1Department of Biology, Faculity of Science, King Abdulaziz Univirsity, Saudi Arabia,
2Department of Biology, Faculity of Science, Jeddah Univirsity, Saudi Arabia
3Botany and Microbiology Department, Faculty of Science, Kafrelsheikh University, Egypt
Corresponding Author email: magdammali@hotmail.com
Article Publishing History
Received: 12/12/2020
Accepted After Revision: 27/03/2021
The biosurfactant produced by Pseudomonas balearica isolated from oil-contaminated sea water was studied in the present research. A range of different growth conditions, such as temperature, pH, incubation period, inoculum size and different carbon/nitrogen sources and different C/N ratios were investigated to determine the optimum conditions for maximum production of a biosurfactant by the selected bacterial strain using a mineral salt medium. The best carbon andnitrogen sources were olive oil and urea, giving biosurfactant yields of 6.23±0.06 and 6.33±0.10 mg/ml, respectively.
The maximum rhamnolipid production was 6.40±0.14 mg/ml at C/N (olive oil/ urea) of 30. The highest biosurfactant yield was at pH 7 (6.37±0.06 mg/ml), with inoculum size 2% (6.29±0.16 mg/ml), and incubation temperature 30ºC (6.18±0.14 mg/ml) and after a 312 hrs incubation period (6.30±0.09). The previous investigated nutritional and environmental factors showed the highest emulsification activity. The produced rhamnolipid biosurfactant reduced the surface tension of water from 72 to 34 mN/m. In conclusion, the findings herein demonstrated that the rhamnolipid biosurfactant production by P. balearica can be used during oil hydrolysis.
Biosurfactant, Pseudomonas balearica, Contamination, Degradation, Oil Hydrolysis
Motwali E. A, Aly M. M, Qari H. A, Amasha R. H, Zabermawi N. M . Effect of Growth Conditions on Biosurfactant Production by Pseudomonas Balearica Isolated from Oil Contaminated Sea Waters from Jeddah Saudi Arabia. Biosc.Biotech.Res.Comm. 2021;14(1).
Motwali E. A, Aly M. M, Qari H. A, Amasha R. H, Zabermawi N. M. Effect of Growth Conditions on Biosurfactant Production by Pseudomonas Balearica Isolated from Oil Contaminated Sea Waters from Jeddah Saudi Arabia. Biosc.Biotech.Res.Comm. 2021;14(1). Available from: <a href=”https://bit.ly/3ev3WGT“>https://bit.ly/3ev3WGT</a>
Copyright © Motwali et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Biosurfactants are important materials produced by microorganisms and are used in various industries. The production of biosurfactants not only depends on the producer’s strain but also on the culture conditions. Additionally, many parameters affect not only the amount of biosurfactant but also the type of product produced (Salihu et al., 2009). These parameters include environmental (pH, temperature, and aeration), and nutritional factors (carbon source and nitrogen source). It is important to know the suitable nutrients and cultural conditions required to achieve higher productivity (Reddy et al., 2011, El-sersy, 2012). First of all, the carbon source plays a vital role in the growth and production of biosurfactants by microorganisms.
The substrates required for biosurfactant generations are typically formed of either carbohydrates or hydrocarbons, though they can also be used consecutively. Sarubbo et al. (2007) reported that the maximum yield of sophorolipids was obtained from Candida lipolytica when canola oil and glucose were used as carbon sources at concentrations of 10% for each. The type of carbon substrate used for production has been reported to influence both the quality and quantity of biosurfactants (Abouseoud et al., 2008). Waste cooking oil and coffee wastewater have also been used as a carbon source for biosurfactant production (Yañez-Ocampo et al., 2017, Nejad et al., 2020).
Nitrogen is the second most important supplement required to produce biosurfactants in microorganisms. Low nitrogen levels limit bacterial growth, pushing cell metabolism towards the production of metabolites. In contrast, excessive nitrogen leads to the synthesis of cellular material and limits the build-up of products (Robert et al., 1989, Fakruddin, 2012). It has been reported that nitrogen limitation enhances biosurfactant production (Kim et al., 2006). Different nitrogen compounds have been used to produce biosurfactants including urea, peptone, yeast extract, ammonium sulphate, ammonium nitrate, sodium nitrate, meat extract, and malt extracts. Additionally, environmental parameters such as pH, temperature, and incubation period all influence both microbial growth and biosurfactant production.
In a low pH culture medium, bacteria cannot efficiently synthesise biosurfactants (Saikia et al., 2012). Likewise, different microbial processes are affected by even a small change in temperature. Most biosurfactant producers are reported to perform in a temperature range of 25–30ºC (Desai and Banat, 1997). However, Hmidet et al. (2017) found that the production of biosurfactants called surfactin and fengycin by Bacillus mojavensis A21 was at 20–30oC. As microorganism production rates are themselves a function of time, adjustments to the length of incubation can radically affect the resultant products. The optimum condition for maximum biosurfactant productivity in B. brevis was 10 days (Mouafi et al., 2016).
The growth and biosurfactant formation by B. subtilis and B. tequilensis were increased with the incubation period which was associated with reduction in surface tension (Marajan et al., 2018). The current study estimates the effects of different environmental parameters (pH value, temperature, inoculum size, and incubation period) and also nutritional factors (carbon source, nitrogen source, carbon to nitrogen ratio) on biosurfactant production by the selected bacterial strain, isolated from oil-contaminated water from the southern sea-shores of Jeddah, Saudi Arabia. The emulsification ability and decrease in surface tension were also evaluated.
MATERIAL AND METHODS
Isolation and Screening method for biosurfactant producing bacteria: The mineral salt medium contained 1% model hydrocarbon compounds (diesel oil) as the sole source of carbon was used for bacterial isolation using the method described by Motwali et al., (2020). After four subcultures, samples were serially diluted using sterile saline solution (0.85% NaCl) and spread ontonutritional agar plates (Kumar et al., 2016). After 24 hours’ incubation at 37℃, the chosen isolates were repeatedly purified before preservation in nutrient agar slants.The isolated bacterial strains were then screened for biosurfactant production using an oil displacement test, drop collapse, CTAB assay and surface tension measurements, applying the same procedure as described by Motwali et al. (2020).
Characterisation and Identification of the Selected Isolates of Bacteria: The purified isolated bacterial cells’ morphological shapes were observed with Gram staining under a microscope (oil immersion, 100×). The bacterial isolate was identified at Macrogen, Korea. Genomic DNA of the selected isolate was extracted according to the method described by Asubel et al. (1987). Universal bacterial primers corresponding to Escherichia coli positions 27F (5′ (AGA GTT TGA TCM TGG CTC AG) 3) and 1492R (5′ (TAC GGY TAC CTT GTT ACG ACT T) 3′) were used for PCR amplification of the 16S rRNA gene.
Microorganism, inocula and production medium: The inoculum was prepared by the transferal of1% of the fresh bacterial subculture to the production medium. The production medium was a mineral salt medium that reported before for bacterial isolation containing 1% model hydrocarbon compounds (diesel oil) as the sole carbon and energy source.
Effects of carbon and nitrogen sources and C/N ratio on biosurfactant production: Different carbon sources were tested to determine the one most suitable for maximum biosurfactant production (Table 1). The carbon source was added separately to the mineral salt medium at approximately 1% concentration. Also, the effect of different nitrogen sources was also studied (Table 1) and each source was added to the medium at 0.1%. In addition, different carbon to nitrogen concentrations were investigated (Table1).
The C/N ratio was varied from 10 to 50 (Kiran et al., 2009; Khopade et al., 2012). The effect of different pH of the medium and different incubation temperature was determined. Each flask was inoculated with 1% of the pre-culture of the selected bacterial isolate (5×106 CFU/ml) and incubated at 120 rpm for 7 days. The impact of changing inoculum volume and optimum incubation period on biosurfactant production were studied (Table 1). At the end of the growth period, emulsification index, bacterial dry weight and biosurfactant production were determined.
Table 1. Factors considered for the optimisation of biosurfactant production by the selected bacterial isolate.
| Factor | Range |
| Carbon source | Glucose, olive oil, corn oil, sunflower oil, mustard oil,
sesame oil, lubricant oil, xylene, toluene, and diesel. |
| Nitrogen source | Urea, peptone, yeast extract, NaNO3, KNO3,
and NH4NO3. |
| C/N ratio | 10, 20, 30, 40, 50. |
| pH value | 3, 5, 7, 9, 11. |
| Temperature | 20, 25, 30, 35, 37, 40, 45, 50ºC. |
| Inoculum size | 0.5, 1, 2, 3, 4, 5, 6, 7, 8 %. |
| Incubation period | 96, 168, 240, 312hrs |
Detection of biosurfactant concentration: At the end of the incubation period, the bacterial culture was centrifuged at 4500 rpm for 30 min at 4°C. The filtrate was used to measure biosurfactant (Rhamnolipid) concentration using an orcinol assay as the method described by Pathaka and Nakhate, (2015). A standard curve for 10 mg/ml L-rhamnose (Sigma) was prepared.
Measurement of bacterial dry weight: For bacterial dry weight measurement, 50 ml of each sample was centrifuged at 4500 rpm for 30 min at 4°C and the cell pellet was dried in an oven at 70°C for 24 h or until recording constant weight.
Detection of biosurfactant activity :The emulsification index (EI24) was calculated using the method described by Gagelidze et al., (2016). The EI24 of culture samples was determined by adding 2 ml diesel oil to 2 ml of the cell free broth in a test tube (15×15 mm) and vortexed at high speed for 2 minutes. Then, it was calculated after 24 hours at room temperature using the following equation:
EI24= (height of emulsion formed (cm)/ total height of solution (cm)) × 100
Measurement of the surface tension: The surface tension of the culture supernatant is the most upfront screening method of biosurfactant producing microbes. This measurement was performed using the Du-Nouy-Ring assay (Carrillo, 1996). The surface tension of the bacterial supernatant was then measured at room temperature using a tensiometer (Kruss Force K6).
RESULTS AND DISCUSSION
Screening the selected bacteria strain for biosurfactant production: The selected bacterial strain Emb39 was screened for its biosurfactant production ability. It showed positive activity on both the CTAB assay and drop collapse test. In addition, it was able to spread the oil in an oil spreading test by 3.05 ± 0.4 cm and to reduce surface tension to 42 mN/m
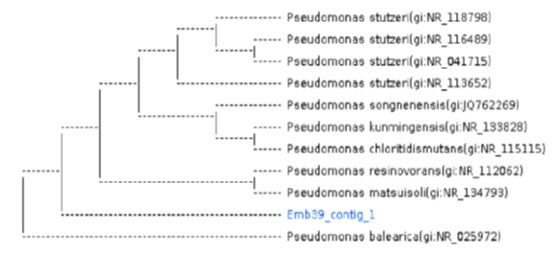
Characterisation and molecular identification of the bacterial strain selected: The selected bacterial strain was characterised as an aerobic gram negative non spore forming bacteria (Figure1). Molecular identification of the selected isolate was performed using the GenBank BLAST tool on the 16S rRNA gene sequences. It was found that the selected bacterial strain was closely related (99%) to P. balearica (Figure 2). The bacteria accession number is NR_025972.1.
Figure 1: A: Bacterial culture on nutrient agar plate, B: Cells of P. balearica under light microscope X1000
Figure 2: The phylogenetic tree of the bacterial strain P. balearica. Emb39.
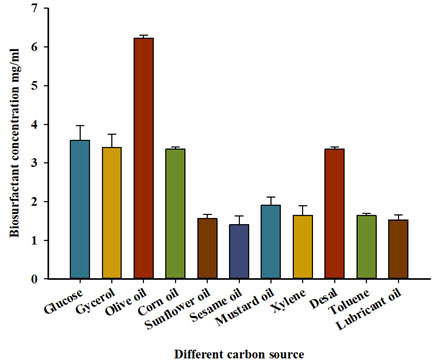
Effect of different carbon sources on biosurfactant production: The bacterial strain P. balearica was selected to determine the effects of different carbon sources on biosurfactant production. Olive oil provides the greatest level of emulsification and greatest quantities of both biosurfactant and dry weight of P. balearica cells (Table 2 and Figure 3).
Table 2. Effect of different carbon sources on EI 24, biosurfactant concentration and bacterial dry weight (DW).
| Carbon source | P. balearica | ||
| EI 24% | Rhamnose con. mg/ml | DW g/ 50ml | |
| Glucose | 25±3 | 3.58±0.4 | 0.21±0.02 |
| Glycerol | 0 | 3.40±0.4 | 0.17±0.03 |
| Olive oil | 64±2 | 6.23±0.06 | 0.21±0.04 |
| Corn oil | 35±2 | 3.35±0.05 | 0.19±0.04 |
| Sunflower oil | 0.0 | 1.56±0.11 | 0.18±0.03 |
| Sesame oil | 0.0 | 1.40±0.23 | 0.20±0.03 |
| Mustard oil | 9.5±2 | 1.90±0.21 | 0.14±0.03 |
| Xylene | 0.0 | 1.64±0.24 | 0.10±0.01 |
| Diesel | 21±2 | 3.35±0.06 | 0.20±0.02 |
| Toluene | 0.0 | 1.64±0.04 | 0.11±0.01 |
| Lubricating oil | 0.0 | 1.52±0.14 | 0.12±0.02 |
Figure 3: Effect of different carbon source on biosurfactantproduction
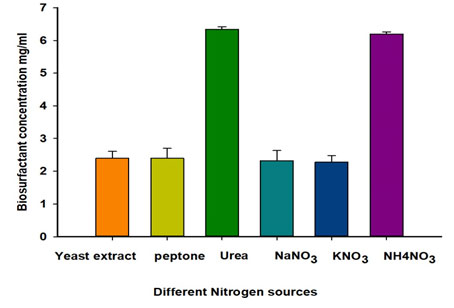
Effect of different nitrogen sources on biosurfactant production: The effect of changing the nitrogen source on biosurfactant production was also investigated. It is clear from Table 3 that using urea as the medium’s nitrogen source provided optimum results in all the three measured parameters. Likewise, ammonium nitrate (NH4NO3) was similarly suitable for biosurfactant production (Table 3 and Figure 4). Urea as a nitrogen source gave anemulsification index of 57±2.3%.
Table 3. Impact of different nitrogen sources on biosurfactant concentration, EI24, and bacterial dry weight (DW).
| Nitrogen source | P.balearica | ||
| EI24% | Rhamnose concentration mg/ml | DW g/50ml | |
| Yeast extract | 0 | 2.40±0.22 | 0.23±0.02 |
| Peptone | 0 | 2.40±0.31 | 0.27±0.04 |
| Urea | 57±2.3 | 6.33±0.10 | 0.27±0.03 |
| NaNO3 | 0 | 2.7±0.31 | 0.19±0.02 |
| KNO3 | 0 | 2.30±0.20 | 0.17±0.05 |
| NH4NO3 | 51±2.3 | 6.20±0.07 | 0.22±0.04 |
Figure 4: Effect of different nitrogen source on biosurfactant production.
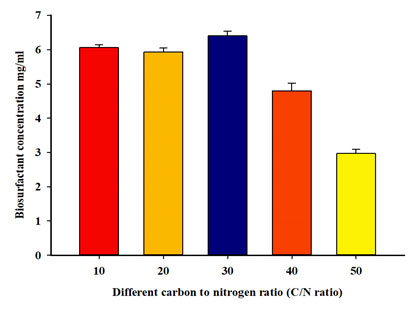
Effect of different C/N ratios on biosurfactant production: A carbon source (olive oil) suitable for the tested strain was added to the production medium with different concentrations, along with aconstant concentration of a nitrogen source (Urea). The ultimate EI24 for diesel oil by P.balearicawas recorded when the C/N ratio was 30 (Table 4). Likewise, the highest biosurfactant (rhamnolipid) concentration produced by this strain also occurred at a C/N ratio of 30 (Table 4 and Figure 5). A strong negative correlation (-0.82) apparent between C/N ratio and biosurfactant concentration.
Table 4. Outcome of different carbon/ nitrogen ratios on biosurfactant concentration, EI24, and bacterial dry weight (DW).
| C/Nratio | P.balearica | ||
| EI24% | Rhamnose concentration mg/ml | Bacterial DW g/50ml | |
| 10 | 43±5 | 6.05±0.09 | 0.24±0.04 |
| 20 | 44±7 | 5.93±0.12 | 0.27±0.02 |
| 30 | 60±4 | 6.40±0.14 | 0.25±0.05 |
| 40 | 46±5 | 4.79±0.23 | 0.25±0.02 |
| 50 | 14±2 | 2.97±0.12 | 0.17±0.04 |
Figure 5: The effect of different C/N ratio on biosurfactant concentration.
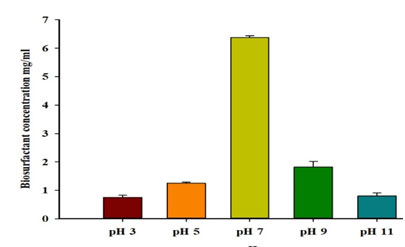
Effect of initial pH value on biosurfactant production: The pH of the production media for the tested strain was adjusted to different values, with amaximumrecorded emulsification index for diesel oil at pH 7 (Table5). Similarly, the highest biosurfactant production (6.37±0.06 mg/ml) as showed in Figure 6, and highest bacterial cell dry weight (0.27±0.04 g/50 ml) were recorded at pH 7 (Table 5). The correlation between biosurfactant concentration and pH is only weakly positive (+0.04).
Table 5. Effect of different pH values on biosurfactant concentration, EI24, and bacterial dry weight (DW).
| pH | P.balearica | ||
| EI24% | Rhamnose concentration mg/ml | DW g/50 ml | |
| 3 | 0.0 | 0.75±0.08 | 0.02±0.01 |
| 5 | 0.0 | 1.25±0.04 | 0.08±0.03 |
| 7 | 57±6 | 6.37±0.06 | 0.27±0.04 |
| 9 | 0.0 | 1.81±0.20 | 0.19±0.04 |
| 11 | 0.0 | 0.80±0.10 | 0.08±0.04 |
Figure 6: Effect of different pH values on biosurfactantproduction by P. balearica.
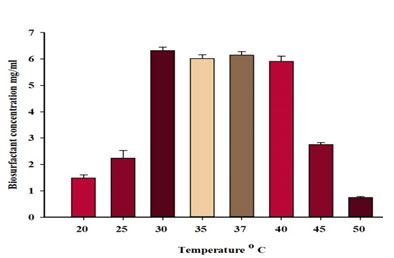
Effects of different incubation temperatures on biosurfactant production: The effect of incubation temperature on biosurfactant production was determined. The maximum emulsification index for diesel oil (67±6 %) and the highest biosurfactant production (6.35±0.14 mg/ml) with P.balearica were occurred at 30ºC (Figure 7 and Table 6). For bacterial cell dry weight, the highest value was at 37ºC (Table 6). Based on the correlation results, there appears to be a low negative correlation (-0.02) between incubation temperature and biosurfactant concentration.
Table 6. Results of different incubation temperatures on biosurfactant concentration, EI24 and bacterial dry weight (DW).
| Temperature (oC) | P.balearica | ||
| EI24% | Rhamnose co. mg/ml | DW g/50 ml | |
| 20 | 0 | 1.52±0.12 | 0.13±0.02 |
| 25 | 13±2 | 2.17±0.30 | 0.18±0.04 |
| 30 | 67±6 | 6.35±0.14 | 0.22±0.06 |
| 35 | 53±2 | 6.00±0.17 | 0.24±0.04 |
| 37 | 61±6 | 6.18±0.14 | 0.27±0.04 |
| 40 | 59±5 | 5.96±0.20 | 0.21±0.03 |
| 45 | 0 | 2.75±0.08 | 0.13±0.03 |
| 50 | 0 | 0.75±0.03 | 0.11±0.02 |
Figure 7: Effect of temperature on biosurfactantproduction by P. balearica.
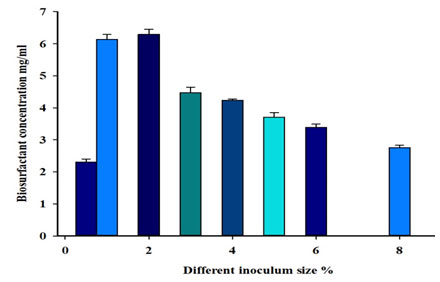
Effects of changing the inoculum size on biosurfactant production: Effect of various inoculum sizes on biosurfactant concentration was studied. The results in table (7) indicate that the greatest emulsification index for diesel oil, bacterial cell dry weight and the highest biosurfactant production occurred with an inoculum size of 2% (Figure 8). Statistical analysis showed weak negative correlation (-0.45) between inoculum size and biosurfactant production.
Table 7. Effect of inoculum size on biosurfactant concentration, EI24, and bacterial dry weight.
| Inoculum size% | P. balearica | ||
| EI24% | Rhamnose con. mg/ml | Dry weight g/50ml | |
| 0.5 | 0 | 2.31±0.08 | 0.13±0.02 |
| 1 | 46±6 | 6.14±0.15 | 0.19±0.02 |
| 2 | 62±2 | 6.29±0.16 | 0.17±0.02 |
| 3 | 52±4 | 4.47±0.17 | 0.19±0.03 |
| 4 | 42±5 | 4.23±0.04 | 0.14±0.03 |
| 5 | 37±5 | 3.71±0.14 | 0.13±0.02 |
| 6 | 32±4 | 3.39±0.09 | 0.15±0.03 |
| 8 | 18±2 | 2.75±0.07 | 0.15±0.04 |
Figure 8: Effect of different inoculum size on biosurfactant concentration.
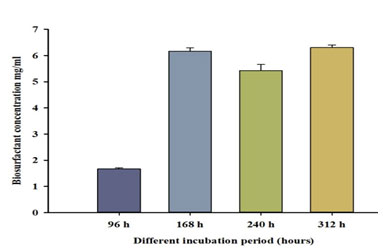
Effects of different incubation periods on biosurfactant production: balearica was incubated in the production medium with suitable selected nutritional factors for different time periods. The best diesel oil emulsification index, highest biosurfactant production, and highest bacterial cell dry weight were recorded after 312 hours (Table 8, Figure 9). The results show a strongly positive correlation (0.78) between incubation and biosurfactant production.
Table 8. Effect of different incubation periods on biosurfactant concentration, EI24, and bacterial dry weight.
| hours | P.balearica | ||
| EI24% | Rhamnose conc. (mg/ml) | Dry weight (g/50 ml) | |
| 96 | 0 | 1.67±0.04 | 0.13±0.03 |
| 168 | 49±2 | 6.16±0.13 | 0.23±0.02 |
| 240 | 56±4 | 5.42±0.23 | 0.20±0.03 |
| 312 | 63±2 | 6.30±0.09 | 0.23±0.03 |
Figure 9: Effect of different incubation period on biosurfactant concentration
The optimum carbon and nitrogen sources were Olive oilandUrea, respectively. The best C/N ratio was 30 and optimummedium pH was 7. The used inoculum size was 2%, incubation temperaturewas 30ºC and incubation time was 312 hrs. At the previous optimum temperature, the supernatant surface tension was reducedto 34 mN/m in comparison with distilled water (74 mN/m). The isolation of bacterial strains with biosurfactant producing capabilities from sites subject to oil contamination has been undertaken across the globe, including P. aeruginosa SG from Xinjiang oil field, China (Zhao et al., 2016).
B. subtilis MG495086 oil reservoir in Assam, India (Balan et al., 2017) Ochrobactrum anthropi HM-1 and Citrobacter freundii HM-2 from engine oil contaminated soil in Egypt (Datta et al., 2018) and both P. aeruginosa and klebsiella quasivariicola from oil contaminated sea shores in Jeddah, Saudi Arabia (Ibrahim, 2018). This indicates the presence and suitability of distinct biosurfactant producing microorganisms across numerous geographical oil contaminated locations (Motwali et al., 2020).
Assessing oil displacement and droplet collapse allow for rapid screening of a bacteria’s efficacy as a biosurfactant producer. The strain presented herein was able to create over 2 cm spread in an oil displacement test (3.0 ± 0.4) and form flat drop in a drop collapse assay. The present value of oil displacement test was higher than that observed by Sharma et al., (2019) who recorded a value of 1.5 ± 0.3 cm by some screened bacterial strains. The present result of dropcollapse assay is in accordance with Vaijayanti (2020) who found that all tested bacterial isolates showed positive results for drop collapse test indicated the occurrence of biosurfactant.
The results also show that the selected bacterium Emb 39 has positive activity on CTAB agar test which considered as evidence of rhamnolipid-production (Verma et al., 2006). The application of quantitative techniques such as surface activity measurement corroborates and adds confidence to the previously offered qualitative assessments. The screened bacterial strain in the current study was able to reduce surface tension to 42±0.3 mN/m, which reveals the ability of this strains to produce surface-active molecules. These results are in accordance with previous reports and Sharma et al., (2015), Eslami et al., (2020) which have suggested a direct relationship between the production of surface-active compounds and a reduction in surface tension.
The selected bacterial strain isolated from an oil-contaminated marine environment was identified as P. balearica. Marine bacteria are halo tolerant and can produce novel metabolites, such as biosurfactants to live in such habitats. Strains that belong to genus Pseudomonas are the greatest biosurfactant producers (Sharma et al., 2015). The present finding is also in consistent with those of Nejad et al. (2020) who proved that P. balearica is a biosurfactant producing bacteria.
According to Noh et al., (2014) the most important factors in increasing biosurfactant yield is the solubility of the carbon source in the culture medium. For instance, palm oil and diesel, as insoluble carbon sources, generally produce more rhamnolipids in comparison with water-soluble carbon sources such as glucose. It has been shown that the highest biosurfactant concentration was produced by P. balearica when olive oil was used as the carbon source. Almatawah (2017) and Tan and Li (2018) noted the suitability of olive oil as a substrate for biosurfactant production.
Recently, Sun et al., (2021) have indicated that the highest biosurfactant yields were obtained by Pseudomonas sp. using plant oils such as olive oil, soybean oil, and peanut oil as carbon sources. However, the current result disagrees with Wu et al. (2008) who reported a lower biosurfactant yield from P. aeruginosa using olive oil than that from glucose and glycerol. Moreover, using olive oil as a carbon source gave the highest emulsion stability. Ezebuiro et al. (2019) concluded that the biosurfactant produced with olive oil showed a high emulsification index. Wasoh et al., (2017) observed emulsification activity against diesel ranging from 55- 60 % by P. aeruginosa when sunflower oil was used as a carbon source.
Additionally, the highest biosurfactant concentration was obtained using urea or NH4NO3 as nitrogen sources. Also, Adamczak and Bednarski (2000) found that urea and ammonium salts are optimum nitrogen reservoirs for biosurfactant production. Prieto et al., (2008) reported urea as a best nitrogen source for biosurfactant production by P. aeruginosa with emulsification index about 60 while and Saikia et al., (2012) Alyousif et al., (2020) reported NaNO3 as the best nitrogen source for biosurfactant production by P. aeruginosa.
The C/N ratio is known to be a vital factor influencing the performance of bacteria in rhamnolipid production (Santos et al., 2002). The highest biosurfactant production and emulsification index for P. balearica in this study was obtained at a C/N ratio of 30, while the least yield was recorded at a ratio of 50. Negative correlation (-0.82) was recorded between the C/N ratio and biosurfactant concentration, which agrees with the results of Onwosi and Odibo (2012). Heryani and Putra (2017) reported that the C/N ratio of 12.4 was optimum for the production of a biosurfactant by Bacillus sp. and resulted in the highest decrease in surface tension. Khopade et al. (2012) indicated that lower value of C/N ratio (C/N= 20) was improved the emulsification activity by Nocardiopsis sp.
The current research found that the best production of biosurfactant and emulsification activity by P. balearica was at pH 7, with weak positive (+0.04) correlation detected between pH and biosurfactant concentration. This finding is in agreement with Fouda et al. (2016) who observed that the subsequent increase in pH from 8–10 was followed by a decrease in biosurfactant productivity in P. aeruginosa and B. cereus. Similarly, maximum biosurfactant from mutated strain of B. subtilis and Pseudomonas sp. was obtained at pH 7 (Kannahi and Sherley, 2012, Onwosi and Odibo 2012, Alyousif et al., 2020).
However, Elazzazy et al. (2015) reported the maximum biosurfactant production by Virgibacillus salaries was achieved at pH 9. The optimum temperature of operation for P. balearica is reported to be 30°C which was in accordance with Chander et al. (2012). Patil et al. (2014) reported maximum biosurfactant production was at 30◦C while the best inoculums size was 2% (Roy et al., 2017). Hema et al. (2019) have indicated that the optimum growth and emulsification activity for Planococcus sp. was 48 hours of incubation and that emulsification activity decreased by further incubation. After optimisation of the biosurfactant production of P.balearica, surface tension was measured to confirm the effect of the selected optimum conditions on biosurfactant activity. The results showed a decrease in surface tension which is in accordance with the data of Asgher et al., (2020).
CONCLUSION
Marine environments polluted with oil contain microorganisms able to produce surface-active agents (biosurfactant). P. balearica was the most active isolate and the use of olive oil and urea as carbon and nitrogen sources, pH 7, inoculum size 2% and incubation temperature at 37ºC temperature for 312 hr enhanced biosurfactant production.
REFERENCES
Adamczak, M. and Bednarski, W. (2000) Influence of medium composition and aeration on the synthesis of biosurfactants produced by Candida antartica. Biotechnology Letters, 22: 313-316.
Almatawah, Q. (2017) An Indigenous Biosurfactant Producing Burkholderia cepacia with High Emulsification Potential towards Crude Oil. Journal of Environmental and Analytical Toxicology, 7: 528-534.
Alyousif, N.A.; Al-Tamimi, W.H. and Al-Luaibi, Y.Y.Y. (2020). Screening enhances production and characterization of biosurfactant produced by Pseudomonas aeruginosa isolated from hydrocarbon contaminated soil. Eurasian Journal of Bioscience, 14: 4377-4391.
Asgher, M.; Afzal, M.; Qamar, S.A. et al. (2020) Optimization of biosurfactant production from chemically mutated strain of Bacillus subtilis using waste automobile oil as low-cost substrate. Environmental Sustainability, 3: 405-413.
Asubel, F.M.; Brent, R.; Kingston, R.E.et al. (1987). Current Protocols in Molecular Biology, Unit 24. Wiley, New York.
Balan, S.S.; Kumar, C.G.; Jayalakshmi, S. (2017) Aneurinifactin, a new lipopeptide biosurfactant produced by a marine Aneurinibacillus aneurinilyticus SBP-11 isolated from Gulf of Mannar: purification, characterization and its biological evaluation. Microbiological Research, 194: 1–9.
Carrillo, P.G.; Mardaraz, C.; Pitta-Alvarez, S.I.et al. (1996) Isolation and selection of biosurfactant-producing bacteria. World Journal of Microbiology and Biotechnology,12: 82-84.
Chander, S.; Lohitnath, C.R.; Mukesh, T. et al. (2012) Production and characterization of biosurfactant from Bacillus subtilis MTCC441 and its evaluation to use as bioemulsifier for food bio–preservative. Advances in Applied Science Research, 3(3):1827–1831.
Dattaa, P.; Tiwaria, P.; Pandey, L.M. (2018) Isolation and characterization of biosurfactant producing and oil degrading Bacillus subtilis MG495086 from formation water of Assam oil reservoir and its suitability for enhanced oil recovery. Bioresource Technology, 270: 439–448.
Desai, J.D. and Banat, I.M. (1997) Microbial production of surfactants and their commercial potential. Microbiology and Molecular Biology Reviews, 61: 47–64.
Elazzazy, A.M.; Abdelmoneim, T.S. and Almaghrabi, O.A. (2015) Isolation and characterization of biosurfactant production under extreme environmental conditions by alkali-halo-thermophilic bacteria from Saudi Arabia. Saudi Journal of Biological Sciences, 22: 466–475.
El-Sersy, N.A. (2012). Plackett-Burman Design to Optimize Biosurfactant Production by Marine Bacillus subtilis N10. Romanian Biotechnological Letter, 17(2): 7049-7064.
Eslami, P.; Hajfarajollah, H. and Bazsefidparc, S. (2020) Recent advancements in the production of rhamnolipid biosurfactants by Pseudomonas aeruginosa. The Royal Society of Chemistry, 10: 34014–34032.
Ezebuiro, V., Jonathan Otaraku, I.; Oruwari, B. et al., (2019) Effects of Nitrogen and Carbon Sources on Biosurfactant Production by Hydrocarbon-utilizing Stenotropho monas sp. Microbiology Research Journal International, 29(5), 1-10. https://doi.org/10.9734/mrji/2019/v29i530177.
Fakruddin, M.d. (2012) Biosurfactant: Production and application. Journal of Petroleum and Environmental Biotechnology, 3(4):124-129.
Fouda, A.; El-Gamal, M.S.; Abdel-Shakour, E.H. et al. (2016) Optimization and improvement of biosurfactant production for Pseudomonas aeruginosa4.2 and Bacillus cereus 2.3 strains isolated from oily polluted soil sample. International Journal of Advanced Research in Biological Sciences, 3(1): 76-87.
Gagelidze, N.A.; Amiranashvili, L.L.; Varsimashvili, K.I.et al. (2016) Selection of effective biosurfactant producers among Bacillus strains isolated from soils of Georgia. Annals of agrarian science, (14): 72-75.
Hema, T.; Seghal Kiran, G.; Sajayyan, A.et al., (2019) Response surface optimization of a glycolipid biosurfactant produced by a sponge associated marine bacterium Planococcus sp. MMD26. Biocatalysis and Agricultural Biotechnology, 18: 1-8.
Heryani, H. and Putra, M.D. (2017) Kinetic study and modeling of biosurfactant production using Bacillus sp. Electronic Journal of Biotechnology, 27:49–54.
Hmidet, N.; Ben Ayed, H.; Jacqueset al. (2017) Enhancement of Surfactin and Fengycin Production by Bacillus mojavensis A21: application for Diesel Biodegradation. BioMed Research International, 1-8.
Ibrahim, H.M.M. (2018) Characterization of biosurfactants produced by novel strains ofOchrobactrum anthropic HM-1 and Citrobacter freundii HM-2 from usedengine oil-contaminated soil.Egyptian Journal of Petroleum, 27:21-29.
Kannahi, M. and Sherley, M. (2012) Biosurfactant production by Pseudomonas putida and Aspergillus niger from oil contaminated site. International Journal of Chemical and Pharmaceutical Sciences, 3(4): 37-42.
Khopade, A.; Ren, B.; Liu, X. Y.et al. (2012). Production and characterization of biosurfactant from marine Streptomyces species B3. Journal of Colloid and Interface Science, 367: 311–318.
Kim, H.S.; Jeon, J.W.; Kim, B.H.et al. (2006) Extracellular production of a glycolipid biosurfactant, mannosylerythritol lipid, by Candida sp. SY16 using fed-batch fermentation. Applied Microbiology and Biotechnology, 70:391–396.
Kiran, G.S.; Hema, T.A.; Gandhimathi, R. et al. (2009) Optimization and production of a biosurfactant from the sponge-associated marine fungus Aspergillus ustus MSF3. ColloidsandSurfaces, 73: 250–256.
Kumar, A.; Alam, A.; Rani, M.et al. (2017) Biofilms: survival and defense strategy for pathogens. International Journal of Medical Microbiology, 307: 481–489.
Marajan, C.; Alias, S.; Ramasamy, K. et al. (2018) The Effect of Incubation Time, Temperature and pH Variations on the Surface Tension of Biosurfactant Produced by Bacillus spp. AIP Publishing, 020047-020055.
Mouafi, F. E.; Abo Elsoud, M. M. and Moharam, M.E. (2016) Optimization of biosurfactant production by Bacillus brevis using response surface methodology. Biotechnology reports, 9:31-37.
Motwali, E.A.; Aly, M.M.; Qari, H. et al. (2020) Screening and Identification of Efficient Biosurfactant Producing Bacteria for some Medical Applications. La Prensa Medica Argentina, 2:005: 6.
Nejad, Y.S.; Jaafarzadeh, N.; Ahmadi, M. et al. (2020) Remediation of oily sludge wastes using biosurfactant produced by bacterial isolate Pseudomonas balearica strain Z8. Journal of Environmental Health Science and Engineering, 18:531–539
Noh, N.A.; Salwa, M.S.; Ahmad Ramli, M.Y. (2014) Enhanced rhamnolipid production by Pseudomonas aeruginosa USM‐AR2 via fed‐batch cultivation based on maximum substrate uptake rate. Letters in Applied Microbiology, 58: 617–623.
Onwosi, C.O. and Odibo, F.J.C. (2012) Effects of carbon and nitrogen sources on rhamnolipid biosurfactant production by Pseudomonas nitroreducens isolated from soil. World Journal of Microbiology and Biotechnology, 28:937–942.
Pathaka A.N. and Nakhate, P.H. (2015) Optimisation of Rhamnolipid: A New Age Biosurfactant from Pseudomonas aeruginosa MTCC 1688 and its Application in Oil Recovery, Heavy and Toxic Metals Recovery. Journal of Bioprocess Biotechnology, 5: 229-243.
Patil, S.; Anuradha, P. and Aruna, K. (2014) Studies on optimization of biosurfactant production by Pseudomonas aeruginosa F23 isolated from oil contaminated soil sample. International Journal of Current Biotechnology, 2(4):20–30.
Prieto, L.M.; Michelon, M.; Burkert, J.F.M.; Kalil, S.J. and Burkert, C.A.V. (2008) The production of rhamnolipid by a Pseudomonas aeruginosa strain isolated from a southern coastal zone in Brazil. Chemosphere, 71:1781–1785.
Reddy, M. N.; Ganesh Kumar, C.; Swathi, K.; Nagamani, B.; Venkateshwar, S. and Rao, L.V. (2011) Extracellular alkaline protease production from isolated bacillus subtilis SVR-07 by using submerged fermentation. International Journal of pharma Research and Development, 3:126-223.
Robert, M.; Mercadé, M.E.; Bosch, M.P.; Parra, J.L.; Espiny, M.J.; Manresa, M.A. and Guinea, J. (1989) Effect of the carbon source on biosurfactant production by Pseudomonas aeruginosa 44T1. Biotechnology Letters, 11: 871–874.
Roy, A. (2017) Review on the Biosurfactants: Properties, Types and its Applications. Journal of Fundamentals of Renewable Energy and Applications. 8: 248-253.
Saikia, R.R.; Deka, S.; Deka, M. and Banat, I.M. (2012) Isolation of biosurfactant-producing Pseudomonas aeruginosa RS29 from oil-contaminated soil and evaluation of different nitrogen sources in biosurfactant production. Annals of Microbiology, 62:753–763.
Salihu, A.; Abdulkadir, I. and Almustapha, M.N. (2009) An investigation for potential development on biosurfactants. Biotechnology and Molecular Biology Reviews, 3: 111-117.
Santos, A.S.; Sampaio, A.W.; Vasquez, G.S.et al. (2002) Evaluation of different carbon and nitrogen sources in production of rhamnolipids by a strain of Pseudomonas aeruginosa. Applied Biochemistry and Biotechnology, 98(100):1025–1035.
Sarubbo, L.A.; Farias, C.B.B. and Campos-Takaki, G.M. (2007) Co-utilization of canola oil and glucose on the production of a surfactant by Candida lipolytica. Current Microbiology, 54: 68–73.
Sharma, D.; Ansari, M.J.; Al-Ghamdi, A. et al. (2015) Biosurfactant production by Pseudomonas aeruginosa DSVP20 isolated from petroleum hydrocarbon-contaminated soil and its physicochemical characterization. Environmental Science and Pollution Research, 22:17636–17643.
Sharma, S.; Verma, R. and Pandey, L.M. (2019) Crude oil degradation and biosurfactant production abilities of isolated Agrobacterium fabrum SLAJ731. Biocatalysis and Agricultural Biotechnology, 21:10 pages.
Sun, W.; Zhu, B.; Yang, F.et al. (2021) Optimization of biosurfactant production from Pseudomonas sp. CQ2 and its application for remediation of heavy metal contaminated soil. Chemosphere, 265: 1-12.
Tan, Y.N. and Li, Q. (2018) Microbial production of rhamnolipids using sugars as carbon sources.Microbial Cell Factories, 17: 089-102.
Vaijayanti, M. (2020) Comparative study of antimicrobial efficiency of biosurfactant producing Pseudomonas spp. from different soil samples. Journal of Applied and Advanced Research, 5: 1-5.
Verma, S.; Bhargava, R. and Pruthi, V. (2006) Oily sludge degradation by bacteria from Ankleshwar, India. International Biodeterioration and Biodegradation, 57:207-213.
Wasoh, H.; Baharun, S.; Halim, M; Lajis, A.F.; Ariff, A. and Lai, O. M. (2017) Production of rhamnolipids by locally isolated Pseudomonas aeruginosa using sunflower oil as carbon source. Bioremediation Science and Technology research, 5(1):1-6.
Wu, J.Y.; Yeh, K.L.; Lu, W.B.; Lin, C.L. and Chang, J.S. (2008) Rhamnolipid production with indigenous Pseudomonas aeruginosa EM1 isolated from oil-contaminated site. Bioresource Technology, 99:1157–1164.
Yañez-Ocampo, G.; Somoza-Coutiño, G.; Blanco-González, C. et al. (2017) Utilization of agroindustrial waste for biosurfactant production by native bacteria from Chiapas. Open Agriculture, 2: 341–349.
Zhao, F.; Li, P.; Guo, C.et al. (2018) Bioaugmentation of oil reservoir indigenous Pseudomonas aeruginosa to enhance oil recovery through in-situ biosurfactant production without air injection. Bioresource Technology, 251: 295–302.


