central Asia (Mascarenhas et al. 2012).In 2012, data were obtained and analyzed for 457 patients with infertility in the eastern region of Saudi Arabia. The prevalence of infertility was estimated to be 18.93 percent, higher than the prevalence in developing countries (3.5 percent to 16.7 percent) within one year (Al-Turki, 2015). The overall birth rate per woman in the world fell from 4,979 in 1960 to 2,432 in 2017, (Al-Turki, 2015; World Bank, 2017).
Infertility is now recognized as a major problem in Saudi society and a cause of concern. Many people, therefore, choose to have an assisted reproductive technology (ART) for having a child. One such technique is the treatment of IVF (Aoun & Moawed, 2012). In 2016, global data from 178 ART cycle centers in 15 Latin American countries were collected. They recorded 3931 IVF cycles, 1113 pregnancy rates, and 859 delivery rates at that time. Overall, the perinatal mortality rate was 8.2 percent in singletons, 19.31 percent in twins, and 63.2 percent in multiples of a high order. Compared to the previous year, the number of registered cycles increased by 14 percent, and the number of transferred embryos decreased with a decline in several births (Zegers-Hochschild et al., 2009).
One of the most difficult situations when embryologists consider that after investing a lot of money and time, the embryo polluted. A second possible harmful effect when the microorganisms transmitted to the female reproductive tissue from the infected embryo culture media that can contribute to adverse pregnancy outcomes and several cases of embryo contamination are attributed to microorganisms from a follicular fluid (Pomeroy, 2010). In this fluid, the oocyte matures and the consistency of the oocyte influences embryo development and can be used as an embryo consistency biomarker (Chen et al., 2016).
Various micro-organisms may colonize the FF.These microorganisms are thought to have spread to the FF by hematogenic invasion from other body locations, such as the oral cavity and the respiratory tract (Pelzer et al., 2013a). Follicular fluid may also be infected by vaginal microorganisms when a large needle passed through the vagina into the ovary begins extracting the sample from the clinical embryologist. These microorganisms can produce many toxic substances, including endotoxins, alpha-hemolysin, Shiga-like, and other lipopolysaccharides and peptidoglycans, which may affect the medium of embryo culture and may result in DNA fragmentation of gametes, low-quality embryos and premature birth ( Pomeroy 2010, Borges & Vireque, 2019).
Some microorganisms may be in low concentrations which affect the culture of the embryo but do not produce any obvious signs such as flocculants or embryo necrosis (Pomeroy, 2010). Traditionally, the embryo culture media contains antibiotics such as penicillin, streptomycin or gentamycin in an attempt to prevent the growth of pathogenic microorganisms while other forms of bacteria may be immune to these particular antibiotics and other microorganisms such as mycoplasms and anaerobic bacteria escape from the antibiotics placed on the culture of embryos (Pelzer & Allan, 2011 Borges &Vireque, 2019).
Antimicrobials also have little inhibition of the potentially large number of bacteria in the culture medium (Moore et al. 2000). Also, in patients who received antibiotics before IVF cycles, the frequency of microbial infection after recovery declined from 0.4 percent to 0 percent (Gardner & Simón, 2017).Previous experiments associated FF microorganisms with declining or growing outcomes of IVF (Hamad et al., 2018; Ibadin & Osemwenkha, 2014; Pelzer et al. 2011; Pelzer et al. 2013a Kim et al. 2018).
The microbiome of semen has been studied mostly in connection with male infertility or prostatitis, Monteiro et al. (2018). Just a few microbial experiments of IVF have demonstrated that high-variety bacterial infection of the culture media in IVF induces injury or even destruction of oocytes and embryos produced. We aimed to determine and associate the prevalence and count of bacteria in IVF samples with clinical outcomes (Štšepetova et al., 2020). This research aims to obtain an overview of human follicular fluid microorganisms and their effects on in vitro fertilization outcomes.
The ovary and function: One coat of epithelium coats the ovary. The region pellucida is a sheet of glycoprotein encompassing the oocyte plasma membrane. The ovarian function is regulated by a nerve cell gonadotrophin hormone which sends its messages to the anterior pituitary gland to produce LH and FSH to grow follicles and ovarian steroid hormone output. In the center of each menstrual period, the ovaries produce one egg (oocyte), or occasionally two. This is called ovulation. The ovary has two main body reproduction features. (Bradford, 2017; Speroff & Fritz, 2005). The ovarian follicle comprises of an oocyte, enclosed by cell layers of granulosa, and an exterior basement membrane enclosed by additional layers of thecal cells and functioning together to synthesize the hormone to regulate the maturation of additional follicles. Once the reproductive hormones activate a certain egg for maturation, and the ovarian follicle goes through the following stages: primordial, main, secondary (pre-antral) and the final step is the pre-ovulatory follicle level. Mature follicles, known as Graafian follicles, can expand up to around 1.2 inches (30 millimeters) in diameter and the fluid that occupies the oocyte’s cavity called follicular fluid. (Bradford, 2017; Fritz & Speroff, 2005).
Evaluation of oocyte quality: Before the IVF procedure evaluation of the infertile couple is important to achieve the best results and avoid complications. Women must then undergo blood tests for FSH, LH, prolactin, E2 (estrogen content in women’s blood), and inhibit B rates, AMH, and Antral Follicle Count (AFC) with a high-quality trans-vaginal ultrasound scan that will provide the doctor with details on egg size and condition, ovarian reaction, and the appropriate way to implant the embryos. When the IVF process is completed with an elevated FSH level, the efficiency of ovarian stimulation is not enhanced, so women have a decreased cancelation risk. During the IVF process, too, most IVF practitioners undergo regular son hysterogram or hysteroscopy. The downside of hysteroscopy is that minor polyps or symptoms suggesting persistent endometritis are visualized (Gardner & Simón 2017). Pairs are screened for the presence of sexually transmitted infections (STIs) including C before IVF procedure. Trachomatis, that is N. gonorrhea, hepatitis B and C, human immunodeficiency virus (HIV), syphilis, and cytomegalo virus (CMV), but microbiological monitoring procedure for the IVF process is not done (Pelzer, 2011).
Upper genital tract (UGT): The women’s reproductive organs’ upward genital tract infection (UGTI) involving the endometrium, fallopian tubes, and ovaries is a prevalent disease among reproductive-age women. UGTI is typically triggered by an ascending infection of the form, where N is the most common cause. C or gonorrhea Trachomatis, but approximately 30 and 40% of cases are triggered by polymicrobial (Schiappacasse, 2014). Many experiments have reported that in the absence of symptomatic infection, micro-organisms colonize the female UGT (Pelzer et al. 2013a). Microbial contamination of UGT occurs due to LGT, especially while using a sample selection transcervical technique. The endometrial cultures lead obtained one or more microbes with Lactobacillus spp., M. Homa, G. Vaginalis, and Enterobacter spp. and another infection was induced by the tendency of such microbes to bind to human spermatozoa and then transmitted through the intrauterine area via the cervix. (Rampersaud et al. 2012). The microorganisms which were extracted from PID cases were S. horny, S. cohnii, Megaterium bacillus, Brevibacterium epidermidis, Francisella philomiragia, E. coli, Citrobacter freundii (Okiki et al.2015).
Many cervical swab experiments in people with bacterial vaginosis have shown that the first community of independent species is E. coli followed by Bacillus subtilis, Proteus mirabilis, and Actinomyces israelii (Jabuk, 2014). Group B streptococci colonize 20-25% of pregnant women’s maternal genital tract, which is a significant cause of neonatal illness which mortality (Stoll et al. 2011). Microorganisms inside the UGT, including those that contaminate the IVF culture method, can result in reduced oocyte content, embryo content (possibly due to fragmentation of oocyte DNA), And the failure of early infancy (Pelzer & Allan 2012). Patients with UGTI have an elevated chance of ectopic pregnancy and infertility correlated with damaged Fallopian tubes that develop after UGTI (Schiappacasse, 2014).
The placenta, fetal membranes, and cervical mucus function together during pregnancy to shield the baby from invasion by microorganisms. Inflammation of fetal membranes and placental chorion typically means that bacterial contamination is on the rise. Vaginal organisms are believed to initially invade the space between the tissues of the mother and the fetal membranes and afterward infect the amniotic fluid. (Rampersaud et al., 2012).Infertility described as women’s inability to get pregnant during unsafe intercourse for 12 months (World Health Organization, 2016). It can be either primary infertility, which is a wait for a couple to conceive with no prior pregnancies or secondary infertility after one year or more, which is a pause for a couple who have produced children before (Anwar & Anwar, 2016).
This disorder will lead the infertile couple to depression and other psychiatric psychological disorders (Abolfotouh et al., 2013). There are two gonadotropin hormones produced in the pituitary gland, and the gonadotropin-releasing hormone (GnRH) [follicle-stimulating hormone (FSH) and luteinizing hormone (LH)] controls their secretion. The GnRH works on the pituitary gland to produce FSH and LH at the start of a new process. These hormones activate ovarian follicles and they grow them. Around 30-40 follicles begin to develop each month in response to FSH with the ability to release one single mature egg at fertilization ovulation (Anwar & Anwar, 2016).
The follicular fluid within the ovarian follicle
Follicular fluid is a substance that covers the ovum and develops from two outlets, the flow of the blood plasma portion connected with some thecal capillaries in the ovary’s cortical area, and the secretory operation of granulosa and thecal cells. It includes many hormones including FSH, LH, GH, human chorionic gonadotropin (hCG), progesterone and estradiol (E2); enzymes; anticoagulants; electrolytes; reactive oxygen species; growth factors such as epidermal growth factor ( EGF), EGF like growth factor (EGF), vascular endothelial growth factor ( VEGF) and transforming growth factor-alpha (TGF-α); cytokines; antioxidants and metabolites and Multi-effecting fatty acids on ovarian development and oocyte maturation (Basuino& Silveira, 2016; Revelli et al., 2009).
FF pH is stated to be between 7.2–7.3 (Swain, 2012). The Fallopian tube plays a crucial role in fertilization and early development of the fetus. FF is released into the peritoneal cavity at ovulation and inserted into the Fallopian tube to influence reproductive parameters and promote embryo cleavage during IVF (Lyons et al., 2005). The follicular fluid has an essential role in antral follicle contact between cells when transporting nutrients through the oocyte. FF is also a core component of the effectiveness of natural fertilization present at any point in the design process (Basuino & Silveira, 2016; Revelli et al., 2009). Hormones, gonadotropins play a significant role in the secretion of several substances that influence oocyte production and maturation by granulosa cells. The elevated FSH, hCG and LH rates have been related to oocyte maturation and fertilization (Revelli et al., 2009). In comparison, PCOS patients, low levels of FF components such as testosterone, E2, progesterone, and β-hCG, can have a detrimental effect on oocyte production and fertilization rates (Basuino & Silveira, 2016).
Growth hormone (GH), Granulosa cells boost the FSH and develop FSH and LH receptors in certain cells. Growth hormone production also happens in the follicle; thus, it may interact with gonadotropins that increase the amount of estrogen contributing to improved oocytes. There is no clear correlation between GH levels intrafollicular and the rates of pregnancy.Prolactin (PRL): Several types of research found a link between high PRL with fertilization and positive pregnancy, but this was not supported by other tests. Hence FF PRL is not considered a strong sign of oocyte quality at present, (Revelli et al. 2009). Estrogens, progesterone, and androgens: Several types of research have shown that the strong FF estrogens ratio correlates with oocyte maturation may contribute to a higher risk of pregnancy but not supported by others.
Optimal progesterone exposure has beneficial effects on oocyte characteristics although inappropriate exposure contributes to a decrease in oocyte production. In comparison, elevated androgen rates (testosterone) associated with lower-quality oocytes. Inhibin: Granulosa cells manufacture inhibin and are classified into two forms (inhibin A, and B). Throughout the FF, inhibin A improves throughout women with endometriosis during the follicular period, and higher, while inhibin B reduces. Inhibin B in FF correlated with the number of oocytes retrieved, but not with the result of the IVF, may, therefore, be viewed as a symbol of ovarian reaction but not of oocyte efficiency (Revelli et al., 2009). Anti-Mullerian hormone (AMH): There is already an inconsistent association between oocyte production and AMH rates. Some studies noticed that rates of AMH were associated with oocyte production, and others showed that rates of AMH were linked inversely with oocyte maturation (Revelli et al., 2009).
The normal genital tract flora and opportunistic pathogens: Lower genital tract, Lactobacillus species predominate the normal lower genital tract flora (LGT) for most healthy women. The species most common include L. crispato, L. down, L. jensenii & L. gasseri gasseri. Their capacity can provide protective functions through the production of lactic acid to preserve an acid environment, hydrogen peroxide, and other substances that prevent pathogenic microorganisms from overgrowth. When the vagina lacks lactobacilli, other lactic acid bacteria in the vagina, including the species Atopobium, Megasphaera and Leptotrichia (Lamont et al., 2011; Witkinet al., 2007).
In addition, any overgrowth disturbance of the usual vaginal flora, especially anaerobic species Mycoplasma hominins, Gardnerella vaginalis, Bacteroides and Mobiluncus can contribute to bacterial vaginosis (BV). Gray or yellow blood, fishy odor, and stomach pain are the most frequent signs of BV. Up to half of the people are asymptomatic. The clinical treatment describes this variation with low pH>4.5, fishy odor on the introduction of 10 percent KOH, and the presence of hint cells under microscopic vaginal smear inspection (Morris et al., 2001).BV incidence has been associated with various gynecological disorders and pregnancy problems such as pelvic inflammatory disorder (PID), miscarriage, endometriosis, and preterm delivery (Lamont et al., 2011; Nelson & Macones, 2002). Many types of research before and after the ART procedure indicated that the prevalence of BV among infertile women was 4.2-36 percent (Morris et al., 2001; Spandorfer et al., 2001; Wilson et al., 2002). Opportunistic pathogens like Staphylococci, Enterococci, Enterobacteria, Candida, Peptostreptococci, Peptococci fungi, and Gram-negative anaerobic bacteria were the most widespread microorganisms found in women’s vaginal discharge.(Aleshkin et al., 2006).
An anaerobic overgrowth of the regular flora of aerobic microbes including Escherichia coli, group B Streptococci, Enterococci, and Staphylococcus aureus may disrupt the abnormal vaginal flora. Pregnancy risks are the most significant contributing factors for a vaginal infection, such as early membrane breakup, early delivery, and perinatal infection. Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis, Haemophilus influenza, and group B Streptococci are the forms of pathogenic bacteria that may be linked with such complications (Donati et al., 2010).Additionally, Candida spp. It is a natural commensally yeast in the female genital tract and may sometimes cause diseases varying from mild to extremes, such as vaginitis, cervicitis, and persistent vulvo vaginal candidiasis (Pellati et al., 2008). Contamination with candida Albicans happens predominantly (80 to 90 percent) in confirmed patients, whereas contamination with other pathogens happens less often, including C. glabrata and C. tropicalis (Soong & Einarson, 2009).
Microorganisms within Human Follicular Fluid Effects on IVF: In both normal and IVF pregnancies, the existence of opportunistic pathogens in the lower female reproductive tract was correlated with adverse outcomes of pregnancy (McClure and Goldenberg, 2009). Discrepancies in women with colonized and infected follicular fluid were found (Pelzer et al., 2011). Also, multiple reports have reported that, in the absence of asymptomatic infection, microorganisms often and transiently colonize the female upper genital tract (Viniker, 1999). Studies of microorganisms and human follicular fluid were performed predominantly in women engaging in IVF cycles owing to the complexity of the procedures needed to acquire this specimen (Pelzer et al., 2011). Cottell et al., (1996) in their report, examined the impact of microorganisms from the IVF culture method as a whole by pooling the findings obtained for each test form (follicular fluid, oocyte extraction needle wash, semen and culture media) and finding correlations between these tests and IVF outcomes and concluding that no adverse effects occurred (Cottell et al., 1996, Pelzer et al., 2013).
The IVF is not possible in a sterile setting. During semen therapy, the frequency and concentrations of bacteria reduced during the IVF process. The prevalence of Bacilli (Lactobacillus genera) groups in raw semen and IVF culture media, Clostridia in washed sperm, and Bacteroidia in incubated sperm samples was shown. Staphylococcus sp has an appearance. Alphaproteo bacteria and health measures such as semen and embryo production are correlated with this. Potential studies will also concentrate on strategies to help reduce the harmful effects of these microorganisms on the development of IVF embryos and to help deter IVF failure (Monteiro et al., 2018 and Štšepetova, et al., 2020).
Microorganisms and IVF outcomes: Techniques for in vitro fertilization are vulnerable to infection on several levels. Oocyte infection or the developing embryo can occur during IVF procedures. Past research also indicated a link between FF-isolated micro-organisms and IVF outcomes. Kim et al. (2018) analyzed collections of vaginal swab and FF from infertile women completing IVF cycles at the time of ovum processing. The isolated bacterial species is coagulase-negative Staphylococci, Streptococcus agalactiae, E. coli, Kristina kocuria, E. fecalis, pneumonic Klebsiella, and S. aurora. They stated that FF is not sterile but that FF microorganisms do not have any major adverse effects on IVF tests. Studies with a vaginal swab and FF tests in people that have had IVF periods.
They showed that 45.7 percent of FF samples had bacterial organisms, including Lactobacillus spp., Staphylococcus spp., Streptococcus spp., Propionibacterium spp., Actinomyces spp., and Bifidobacterium spp. Haahr et al. (2016) analyzed vaginal swabs for specific Lactobacillus spp., Gardnerella vaginalis, and extracted Atopobium vaginae from women in IVF periods. They revealed the G. vaginaliz, A. vaginae were BV associated. Lactobacillus crispatus, L. jensenii y, L. gasseri were correlated with natural microbiota and the presence of unhealthy vaginal microbes (AVM) in IVF patients could have a detrimental impact on fertility levels and other reproductive outcomes. If there was a significant association between AVM and the results of abortion, patients should be tested and monitored with AVM until the IVF treatment begins.
Pelzer et al. (2013a) isolated FF and vaginal swabs for microorganism detections from women undergoing IVF procedure with various infertility causes at the time of oocyte retrieval. They estimated that the prevalence of FF micro-organisms was 99 percent; infected 71 percent of FF and colonized 29 percent. Propionibacterium spp., Streptococcus spp., Actinomyces spp., Staphylococcus spp., and Bifidobacterium spp. have been correlated with negative IVF results whereas Lactobacillus spp. is present. Heightened embryo transfer levels were consistent with this. Pelzer et al. (2013b) investigated the DNA heterogeneity inside FF of non-fertilized mouse oocytes incubated in vitro from women undergoing IVF procedures. To detect the existence of bacteria, each sample of FF was cultured. They displayed other bacterial forms including Streptococcus anginosus, Peptoniphilus spp., Lactobacillus gasseri, E. fecalis, and acnes containing Propionibacterium. Much fragmentation of DNA occurred with a large dose of P. acnes or L. gasseri. No fragmentation of DNA with a low dose of L was observed. L. gasseri and E. fecalis as shown in Fig 1. They concluded that FF microorganisms can contribute to oocytes of poor quality that lead to reduced IVF outcomes.
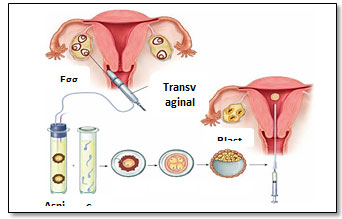
Figure 1: In vitro fertilization procedure (Hoffman et al., 2016).
Association between anti-chlamydial immunity and IVF outcome: Chlamydial inflammation is one of the main sources of occlusion of the fallopian tube. The latest studies have shown that serum anti-chlamydial antibodies are present in almost every second where individual receiving in vitro fertilization (IVF) therapy for tubal infertility (de Barbeyrac Papaxanthos-Roche, 2006 and Muller et al., 2015). Several reports have examined the connection between the immune reaction to the Chlamydia trachomatis and the result of IVF. Neuer et al.(1997) reported a decrease in pregnancy levels for anti-chlamydial IgA in follicular fluid in women with IVF positive. Liccardi et al. (1992) identified a correlation between serum positivity of the chlamydial antibody and spontaneous abortion. In comparison, a comparable number of publications, Gaudoin et al. (1999) and Muller et al. (2015) reported no association between anti-chlamydial immunity and IVF loss. Data are present in various research on potential pathways involved in the production of infertility following chlamydial infection, although it remains contentious. Hence, our study’s key objective was to evaluate the effects of microorganisms on in vitro fertilization of human follicular fluid.
Endocrine disorders: The pituitary gland was split into two lobes, the pituitary posterior (neurohypophysis) and the pituitary anterior (adenohypophysis). The anterior pituitary controls the release of the thyroid-stimulating hormone (TSH), the adrenocorticotropic hormone (ACTH), the thyrotropin, the corticotrophic, and the gonadotrophic cells respectively. The anterior pituitary also secretes growth hormone (GH) and prolactin, respectively, from the somatotroph and lactotroph cells. Several factors, such as genetic abnormalities, diet, drugs, inflammatory processes, and certain tumors may affect the pituitary role. The abnormality or deficiency of the pituitary gland, which can contribute to excessive prolactin (hyperprolactinemia) will inhibit ovulation. Thyroid conditions(hyperthyroidism and hypothyroidism) can also interfere with ovaries that can trigger ovulation delay ( Pauli and Kallen 2011, Anwar & Anwar 2016).
Premature ovarian failure: In people less than 40 years old, this is a disease of discontinues usual ovarian activity. It is normally triggered by an allergic reaction or early depletion of ovary eggs probably induced by genetic factors or chemotherapy. In women less than 40 years old, natural ovarian activity stops. The most frequent signs include prolonged or missing cycles, hot flashes, and sweating at night. This disorder is a disease of elevated FSH rates, low estradiol rates, and reduced hormone levels (Unuane et al. 2011).Endometriosis, a common debilitating condition that affects 10 percent of women of reproductive age, triggering pelvic pain, and infertility. The incidence of endometriosis in women with infertility rose by up to 50 percent. It is known as endometrial glands and stroma developing outside the uterus on other pelvic organs such as ovaries or Fallopian tubes. This tissue has no way to leave the body, it gets stuck and cysts grow. The surgical removal of it may induce scarring, which can obstruct Fallopian tubes and prevent the joining of an egg and sperm. Compared to women with unexplained or tubal infertility, the existence of endometriosis may adversely affect spontaneous pregnancy and IVF outcomes, (Khine et al. 2016).
Genetic factors: It is a genetic disorder influencing the role of the ovaries. Girls with Turner’s syndrome have distinctive facial characteristics and reproductive defects of the X chromosomes because of incomplete or anomalies. Any of the girls impacted was unable to reproduce because of an ovarian defect. Patients with Turner’s syndrome get an appropriate level of conception after oocyte donation. A high pregnancy prevalence associated with hypertensive disorders that can contribute to restriction of premature birth and intrauterine development (Bodri et al., 2005). It is the absence of an apparent cause of infertility that occurs in about 15–30 percent of infertile couples. It can only be diagnosed after the male and female partners have completed a fertility assessment (Schattman et al., 2016).
Lifestyle influences are modifiable habits and activities which may influence physical health and lead to infertility. Many factors in lifestyle such as age, nutrition, obesity, exercise, smoking, consumption of caffeine, psychological stress, pollutants in the environment, and others can have an impact on fertility. There is clear proof that sex, weight, and smoking influence general wellbeing and detrimental impact on sexual health, (Homan et al. 2007).These considerations are further explored in-depth below: As women enter the age of 35, their fertility declines, rendering pregnancy more complicated due to the reduction of both oocyte and follicle pool quantity and consistency. When women are younger than 30 years of age, the chances of conception can reach 71%; when they are older than 36, they can only be 41%. With age increases, women are at increased risk of fertility-influencing conditions such as uterine fibroids and endometriosis (Homan et al., 2007; Velde & Pearsom, 2002 Hoffman et al 2016).
Obesity is associated with higher plasma and FF leptin production. Leptin was also found to inhibit the production of LH by the granulosa cells which stimulated estradiol production. Such effects can explain in part the decreased reproductive success of women with overweight (Metwally et al., 2007a). A background of infection with the genital tract All aspects of the female reproductive system may be affected by infectious agents and impair female fertility. Infectious diseases may impact multiple reproductive tract sites and include damage to the cervical, tubal, and peritoneal. The most popular female fertility-related micro-organisms are C. trachomatis and N. Gonorrhoeae, which considered being the most significant cause of tubal lacerations and obstruction, PID, and adhesions.
Microorganisms which are associated with bacterial vaginosis can reach the genital tract in different ways and may result in infertility to the tubal factor (Pellati et al., 2008). In smoking, miscarriage rates in both natural and assisted conception cycles, ectopic pregnancy, early menopause, and infertility are the reproductive risks associated with smoking. Cigarette smoke substances such as nicotine and carbon dioxide and cyanide may influence the follicular microenvironment, modify the hormone rates, increase genetic defects, placental insufficiency, and lack of reproductive ability. In female smokers’ FF, cotinine and cadmium have been found, and this can impact the forming follicle ( Homan et al 2007, Hoffman et al., 2016).
Reactive Oxygen Species and antioxidant factors: Some studies found a positive correlation between levels of FF, Reactive Oxygen Species (ROS) and maturation of the oocytes. The women who were pregnant with IVF had higher rates of ROS in the FF relative to non-pregnant people. On the other hand, the etiology of defective embryo development is associated with oxidative stress. The presence of antioxidants and reactive oxygen species in the FF helps the meiosis cycle to take place (Revelli et al., 2009). Cytokines and growth factors, cytokines are required for ovarian activity, modulating ovarian steroid hormone production, and embryonic growth.
Many studies have examined the association between FF cytokines and ART outcomes: these researchers found that cytokines and VEGF were associated with higher fertilization levels, positive embryo transfer, and clinical pregnancy, whereas interleukin (IL) -12, VEGF and IL-15 were associated with low fertilization and aborted conception (Pelzer et al., 2011). Other contents, the peritoneal fluid associated with FF is an essential factor for the ovulation process, gametes transfer and preservation, and oocyte-sperm involvement in environmental development. In the FF lipoprotein acts as a medium for progesterone production. High-Density Lipoprotein (HDL) also lets the oocyte and embryo grows well (Basuino & Silveira, 2016).
CONCLUSION
There are elevated antibody titers against Chlamydia trachomatis found in up to 70 percent of people with tubal factor infertility (TFI). Determining the influence of past chlamydial agents. Longitudinal retrospective research on the effects of an infection with an IVF procedure was conducted. Detection of an anti-chlamydial antibody is not linked to the formation of oocytes, the growth of eggs, the pregnancy, and the birth of life stages. Thus, past chlamydial infection in TFI patients is correlated to ovarian stimulation and missed risk of abortion with decreased IVF success. The FF was not sterile but had a range of micro-organisms that influenced IVF results, so a broader population of patients had to be tested to further support our hypothesis. Indeed, it would provide an incentive to begin antimicrobial therapy before the next birth to recognize FF bacteria in individuals with multiple missed IVF cycles.
Conflict of interest: The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge assistance from the Department of Biological Sciences, Faculty of Science, King Abdulaziz University (KAU), Jeddah, KSA.
REFERENCES
Abolfotouh, M. A., Alabdrabalnabi, A. A., Albacker, R. B., Al-Jughaiman, U. A., & Hassan, S. N. (2013). Knowledge, attitude, and practices of infertility among Saudi couples. International journal of general medicine, 6, 563.
Aleshkin, V. A., Voropaeva, E. A., & Shenderov, B. A. (2006). Vaginal microbiota in healthy women and patients with bacterial vaginosis and nonspecific vaginitis. Microbial ecology in health and disease, 18(2), 71-74.
Al-Turki, H. A. (2015). Prevalence of primary and secondary infertility from a tertiary center in eastern Saudi Arabia. Middle East Fertility Society Journal, 20(4), 237-240.
Aoun, T. B., & Moawed, S. (2012). Effects of environmental, cultural, and socioeconomic factors on Saudi infertile couples in Riyadh City. Life Science Journal, 9(4).
Basuino, L., & Silveira, J. C. (2016). Human follicular fluid and effects on reproduction. JABRA assisted reproduction, 20(1), 38-40.
Bodri, D., Vernaeve, V., Figueras, F., Vidal, R., Guillen, J., & Coll, O. (2005). Oocyte donation in patients with Turner’s syndrome: a successful technique but with an accompanying high risk of hypertensive disorders during pregnancy. Human Reproduction, 21(3), 829-832.
Borges, E., & Vireque, A. (2019). Microbial Contamination in Assisted Reproductive Technology: Source, Prevalence, and Cost. Advances in Biotechnology & Microbiology, 13(4), 555869.
Bradford, A. (2017). Ovaries: Facts, Function & Disease. Live Science
DeBarbeyrac B, Papaxanthos-Roche A. Chlamydia trachomatis in subfertile couples undergoing an in vitro fertilization program: a prospective study. Eur J ObstetGynecolReprodBiol 2006; 129:46–53.
Donati, L., Di Vico, A., Nucci, M., Quagliozzi, L., Spagnuolo, T., Labianca, A., Paradisi, G. (2010). Vaginal microbial flora and outcome of pregnancy. Archives of gynecology and obstetrics, 281(4), 589-600.
Chen, F., Spiessens, C., D’Hooghe, T., Peeraer, K., & Carpentier, S. (2016). Follicular fluid biomarkers for human in vitro fertilization outcome: Proof of principle. Proteome Science, 14(1), 17.
Cottell E, McMorrow J, Lennon B, Fawsy M, Cafferkey M, et al. (1996)Microbial contamination in an in vitro fertilization-embryo transfer system Fertil Steril 66: 776–780.
Gardner, D. K., & Simón, C. (2017). Handbook of in vitro fertilization: CRC press.
Gaudoin M, Rekha P, Morris A, et al. (1999) Bacterial vaginosis and past chlamydial infection are strongly and independently associated with tubal infertility but do not affect in vitro fertilization success rates. Fertil Steril 72:730–2.
Gurgan T, Urman B, Diker KS, Delilbasi Land Kisnisci HA (1993) Human follicular fluid from pre-ovulatory follicles in patients undergoing in-vitro fertilization inhibits the in-vitro growth of gram-positive micro-organisms. Hum Reprod 8: 508–510.
Haahr, T., Jensen, J., Thomsen, L., Duus, L., Rygaard, K., & Humaidan, P. (2016). Abnormal vaginal microbiota may be associated with poor reproductive outcomes: A prospective study in IVF patients. Human Reproduction, 31(4), 795-803.
Hamad, T. A., Ahmed, A. T., Sadeq, S. M., & Ismaeel, B. (2018). Microbial colonization of human follicular fluid and adverse outcome on in vitro fertilization cases in Kamal al-Samarrai’s Hospital for fertility and In vitro fertilization treatment in Baghdad, Iraq. Dental and Medical Sciences 17(5), 80-87.
Hoffman, B., Schorge, K., Bradshaw, L., & Halvorson, J. (2016). Schaffer, & M.M Corton, Williams Gynecology. In: McGraw Hill Professional.
Ibadin, K. O., & Osemwenkha, A. P. (2014). Microbiological study of infertile women programmed for in-vitro fertilization-embryo transfer in a tertiary health institution In Nigeria.
Jabuk, S. I. A. (2014). Prevalence of aerobic bacterial vaginosis among Intrauterine Contraceptive Device users women in Hilla city. Journal of the University of Babylon, 22(9), 2431-2424.
Keltz MD, Gera PS, Moustakis M. Chlamydia serology screening in infertility patients. Fertil Steril 2006; 85:752–4.
Khine, Y. M., Taniguchi, F., & Harada, T. (2016). Clinical management of endometriosis-associated infertility. Reproductive medicine and biology, 15(4), 217-225.
Kim, S., Won, K., Lee, J., & Suh, C. (2018). The incidence of positive bacterial colonization in human follicular fluids and its impact on clinical in vitro fertilization outcomes. Fertility and Sterility, 110(4), e149.
Kumar, N., & Singh, A. K. (2015). Trends of male factor infertility, an important cause of infertility: A review of the literature. Journal of human reproductive sciences, 8(4), 191.
Lamont, R. F., Sobel, J. D., Akins, R. A., Hassan, S. S., Chaiworapongsa, T., Kusanovic, J. P., & Romero, R. (2011). The vaginal microbiome: new information about genital tract flora using molecular-based techniques. BJOG: An International Journal of Obstetrics & Gynaecology, 118(5), 533-549.
Lessing JB, Kletter Y, Amster R (1991). Success rates in vitro fertilization treatment and its correlation with high titer antibodies for Chlamydia trachomatis. Isr J Med Sci 27:546–9.
Licciardi F, Grifo JA, Rosenwaks Z, Witkin SS. (1992) Relation between antibodies to Chlamydia trachomatis and spontaneous abortion following in vitro fertilization. J Assist Reprod Genet 9: 207–10.
Lyons, R., Saridogan, E., & Djahanbakhch, O. (2005). The effect of ovarian follicular fluid and peritoneal fluid on Fallopian tube ciliary beat frequency. Human Reproduction, 21(1), 52-56.
Mascarenhas, M. N., Flaxman, S. R., Boerma, T., Vanderpoel, S., & Stevens, G. A. (2012). National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS medicine, 9(12), e1001356.
McClure EM and Goldenberg RL (2009) Infection and stillbirth. Semin Fetal Neonatal Med 14: 182–189.
Metwally, M., Li, T., & Ledger, W. (2007a). The impact of obesity on female reproductive function. Obesity Reviews, 8(6), 515-523.
Moller BR, Taylor-Robinson D, Furr PM, Toft Band Allen J (1985) Serological evidence that chlamydiae and mycoplasmas are involved in infertility of women. J Reprod Fertil 73: 237–240.
Monteiro C, Marques PI, Cavadas B, Damiao I, Almeida V, Barros N, et al. (2018) Characterization of microbiota in male infertility cases uncovers differences in seminal hyperviscosity and oligoasthenoterato zoospermia possibly correlated with increased prevalence of infectious bacteria. Am J Reprod Immunol. 79(6):e12838.
Moore, D., Soules, M., Klein, N., Fujimoto, V., Agnew, K., & Eschenbach, D. (2000). Bacteria in the transfer catheter tip influence the live-birth rate after in vitro fertilization. Fertil Steril, 74(6), 1118-1124. DOI:10.1016/s0015-0282(00)01624-1.
Morris, M., Nicoll, A., Simms, I., Wilson, J., & Catchpole, M. (2001). Bacterial vaginosis: a public health review. BJOG: An International Journal of Obstetrics & Gynaecology, 108(5), 439-450.
Nelson, D. B., & Macones, G. (2002). Bacterial vaginosis in pregnancy: current findings and future directions. Epidemiologic Reviews, 24(2), 102-108.
Neuer A, Lam KN, Tiller FW, et al. (1997) Humoral immune response to membrane components of Chlamydia trachomatis and expression of human 60 kDa heat shock protein in follicular fluid of in-vitro fertilization patients. Hum Reprod 12:925–9.
Okiki, A., Nosazena E, A., & Oyinloye, J. M. (2015). Evaluation of Microorganisms Associated with Vaginal Infections in Owo, Nigeria (Vol. 6).
Pellati, D., Mylonakis, I., Bertoloni, G., Fiore, C., Andrisani, A., Ambrosini, G., & Armanini, D. (2008). Genital tract infections and infertility. European Journal of Obstetrics & Gynecology and Reproductive Biology, 140 (1), 3-11.
Pauli, S. A., & Kallen, C. B. (2011). Endocrine Disorders and Infertility. Infertility, 90-101.
Pelzer, E. S. (2011). Microbial colonization of human follicular fluid and adverse in vitro fertilization outcomes. The Queensland University of Technology.
Pelzer, E. S., Allan, J. A., Cunningham, K., Mengersen, K., Allan, J. M., Launchbury, T., Knox, C. L. (2011). Microbial colonization of follicular fluid: alterations in cytokine expression and adverse assisted reproduction technology outcomes. Human Reproduction, 26(7), 1799-1812.
Pelzer, E. S., & Allan, J. A. (2012). The isolation and identification of microorganisms in the reproductive environment: the potential impact on the IVF culture system and IVF outcomes. Journal of Clinical Embryology, 15(3), 44-53.
Pelzer, E. S., Allan, J. A., Waterhouse, M. A., Ross, T., Beagley, K. W., & Knox, C. L. (2013a). Microorganisms within human follicular fluid: effects on IVF. PloS one, 8 (3), e59062.
Pomeroy, K. (2010). Contamination of human IVF cultures by microorganisms: a review. J Clin Embryo, 13, 11-30.
Pelzer ES, Allan JA, Cunningham K, Mengersen K, Allan JM, et al. (2011)
Microbial colonization of follicular fluid: alterations in cytokine expression and adverse assisted reproduction technology outcomes. Hum Reprod 26: 1799–1812.
Pelzer ES, Allan JA, Waterhouse MA, Ross T, Beagley KW, et al. (2013) Microorganisms within Human Follicular Fluid: Effects on IVF. PLoS ONE 8(3): e59062.
Pelzer, E. S., Harris, J. E., Allan, J. A., Waterhouse, M. A., Ross, T., Beagley, K. W., & Knox, C. L. (2013b). TUNEL analysis of DNA fragmentation in mouse unfertilized oocytes: the effect of microorganisms within human follicular fluid collected during IVF cycles. Journal of reproductive immunology, 99(1-2), 69-79.
Rampersaud, R., Randis, T. M., & Ratner, A. J. (2012). Microbiota of the upper and lower genital tract. Paper presented at the Seminars in Fetal and Neonatal Medicine.
Revelli, A., Delle Piane, L., Casano, S., Molinari, E., Massobrio, M., & Rinaudo, P. (2009). Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reproductive biology and endocrinology, 7(1), 40.
Schattman, G. L., Esteves, S., & Agarwal, A. (2016). Unexplained infertility: Springer.
Swain, J. (2012). Is there an optimal pH for culture media used in clinical IVF? Human reproduction update, 18(3), 333-339.
Schiappacasse, G. (2014). Infection of the female upper genital tract: Pictorial review by computed tomography according to clinical classification. Revista Chilena de Radiología, 20(1), 31-37.
Speroff, L., & Fritz, M. A. (2005). Clinical gynecologic endocrinology and infertility: Lippincott Williams &Wilkins.
Spence MR, Blanco LJ, Patel J and Brockman MT (1982) A comparative evaluation of vaginal, cervical, and peritoneal flora in normal, healthy women: a preliminary report. Sex Transm Dis 9: 37–40.
Soong, D., & Einarson, A. (2009). Vaginal yeast infections during pregnancy. Canadian family physician, 55(3), 255-256.
Spandorfer, S. D., Neuer, A., Giraldo, P. C., Rosenwaks, Z., & Witkin, S. S. (2001). Relationship of abnormal vaginal flora, proinflammatory cytokines, and idiopathic infertility in women undergoing IVF. The Journal of reproductive medicine, 46(9), 806-810.
Stoll, B. J., Hansen, N. I., Sánchez, P. J., Faix, R. G., Poindexter, B. B., Van Meurs, K. P., Hale, E. C. (2011). Early-onset neonatal sepsis: the burden of group B Streptococcus and E. coli disease continues. Pediatrics, 127(5), 817-826.
Štšepetova J., Baranova J., Simm J., Parm Ü, Rööp T, Sokmann S., Korrovits P, Jaagura M, Rosenstein K, Salumets A, and Mändar R, (2020) The complex microbiome from native semen to embryo culture environment in human in vitro fertilization procedure, Reproductive Biology and Endocrinology, 18:3, 1-13.
Torode HW, Wheeler PA, Saunders DM, et al. The role of chlamydial antibodies in an in vitro fertilization program. Fertil Steril 1987; 48:987–90.
Unuane, D., Tournaye, H., Velkeniers, B., & Poppe, K. (2011). Endocrine disorders & female infertility. Best Practice & Research Clinical Endocrinology & Metabolism, 25(6), 861-873.
Velde, E. R. t., & Pearson, P. L. (2002). The variability of female reproductive aging. Human reproduction update, 8(2), 141-154.
Viniker DA (1999) Hypothesis on the role of sub-clinical bacteria of the endometrium (bacteria endometrial) in gynecological and obstetric enigmas.
Hum Reprod Update 5: 373–385.
Wilson, J. D., Ralph, S. G., & Rutherford, A. J. (2002). Rates of bacterial vaginosis in women undergoing in vitro fertilization for different types of infertility. BJOG: An International Journal of Obstetrics & Gynaecology, 109(6), 714-717.
Witkin, S. S., Linhares, I. M., & Giraldo, P. (2007). Bacterial flora of the female genital tract: function and immune regulation. Best Practice & Research Clinical Obstetrics & Gynaecology, 21(3), 347-354.
World Bank, W. b. (2017). Fertility rate, total (births per woman). In: United Nations Population Division.
World health organization, W. (2016). Multiple definitions of infertility. Geneva: World Health Organization.
Zegers-Hochschild, F., Adamson, G. D., de Mouzon, J., Ishihara, O., Mansour, R., Nygren, K.,Van der Poel, S. (2009). The international committee for monitoring assisted reproductive technology (ICMART) and the world health organization (WHO) revised glossary on ART terminology, 2009. Human Reproduction, 24(11), 2683-2687.