Department of Biochemistry, Faculty of Sciences, King Abdulaziz University, Jeddah, Saudi Arabia
Corresponding Author email: uomer@kau.edu.sa
Article Publishing History
Received: 20/12/2020
Accepted After Revision: 20/03/2021
Stress can initiate by many factors incorporated in the daily life such as long-term health issues, immune system suppression and obesity. Free radicals as well as endogenous antioxidants are in balance as part of body’s natural functioning. Oxidative stress occurs when endogenous defence system not capable to maintain the balance, therefore natural antioxidant was in-need to help the endogenous antioxidant in scavenging the excess of free radicals. Punicalagin is a natural antioxidant compound that extracted from pomegranate husk. Punicalagin affect the activities of endogenous antioxidant enzymes which was investigated by measuring the level of catalase, superoxide dismutase (SOD), glutathione peroxidase and reductase of Caco-2 cells under stress conditions. Caco-2 cells were exposed to 3 mMtert-butyl hydroperoxide (T-BOOH) for 2 hours to initiate oxidative stress when pretreated with 5 and 10 µM punicalagin for 24 hours. Both doses (5 µM and 10 µM) of punicalagin significantly elevated the level of catalase (15.05 U/ml; p <0.05 and 20.95 U/ml; p < 0.001, respectively) compared to cells treated with only T-BOOH (11.79). On the other hand, punicalagin had no effect SOD, glutathione peroxidase and glutathione reductase. In conclusion, under stress conditions punicalagin significantly enhanced endogenous antioxidant enzymes of Caco-2 cells.
Punicalagin, Oxidative Stress, Glutathione, Superoxide Dismutase, Reactive Oxygen Species
Omar U.M . Effect of Punicalagin on Antioxidant Enzymes in Colon Cancer Cells Stimulated with T-BOOH. Biosc.Biotech.Res.Comm. 2021;14(1).
Omar U.M . Effect of Punicalagin on Antioxidant Enzymes in Colon Cancer Cells Stimulated with T-BOOH. Biosc.Biotech.Res.Comm. 2021;14(1). Available from: <a href=”https://bit.ly/3cnI3H3“>https://bit.ly/3cnI3H3</a>
Copyright © Omar This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Free radicals in living cells are naturally produced as a result of cell metabolism and detoxification process (Halliwel and Gutteridge., 2015). Oxidative stress occurs as consequences of imbalance between antioxidant capacity in biological system and the excess of free radicals initiated in the system. Excessive radical species production causes cell injury by reacting with lipid membrane, cell proteins and DNA (Ahmad et al., 2019). Attacking of biological molecules with excessive radicals leading to pathogenesis and many diseases that include cancers and aging disease ((Valko et al., 2004, Liguori et al., 2018).
Cellular antioxidant system, including antioxidant enzymes are capable to diminish the harmful effect of free radicals and hence protecting cells from being damage (Ray et al., 2012). Some of the important endogenous cellular antioxidant enzymes are catalase, superoxide dismutase (SOD), glutathione peroxidase and glutathione reductase. Superoxide dismutase is one of the endogenous antioxidant enzymes that protecting cells from oxidative stress by catalysing the decomposition of superoxide radicals (O2–) to oxygen (O2) and (H2O2).
Both of catalase and glutathione peroxidase are protecting cells from oxidative damage by catalysing the decomposition of harmful hydrogen peroxide (H2O2) to safe products as water and oxygen. Moreover, Glutathione reductase is one of the important antioxidant defence enzymes at cellular levelas it is responsible for maintaining the reducing environment of the cell by optimizing the concentration level of reduced glutathione (GSH) in cellular system (Aguilar et al., 2016).Under certain conditions, excess reactive species formation overwhelms the oxidant/antioxidant balance despite the presence of cellular antioxidant defense system (Georgieva et al., 2017).
Therefore, scientific researches have been focused heavily on finding natural antioxidants that can protect biological system from free radical-mediated oxidative stress and enhance the action of endogenous antioxidant system to overcome the side effect of excessive free radicals (Jamshidi-Kia et al., 2020). Some plant polyphenol has been suggested to have antioxidant effect by upregulating intracellular defence systems or/and intercepting free radical formation (Losada-Barreiro et al., 2017). Polyphenols have also shown ability to diminish the decrease of GSH levels that induced by T-BOOH (Lima et al., 2006, Cianfruglia et al., 2020).
One of the plant polyphenol naturaly rich with antioxidant compounds is pomegranatefruit.The high antioxidant properties of pomegranate are due to the presence of punicalagin isomers which form when both gallic and ellagic acid link to glucose molecules, and hydrolysable tannins ( Gil et al., 2000). Punicalagin is a yellowwater-soluble compound that is mainly present in the pomegranate husk. Omar et al., (2016) stated that punicalagin from pomegranate has regulating effect on total glutathione in Caco-2 cell stressed by T-BOOH. The current study has been carried out on human intestinal Caco-2 cell line which displays morphological and physiological characteristics that are similar to intestinal epithelial cells when differentiated (Meunier et al., 1995).
T-BOOH was also used to generate oxidative stress that results in cell injury (Lashpina et al., 2005). Once T-BOOH gets penetrated in the cell membrane and is easily decomposed to produce alkoxyl and peroxyl radicals which subsequently continue to generate reactive species (Kim et al., 2013). Prevention or delaying the adverse effect of excess ROS on cells is important to protect cellular membrane and its content (Cianfruglia et al., 2020). Therefore, the aim of this study was to investigate the effect of punicalaginas a natural plant derived antioxidant on the activity of catalase, SOD, glutathione peroxidase and glutathione reductase using stressed Caco-2 cells as an epithelial model.
MATERIAL AND METHODS
Cell culture and treatment:Human colon epithelial cells (Caco-2) was obtained from European Collection of Cell Cultures (ECACC, UK). Cells were stressed for 2 hours with 3 mM tert-butyl hydroperoxide (T-BOOH) and treated with 5 and 10 µM Punicalagin according to (Omar et al., 2016).
Preparation of cell lysate: Cells were collected in 15 ml centrifuge tubes and centrifuged for 3 minutes at 150 x g. The supernatant was decanted and cell pellets were lysed using 300 μl lysis buffer (50 mM Tris HCl, 1% Nonidet P40 (NP-40), 150 mM NaCl, 0.2% SDS solution, 1 μg/ml Aprotinin, 20 μM PMSF, 1 μg/ml Leupeptin, and 1mM Na3VO4). Cells were then kept in ice for 20 minutes and stored at – 80°C for further use in the following experiments.
Catalase: Catalase level was measured in Caco-2 cell lysate using the OxiSelectTM Catalase activity Assay Colorimetric Kit (catalog number STA-341, Cell Biolabs, INC., Cambridge, UK) according to the manufacturer’s instructions.
Superoxide Dismutase assay: Superoxide dismutase (SOD) activity was measured using the SOD Activity Assay Colorimetric Kit (catalog number ab65354, Abcam®, Cambridge, UK) according to the manufacturer’s instructions.
Glutathione Peroxidase: Glutathione peroxidase was measured quantitatively using Glutathione PeroxidaseAssay Kit (catalog number 703102, Cayman chemical, Michigan, USA) according to the manufacturer’s instructions.
Glutathione Reductase: Glutathione reductase was measured quantitatively using OxiSelectTM Glutathione Reductase Assay Kit (catalog number STA-812, Cell Biolabs, INC., Cambridge, UK) according to the manufacturer’s instructions.
Statistical Analysis: Results were analyzed using GraphPad Prism software version 6.0. Data are presented as the mean (±SD). ANOVA was performed in Graphpad Prism version 6.0, followed by Bonferroni’s multiple comparisons test versus treated cells with T-BOOH alone * = p < 0.05, ** = p < 0.01, *** = p < 0.001.
RESULTS AND DISCUSSION
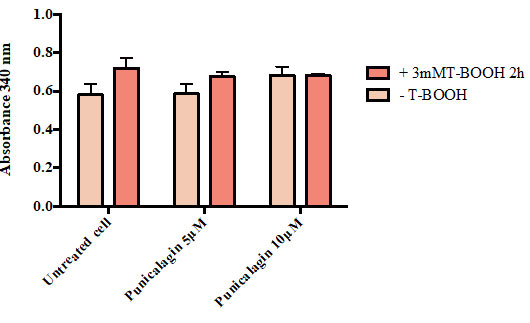
Figure 1 shows that there was a significant depletion of catalase level in cells treated with T-BOOH (11.29 U/ml) as compared with untreated cells (22.42 U/ml; p <0.001). Catalase level was significantly elevated in stressed cells pretreated with 5 µM punicalagin (15.05 U/ml) in comparison to cells treated with T-BOOH alone (p <0.05). Cells treated with 10 µM punicalagin prior to oxidative induction exhibited significantly higher catalase level (20.95 U/ml) when compared with cells treated with t-BHP (p < 0.001).
Figure 1: Effect of Punicalagin onlevel of catalase in Caco-2 cells. Data correspond to the means ± SD of three independent experiments. ANOVA was performed in Graphpad Prism version 6.0. followed by Bonferroni’s multiple comparisons test. * = p < 0.05, ** = p < 0.01, *** = p < 0.001 versus treated cells with T-BOOH alone
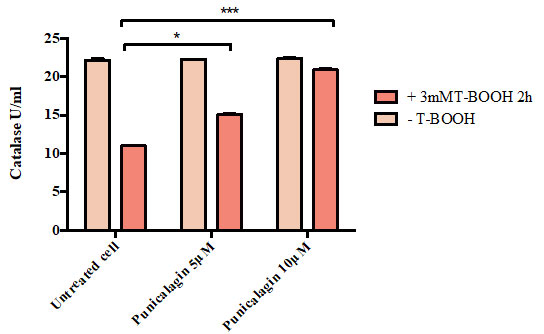
Figure 2 illustrates that there was no significant difference in the percentage of SOD activity between untreated cells (53.19 %),cells treated with T-BOOH alone (49.92 %), cells treated with 5 uMpunicalagin prior to oxidative induction (47.22 %) and cells pretreated with 10 µM punicalagin prior to oxidative conduction (45.86 %).
Figure 2: Effect of Punicalagin on the percentage of superoxide dismutase (SOD) activity in Caco-2 cells. Data correspond to the means ± SD of three independent experiments. ANOVA was performed in Graphpad Prism version 6.0. followed by Bonferroni’s multiple comparisons test.
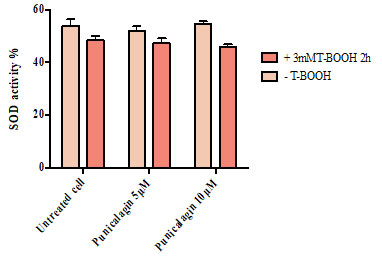
Figure 3 shows that there was no significant difference in the concentration of glutathione peroxidase between untreated cells (0.68), cells treated with T-BOOH alone (0.74), cells treated with 5 µM punicalagin prior to their exposure to T-BOOH (0.71) and cells pretreated with 10 µM punicalagin prior to oxidative conduction (0.70).
Figure 3: Effect of Punicalagin on the amount of glutathione peroxidase in Caco-2 cells. Data correspond to the means ± SD of three independent experiments. ANOVA was performed in Graphpad Prism version 6.0. followed by Bonferroni’s multiple comparisons test.
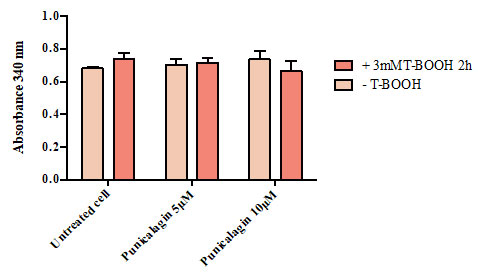
Figure 4 shows that there was no significant difference in the concentration of glutathione reductase between untreated cells (0.62), cells treated with T-BOOH alone (0.72), cells reated with 5 µM punicalagin prior to their exposure to T-BOOH (0.68) andcells pretreated with 10 µM punicalagin prior to oxidative conduction (0.68).
Figure 4: Effect of Punicalagin on the amount of glutathione reductase in Caco-2 cells. Data correspond to the means ± SD of three independent experiments. ANOVA was performed in Graphpad Prism version 6.0. followed by Bonferroni’s multiple comparisons test.
Natural antioxidants play a crucial role in helping the endogenous antioxidants in scavenging the excess of free radicals (Breinholt, 1999; Duthie et al., 2000; Ro ̈hrdanz et al., 2002; Alia et al., 2006, Yusof et al., 2005). Scientific research considering the effect of punicalagin on the activity of antioxidant enzymes in stressed culture cells is scarce. Therefore, this study is considered first to none in measuring the effect of punicalagin in very small doses (5 and 10 µM) on the activity of four main endogenous antioxidant enzymes.
The activity of Punicalagin as an antioxidant was previously highlighted by Omar et al., (2016). Punicalagin at low concentrations (5 and 10 µM) succeeded to protect Caco-2 cells against cell death which exposure to stress using 3 µM T-BOOH. The protection offered by this phenolic compound highlighted by scavenging cellular ROS, preventing lipid oxidation and prohibiting GSH depletion (Omar et al., 2016).
These findings led to further examination of other cellular antioxidant enzymes as catalase, SOD, glutathione peroxidase and glutathione reductase in Caco-2 cells using the same conditions. In a healthy system, cellular antioxidative enzymes can protect cells either their membranes or their cellular content by scavenging free radicals. However, under certain conditions excess radical formation overwhelms the antioxidant/prooxidant balance and then weakens the antioxidant defence system (Cimen, 2008).Catalase is one of the main endogenous antioxidant enzymes that responsible for the dismutation of hydrogen peroxide to safe products such as water and oxygen (Aguilar et al., 2016).
In this study, cells treated with either 5 or 10 µM punicalagin prior to oxidative induction, significantly elevated catalase level to (15.05 U/ml, p<0.05 and 20.98 U/ml, p< 0.001; respectively) as compared with cells treated with T-BOOH alone. This result illustrated the effect of punicalagin on enhancing the activity of Catalase under oxidative stress. In contrast, there was no significant difference in SOD activity between pre-treated stressed cells and untreated stressed cells.Catalase and SOD results might be explained by the effect of stressed condition on the activity of each enzyme. Researcher suggested that 5×10-2 mM T-BOOH organic peroxides can activate catalase enzyme while inactivate SOD, although both enzymes undergo the same stressed condition (Pigeolet et al., 1990). Many studies confirmed that some natural antioxidants either derived from plant or animal source able to enhance the endogenous defence system by increase the enzyme activity level or its gene expression.
Recently Talaei et al., (2020) have suggested that quercetin either alone or with exercise significantly elevated the level of catalase in the heart tissue of rats stressed with 15 mg/kg Azoxymethane in comparison with saline group (p< 0.0001). Another study found significant increase in the expression of catalase and SOD genes was found in HepG2 cells treated with peptide isolated camel milk (Homayouni-Tabrizi et al., 2017). Maalej et al. (2017) suggested that ethanolic olive fruit extract and its phenolic compound exhibited hepatic and renal protection against the toxicity induced by a synthetic pyrethroid (deltamethrin) on Wistar rats. This protection was highlighted by improving the activities of catalase and SOD. Another study revealed that ginger extract (Zingiber officinale) has a possible chemoprotective effect by enhancing the activity of catalase, SOD and glutathione peroxidase in HepG2 cell line (Seyidoglu and Aydin 2020).
Glutathione peroxidase is another enzyme regulating the presence of free radicals in human body. This enzyme may exist in tow forms: selenium-dependent and selenium-independent Glutathione peroxidase reduce hydrogen peroxides or any organic peroxides to water (Maiorino et al., 1995), in the presence of reduced glutathione (GSH) which is converted into oxidised glutathione (GSSG). It is well known that glutathione peroxidase that there is a competition between this enzyme and catalase for scavenging the hydrogen peroxide (Aguilar et al., 2016).
In the current study, although the significant effect of punicalagin on catalase activity, there is no significant difference between the glutathione peroxidase level between pre-treated stressed cells and untreated one. This outcome might be affected by the dose of T-BOOH that used in the current study and/ or incubation time that used for stress. Pigeolet et al., (990) stated that 5×10-2 mM T-BOOH inactivated glutathione peroxidase by 50% when incubated for 11 minutes at 37o C.
Glutathione reductase enzyme is responsible for converting GSSG to GSH which is an important molecule in resisting oxidation stress (Duthie et al., 2000). Under oxidative stress, elevation of peroxideslevel leads to a shift in thiol redox status which defined by a severe reduction in the reduced form of glutathione (GSH) and rise in the level of the oxidised form (GSSG).
Although Omar et al., 2016 stated that punicalagin as a radical scavenger could maintain the GSH level against oxidative stress occur due to T-BOOH in caco-2 cells, in the current study punicalagin did not exhibit any effect on glutathione reductase level and there was no significant difference between untreated stressed cells and treated one. This result can also be linked to the dose of T-BOOH and its incubation time that used while stressing caco-2 cell.
Organic peroxide might inactivate the enzyme activity as stated previously by Pigeolet et al. (1990). Endogenous antioxidants operate together under one system that complements its main constituents to maintain the redox balance in the body. Regulation the level of reactive oxygen species is the target of researcher but not diminished them completely as they may lead to degenerative diseases (Lobo et al., 2010). According to the current research, it is highly likely that punicalagin can enhance the endogenous antioxidant enzyme system by activating catalase enzyme which able to scavenge the excess hydrogen peroxide and hence protect cellular system from damage.
CONCLUSION
Punicalagin enhanced the activity of endogenous antioxidative enzymes causing protective effect to the oxidatively stressed Caco-2 cells. The protective effect of punicalagin was demonstrated by the significant elevation of Catalase level when the stressed cells either treated with 5 µM punicalagin (15.05 U/ml; p <0.05) or with 10 µM punicalagin (20.95 U/ml; p < 0.001) compared to cells treated with T-BOOH alone. Punicalagin has no effect on SOD, glutathione peroxidase and glutathione reductase levels. Highlighting the effect of Punicalagin on the activity of endogenous antioxidant enzymes in addition to previous findings on its antioxidant activity that conducted by the same researcher (Omar et al., 2016) might elucidate the actual mechanism of its protective action. These novel findings may offer punicalagin potential applications in the nutraceutical market.
REFERENCES
Aguilar, T.A.F., Navarro, B.C.H. and Perez, J.A.M. (2016). Endogenous antioxidants: a review of their role in oxidative stress. A master regulator of oxidative stress-the transcription factor nrf2. Jose Antonio Morales-Gonzalez, Angel Morales-Gonzalez and Eduardo Osiris Madrigal-Santillan (eds). IntechOpen Press.
Ahmad, R., Hussain, A. and Ahsan, H. (2019). Peroxynitrite: cellular pathology and implications in autoimmunity. Journal of Immunoassay and Immunochemistry, 40(2), pp.123-138.
Alía, M., Ramos, S., Mateos, R., Granado-Serrano, A.B., Bravo, L. and Goya, L. (2006). Quercetin protects human hepatoma HepG2 against oxidative stress induced by tert-butyl hydroperoxide. Toxicology and applied pharmacology, 212(2), pp.110-118.
Breinholt, V. (1999). Desirable versus harmful levels of intake of flavonoids and phenolic acids. In Natural antioxidants and anticarcinogens in nutrition, health and disease (pp. 93-105). Woodhead Publishing.
Cianfruglia, L., Morresi, C., Bacchetti, T., Armeni, T., and Ferretti, G. (2020). Protection of Polyphenols against Glyco-Oxidative Stress: Involvement of Glyoxalase Pathway. Antioxidants, 9, 1006 .
Çimen, M.B. (2008). Free radical metabolism in human erythrocytes. Clinica chimica acta, 390(1-2), pp.1-11.
Duthie, G.G., Duthie, S.J. and Kyle, J.A. (2000). Plant polyphenols in cancer and heart disease: implications as nutritional antioxidants. Nutrition research reviews, 13(1), pp.79-106.
Georgieva, E., Ivanova, D., Zhelev, Z., Bakalova, R., Gulubova, M. and Aoki, I. (2017). Mitochondrial dysfunction and redox imbalance as a diagnostic marker of free radical diseases. Anticancer research, 37(10), pp.5373-5381.
Gil, M.I., Tomás-Barberán, F.A., Hess-Pierce, B., Holcroft, D.M. and Kader, A.A. (2000). Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. Journal of Agricultural and Food chemistry, 48(10), pp.4581-4589.
Homayouni-Tabrizi, M., Asoodeh, A. and Soltani, M. (2017). Cytotoxic and antioxidant capacity of camel milk peptides: Effects of isolated peptide on superoxide dismutase and catalase gene expression. journal of food and drug analysis, 25(3), pp.567-575.
Jamshidi-Kia, F., Wibowo, J.P., Elachouri, M., Masumi, R., Salehifard-Jouneghani, A., Abolhasanzadeh, Z. and Lorigooini, Z. (2020). Battle between plants as antioxidants with free radicals in human body. Journal of Herbmed Pharmacology, 9(3), pp.191-199.
Kim, Y., Choi, Y., Ham, H., Jeong, H.S. and Lee, J. (2013). Protective effects of oligomeric and polymeric procyanidin fractions from defatted grape seeds on tert-butyl hydroperoxide-induced oxidative damage in HepG2 cells. Food chemistry, 137(1-4), pp.136-141.
Lapshina, E.A., Zavodnik, I.B., Labieniec, M., Rękawiecka, K. and Bryszewska, M. (2005). Cytotoxic and genotoxic effects of tert-butyl hydroperoxide on Chinese hamster B14 cells. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 583(2), pp.189-197.
Liguori, I., Russo, G., Curcio, F. et al. ( 2018). Oxidative stress, aging, and diseases. Clinical interventions in aging, 13, p.757.
Lima, C.F., Fernandes-Ferreira, M. and Pereira-Wilson, C. (2006). Phenolic compounds protect HepG2 cells from oxidative damage: relevance of glutathione levels. Life sciences, 79(21), pp.2056-2068.
Lobo, V., Patil, A., Phatak, A. and Chandra, N., (2010). Free radicals, antioxidants and functional foods: Impact on human health. Pharmacognosy reviews, 4(8), p.118.
Losada-Barreiro, S. and Bravo-Diaz, C., (2017). Free radicals and polyphenols: The redox chemistry of neurodegenerative diseases. European Journal of Medicinal Chemistry, 133, pp.379-402.
Maalej, A., Mahmoudi, A., Bouallagui, Z., Fki, I., Marrekchi, R. and Sayadi, S. (2017). Olive phenolic compounds attenuate deltamethrin-induced liver and kidney toxicity through regulating oxidative stress, inflammation and apoptosis. Food and Chemical Toxicology, 106, pp.455-465.
Maiorino, F.M., Brigelius-Flohé, R., Aumann, K.D., Roveri, A., Schomburg, D. and Flohé, L. (1995). Diversity of glutathione peroxidases. In Methods in enzymology, Vol. 252, pp. 38-53). Academic Press.
Meunier, V., Bourrie, M., Berger, Y. and Fabre, G. (1995). The human intestinal epithelial cell line Caco-2; pharmacological and pharmacokinetic applications. Cell biology and toxicology, 11(3-4), pp.187-194.
Omar, U., Aloqbi, A., Yousr, M. and Yousr, N. (2016). Protective effects of punicalagin on Caco-2 intestine cell line under oxidative stress caused by tert-butyl hydroperoxide. J. Pharmacol. Nutr. Sci, 5, pp.249-256.
Pigeolet, E., Corbisier, P., Houbion, A., Lambert, D., Michiels, C., Raes, M., Zachary, M.D. and Remacle, J. (1990). Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mechanisms of ageing and development, 51(3), pp.283-297.
Ray, P.D., Huang, B.W. and Tsuji, Y. (2012). Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cellular signalling, 24(5), pp.981-990.
Röhrdanz, E., Ohler, S., Tran-Thi, Q.H. and Kahl, R. (2002). The phytoestrogen daidzein affects the antioxidant enzyme system of rat hepatoma H4IIE cells. The Journal of nutrition, 132(3), pp.370-375.
Seyidoglu, N. and Aydin, C. (2020). Stress, Natural Antioxidants and Future Perspectives, The Health Benefits of Foods – Current Knowledge and Further Development, Liana Claudia Salanță, IntechOpen Press.
Talaei, B., Panji, M., Robati, F.N. and Tezerji, S. (2020). Effect of quercetin and intermittent and continuous exercise on catalase, superoxide dismutase, and malondialdehyde in the heart of rats with colon cancer. Basic and Clinical Cancer Research, 12(1), pp.34-41.
Valko, M., Izakovic, M., Mazur, M., Rhodes, C.J. and Telser, J. (2004). Role of oxygen radicals in DNA damage and cancer incidence. Molecular and cellular biochemistry, 266 (1-2), pp.37-56.
Yusof, Y.A.M. and Abdul-Aziz, A. (2005). Effects of Zingiber officinale on superoxide dismutase, glutathione peroxidase, catalase, glutathione and malondialdehyde content in HepG2 cell line. Malaysian Journal of Biochemistry and Molecular Biology, 11, pp. 36-41.


