1King Abdulaziz University, Faculty of Medicine, Jeddah, Saudi Arabia
2Department of Dental Public Health, Faculty of Dentistry, King Abdulaziz University Hospital, Jeddah, Saudi Arabia
3Department of Ophthalmology, King Abdulaziz University Hospital, Jeddah, Saudi Arabia
Corresponding author email: nawaf.almarzouki@gmail.com
Article Publishing History
Received: 24/10/2020
Accepted After Revision: 13/12/2020
Aim of the study was to investigate the effect of chronic hyperglycemia as determined by high glycated hemoglobin (HbA1c) on intraocular pressure (IOP) in patients with diabetes and to recognize the diabetic patients at high risk of developing glaucoma in a tertiary care hospital in western region of Saudi Arabia.This was a retrospective chart review performed at King Abdulaziz University Hospital, Jeddah, Saudi Arabia. Hospital records of diabetic patients in the department of ophthalmology from August 2015 to June 2020 were collected. Patients diagnosed with glaucoma, using intraocular pressure-lowering medications, or using topical or oral steroids were excluded from the study.Overall, 159 participants were enrolled in the study. A significant association between high HbA1c levels and IOP values was observed. Individuals with HbA1c below 6.5, between 9.6 to 10.5, and over 12.6 had a mean IOP of 15.2 ± 2.87, 16.6 ± 5.12, and 19.5 ± 1.88, respectively (p= 0.031). Longer diabetes duration was associated with a higher IOP (p=0.028). Another finding illustrated that female participants had significantly higher IOP compared to males (16.94 ± 3.25 mm Hg, 15.15 ± 3.31 mm Hg, p=0.001, respectively). A significant positive association between high HbA1c levels and IOP values was found, which indicates that diabetes and elevated HbA1c are significant contributing factors for elevated IOP. There was a statistically significant higher IOP in females in which further research is needed with prospective and extensive data collection. Accordingly, a regular diabetic eye examination to monitor intraocular pressure is recommended specially to those with uncontrolled diabetes and high HgA1c to reduce ocular morbidity due to glaucoma.
Diabetes, Intraocular Pressure, Glaucoma, Glycated Hemoglobin
Aljuhani R, Awad A, Bamardouf N, Metwalli E, Obaid H, Ashi H, Almarzouki N. Effect of Glycated Hemoglobin Levels on Intraocular Pressure in Patients with Diabetes Mellitus in Saudi Population. Biosc.Biotech.Res.Comm. 2020;13(4).
Aljuhani R, Awad A, Bamardouf N, Metwalli E, Obaid H, Ashi H, Almarzouki N. Effect of Glycated Hemoglobin Levels on Intraocular Pressure in Patients with Diabetes Mellitus in Saudi Population. Biosc.Biotech.Res.Comm. 2020;13(4). Available from: https://bit.ly/37X6H0u
Copyright © Aljuhani et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Diabetes mellitus (DM) is a metabolic disease associated with chronic hyperglycemia (Mayer-Davis et al .2018). It is a distressing epidemic and is considered one of the leading causes of death worldwide (Glovaci et al. 2019). Globally, the prevalence of DM is estimated to be 9.3% (463 million people) in 2019, increasing to 10.2% (578 million)and 10.9% (700 million) by 2030 and 2045, respectively, with more than 29% incidence in Saudi Arabia alone from 1990 -2015 ( Saeedi et al. 2019) ( Alotaibi et al. 2017). DM is diagnosed according to plasma glucose criteria in the form of fasting plasma glucose (FPG) levels, 2-h plasma postprandial glucose (2-h PPG) levels, or the glycosylated hemoglobin (HbA1c) criteria reflecting the average plasma glucose concentration over the previous 8–12 weeks (Care 2019) ( Nguyen et al.2019). The International Expert Committee recommends HbA1c as a reliable tool for diagnosing type 1 and type 2 DM with a cutoff point of ≥6.5% (Nathan et al. 2009). Al Salamah et al.(2020) have reported that 35% of Saudis aged 55 or more had type 2 diabetes.
HbA1c testing has multiple advantages over plasma glucose measurement, such as pre-analytical stability and less day-to-day variation due to stress or illness (Nathan et al.2009). Therefore, HbA1c is the gold standard for diabetes control. Besides reflecting the glycemic adjustment, Hba1c is used as a predictor 1to assess secondary microvascular complications, including retinopathy, neuropathy, and nephropathy in cases of insufficient glycemic control ( Hasselacher et al. 2014). Diabetes contributes to the risk of developing several types of glaucoma, most commonly, primary open-angle glaucoma (POAG) and neovascular glaucoma (NVG) (Resnikoff et al.2004 Barac et al. 2015, Bahera et al .2020).
POAG is a multifactorial disease that is caused by retinal ischemia, remodeling of the optic nerve head, and altered trabecular meshwork function( Feki et al. 2019) (Faralli et al. 2019) . Diabetic patients are susceptible to retinal ischemia, which is believed to be the main cause of neovascular glaucoma by stimulating the release of vascular endothelial growth factor-A (VEGF-A), leading to vasodilatation and increasing blood flow, which initiates new blood vessel formation leading to NVG (Hayreh 2007, Yang et al .2018). Glaucoma is defined as a group of ocular disorders that are characterized by progressive optic neuropathy and associated visual field loss (Bertaud et al. 2019). Although treatable, it is the most common irreversible blinding disease worldwide (Quigley et al. 2006). Therefore, early detection is required for a good prognosis. Normal intraocular pressure (IOP) is 10-21 mm Hg, which is preserved by a balance between the aqueous humor production and drainage. Any imbalance leads to elevated IOP (Khaw and Elkington 2004), causing both vascular and mechanical stresses ( Song et al. 2016) .Therefore, it is an important risk factor for glaucoma deterioration and progression, and currently, is the only modifiable factor (Asal et al. 2020).
A recent meta-analysis evaluated 47 studies from 16 different countries and found that patients with diabetes had been associated with an average of 0.18 mmHg increase in the IOP ( Zhao et al. 2014) Furthermore, other studies found that patients with increased levels of HbA1c had substantially higher IOP levels compared to the patients with lower levels of HbA1c ( Hymowitz et al. 2016, Perez-Rico et al. 2015, Takahashi et al. 2020).
A study conducted in Riyadh, Saudi Arabia, found that diabetic patients had higher IOP compared to non-diabetic subjects. HbA1c was used as a criterion for diagnosing diabetes; however, the relationship between HbA1c value and IOP has not been studied (Briggs et al. 2016). To the best of our knowledge, there have been no reports evaluating the relationship between HbA1C and IOP among the Saudi population. Therefore, we aimed to investigate the effect of chronic hyperglycemia as determined by HbA1c on IOP in patients with diabetes and identify diabetic patients at risk of developing glaucoma in Saudi Arabia from 2015 to 2020.
MATERIALS AND METHODS
Study design and setting:This retrospective chart review study was conducted at King Abdul-Aziz University Hospital (KAUH), a tertiary center in Jeddah, Saudi Arabia. Medical records from glaucoma and retina clinics in the Department of Ophthalmology between August 2015 and June 2020 were collected.
Sample criteria and diagnostic instrument:Patients aged 15-90 years diagnosed with type 1 or type 2 DM were included Patients diagnosed with glaucoma, using IOP-lowering medications, or using topical or oral steroids were excluded from the study. Of the 383 diabetic patients treated at the department of ophthalmology between 2015 to 2020, 224 subjects were excluded: 73 diagnosed with glaucoma, 138 with previous history of laser or intraocular surgery, and 13 on IOP-lowering medications or topical steroids. Thus, 159 subjects met the inclusion criteria and were enrolled in the study.Data obtained from medical records included demographic data such as age, sex, and nationality. Additionally, type and duration of diabetes, HbA1c levels, IOP in the right and left eye (IOP-OD, IOP-OS, respectively), and body mass index (BMI), which was calculated as weight in kilograms divided by height in meters squared were also collected.
The patient’s IOP was measured using a Goldmann applanation tonometer. The mean IOP was calculated for each patient as the sum of the pressure of both eyes divided by two, using an excel equation. Glycemic control measurement (HgA1c) , was obtained within one year before or after IOP measurement . Patients were categorized according to their glycemic control in three categories: good glycemic control (HbA1c <7%), moderate glycemic control (HbA1c 7-9%), and poor glycemic control (HbA1c >9%) (23). Diabetes duration was defined as the period from the first diagnosis to the day of IOP measurement.
Analysis:Data were registered using an online Google form, and was then imported to Microsoft Excel 2020 for data entry. Statistical analysis was performed using the Statistical Package for the Social Sciences IBM© SPSS© version 21 (IBM© Corp., Armonk, NY, USA). Descriptive statistics (mean and standard deviation) were calculated for normally distributed variables including IOP, HbA1c, BMI, age, and diabetes duration. Frequencies and percentages were calculated for sex, nationality, diabetes type, and population categories. We used the Shapiro-Wilk test to check for normality. An independent-samples t-test was used to compare the IOP in both sexes and both types of diabetes. For multiple comparisons with the IOP, one-way analysis of variance (ANOVA) was performed. All P-value < 0.05 were considered to be statistically significant. Research manuscripts reporting large datasets that are deposited in a publicly available database should specify where the data have been deposited and provide the relevant accession numbers. If the accession numbers have not yet been obtained at the time of submission, please state that they will be provided during review. They must be provided prior to publication.
Research ethics: This research was approved by the Biomedical Ethical Committee at KAUH (ref: 653-19).
RESULTS AND DISCUSSION
There were 73 (45.9%) men and 86 (54.1%) women. The mean age was 58 ± 16 years, with the majority of patients being 50 to 69 years old (49.1%). The majority of patients, 82 (51.6%), were type 2 diabetic patients. The characteristics of the study population are shown in Table 1.
Table 1. mean and standard deviation of the study population characteristics
| Characteristics | IOP (mm hg) | ||||
| N(%) | Mean ± SD | Mean ± SD | p-value | ||
| Gender
Male Female |
73 (45.9%) |
15.15 ± 3.31 |
0.001 | ||
| 86 (54.1%) | 16.94 ± 3.25 | ||||
| Diabetes type
Type 1 Type 2 |
77 (48.4%) |
16.38 ± 3.14 |
0.347 | ||
| 82 (51.6%) | 15.87 ± 3.60 | ||||
| BMI classifications
Underweight Normal weight Pre-obesity Obesity |
4 (2.5%) |
17.53 ± 0.64 |
0.77 | ||
| 34 (21.7%) | 22.49 ± 1.57 | ||||
| 38 (24.2%) | 27.51 ± 1.43 | ||||
| 81 (51.6%) | 35.55 ± 4.76 | ||||
| Age classifications
<29 y 30 – 49 y 50 – 69 y 70 – 84 y |
15 (9.4%) |
23.47 ± 3.96 |
0.42 | ||
| 23 (14.5%) | 42.04 ± 5.42 | ||||
| 78 (49.1%) | 60.90 ± 5.08 | ||||
| 43 (27.0%) | 75.91 ± 4.84 | ||||
Female participants had statistically higher IOP compared to the male participants (16.94 ± 3.25 mm Hg, 15.15 ± 3.31 mm Hg, respectively, p=0.001). We observed no significant difference between Type 1 as well as Type 2 DM and IOP (16.38 ± 3.14, 15.87 ± 3.60, respectively), p=0.347.
When we classified diabetic patients according to their glycemic control, 72 participants (46.5%), almost half of the sample, had moderate glycemic control. The mean HbA1c for good, moderate, and poor controls was 6. ± 1.01, 8.05 ± 0.58, and 10.80 ± 1.40. respectively.The mean IOP was elevated in the poor glycemic control patients (16.78 ± 3.65 mmHg) compared to those with good glycemic control (15.65 ± 3.08 mm Hg). The difference was not statistically significant (p=0.263). Table 2 shows a comparison between the three groups.
Table 2. mean and standard deviation of HgA1c and IOP in glycemic control groups
| good glycemic control: N (%)
38 (24.5%) |
moderate glycemic control: N (%)
72 (46.5%) |
poor glycemic
control: N (%) 45 (29%) |
p-value | |
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| HbA1c | 6.006 ± 1.014 | 8.052 ± 0.581 | 10.809 ± 1.405 | 0.001 |
| IOP | 15.65 ± 3.08 | 15.91 ± 3.39 | 16.78 ± 3.65 | 0.263 |
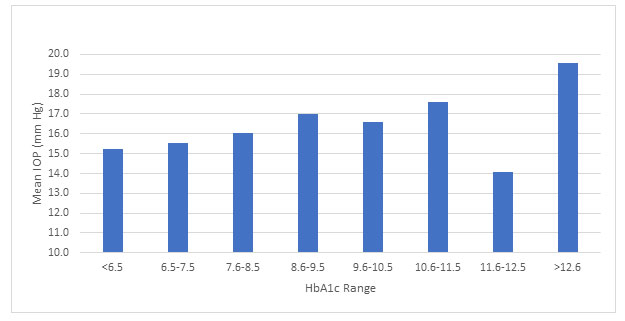
There was a statistically significant difference between the HbA1c range groups and IOP, as determined by one-way ANOVA (F [7,149] = 2.276, p = 0.031). Subjects with HbA1c above 12.6 displayed higher IOPs (19.58 ± 1.88 mm Hg), compared to the subjects with HbA1c between 6.5 to 7.5 (15.52 ± 3.89 mm Hg), and between 7.6 to 8.5 (16.029 ± 2.81 mm Hg), with a mean difference of (4.05 mm Hg), (p=0.006) and (3.55 mm Hg), (p=0.017), respectively (Figure 1).
Figure 1: Comparison between HgA1c range and IOP
One-way ANOVA revealed that with longer duration of diabetes, the IOP significantly increased as well (F (3,143) = 3.117, p = 0.028). Thus, participants with a diabetes duration of less than five years had a mean IOP of 14.75 ± 2.99 mm Hg, while participants with a diabetes duration ranging from 11 to 20 years had a mean IOP of 16.93 ± 3.79 mm Hg, with a mean difference of (2.21 mm Hg), (p=0.006). Table 3 presents the mean IOP values according to the duration of DM.
Table 3. mean and standard deviation of IOP according to duration of DM
| Diabetes duration | N(%) | Mean ±SD | Mean IOP (mmHg) ±SD | P value |
| <5 y
|
26 (17.7%) | 2.04 ± 1.34 | 14.75 ± 2.99 | 0.028 |
| 5 – 10 y | 33 (22.4%) | 8.91 ± 1.73 | 15.74 ± 2.45 | |
| 11 – 20 y | 57 (38.8%) | 16.25 ± 2.708 | 16.93 ± 3.79 | |
| 21 – 33 y | 31 (21.1%) | 24.58 ± 3.09 | 16.82 ± 3.46 |
In this study, we found that individuals with higher levels of HbA1c exhibited significantly higher IOP levels compared to individuals with lower HbA1c levels. This result is consistent with those of previously published studies, (Kang et al. 2019,Takahashi et al. 2020). Hymowitz et al.2016 found an association between poor glycemic control, as evidenced by higher HbA1c and elevated IOP. Baisakhiya et al. 2017 observed an association between higher HbA1c and raised IOP, reporting that poor glycemic control is a risk factor for glaucoma in diabetic patients.
The underlying pathogenesis that explains how DM promotes increased IOP remains unclear. Diabetes is a known cause of microvascular damage and can disturb blood flow at the level of the optic nerve head and retina, which stimulates the invasion of the iris surface and iridocorneal angle of the anterior chamber by a fibrovascular membrane. This fibrovascular membrane initially resists the aqueous outflow, resulting in open-angle glaucoma, and later obstructs the angle and produces secondary angle-closure glaucoma, (Hayreh 2007,Salzy et al.2009, Grzybowski et al. 2020). In addition, higher glucose levels in the aqueous humor of diabetic patients have been observed to upregulate and promote the accumulation of extracellular matrix proteins, particularly fibronectin, thereby blocking aqueous drainage, leading to an increase in IOP. Chronically raised IOP levels sequentially lead to optic nerve head damage, stemming from progressive mechanical compression (Faralli et al .2019).
Oshitari et al. (2007) found a significant association between IOP and glycemic control categories, which is inconsistent with our result, as we could not find an association between glycemic control categories and IOP levels. This is probably due to the use of different HbA1c classifications. Moreover, some researchers have found an alteration in the IOP values following transient blood glucose fluctuations in cases of hypoglycemia or postprandial hyperglycemia that cannot be measured by HbA1c ( Rihan et al.2020). Additionally, comorbidities that falsely lower HbA1c levels on test results by shortening the erythrocyte survival rate could potentially affect the outcome of the study ( Report 2011, Ang et al. 2014).
Our study showed that people with a longer duration of diabetes had higher IOP values compared to patients with a shorter duration. In contrast, a previous study conducted in Riyadh, Saudi Arabia, stated that the duration of diabetes did not vary significantly with IOP levels (Briggs et al. 2016). Recall bias of diabetes duration plays a major role in the inconsistency between the two results. Further, a meta-analysis study reported that each year since diabetes diagnosis increases the risk of glaucoma by 5% (95% CI, 1%–9%), which may be due to the cumulative neuronal damage that progresses with time, (Zhao et al. 2015, Grzybowski et al .2020).
Moreover, we found that female participants had a higher IOP than males, a difference which was statistically significantly. Moreover, Kang et al.2019 reported a significant relationship between IOP and HgA1c in female without diabetes. In contrast, Hymowitz et al.2016 reported no significant difference between two sexes regarding IOP levels. However, variability in the results could stem from differences in the male-to-female ratio between the two sample populations. The sex-related differences in IOP measurement remain incompletely understood. Previous studies reported that anatomical and structural eye differences between the two sexes made the comparison inaccurate, which explains part of this discrepancy (Patel 2018, Chua et al. 2019).
Limitations: This retrospective record review study encountered a few inherent limitations. First, it was not possible to provide precise HbA1C levels and random blood glucose at the exact time point during which the IOP was measured. In addition, open-angle glaucoma has no symptoms initially prior to peripheral vision loss; therefore, many diabetic patients had already presented with glaucoma due to delayed periodic eye screening.
Recommendations: Planning an educational campaign empowering diabetic people to perform periodic eye screening for monitoring IOP to ensure that glaucoma can be diagnosed in the early stage and effectively treated. Further, future studies to assess the confounding factors that may influence the association between HbA1C and IOP, such as central corneal thickness and lens status are warranted.
CONCLUSION
The current study found a significant association between high HbA1c levels and IOP values, which indicates that diabetes and elevated HbA1c are significant contributing factors for elevated IOP. Therefore, a well-established diabetic screening program includes IOP measurement should be applied routinely. Accordingly, diabetics with uncontrolled blood sugar and high HgA1c are recommended to undergo more frequent eye examination to monitor IOP to reduce ocular morbidity due to glaucoma.
Author Contributions: This manuscript has been read and approved by all the authors and the requirements for authorship have been met by all authors. Each author believes that the manuscript represents honest work and the information has not been provided in another form elsewhere.
Funding: This research received no external funding
ACKNOWLEDGEMENTS
This study was a part of the research summer school 2020 project directed by the Road of Change team, which is an institute for teaching students’ research skills. The authors would like to thank the medical students of KAUH, Mawaddah Maghadmi, Abdulkarim Jawhari, and Mohammed Safhi for their valuable guidance throughout the study. The authors also thank Mawaddah Batwa for the critical revision of this research.
Conflicts of Interest: The authors declare no conflict of interest
REFERENCES
Alotaibi A, Perry L, Gholizadeh L, Al-Ganmi A.(2017) Incidence and prevalence rates of diabetes mellitus in Saudi Arabia: An overview. J Epidemiol Glob Health [Internet]. 2017;7(4):211–8. Available from: https://doi.org/10.1016/j.jegh.2017.10.001
Al Slamah T, Nicholl BI, Alslail FY, Harris L, Kinnear D, Melville CA.(2020) Correlates of type 2 diabetes and glycaemic control in adults in Saudi Arabia a secondary data analysis of the Saudi health interview survey. BMC Public Health. 2020;20(1):515. Published 2020 Apr 17. doi:10.1186/s12889-020-08597-6
Ang SH, Thevarajah M, Alias Y, Khor SM.(2014) Current aspects in hemoglobin A1c detection: a review. Clin Chim Acta. 2015 Jan 15;439:202-11. doi: 10.1016/j.cca.2014.10.019. Epub 2014 Oct 22. PMID: 25451954.
Asal A, Alsada A, Ali A, Abdulnoor M, Shakeeb H.(2020) The role of lipid profile and intraocular pressure lowering medications in patients diagnosed with primary open angle glaucoma. Bahrain Med Bull. 2020;42(3):169–70.
Baisakhiya S, Garg P, Singh S.(2017) Association between glycemic control and intraocular pressure in patients with Type II diabetes mellitus. Natl J Physiol Pharm Pharmacol. 2017;7(1):43–6.
Barac IR, Pop MD aniel., Gheorghe AI onu., Taban C.(2015) Neovascular Secondary Glaucoma, Etiology and Pathogenesis. Rom J Ophthalmol. 2015;59(1):24–8.
Bertaud S, Aragno V, Baudouin C, Labbé A.(2019) Primary open-angle glaucoma. Rev Med Interne. 2019;40(7):445–52.
Behera UC, Bhattacharjee H, Das T, et al. (2020) Spectrum of Eye Disease in Diabetes (SPEED) in India: A prospective facility-based study. Report # 4. Glaucoma in people with type 2 diabetes mellitus. Indian J Ophthalmol. 2020;68(Suppl 1):S32-S36. doi:10.4103/ijo.IJO_1948_19
Briggs S, Osuagwu UL, AlHarthi EM.(2016) Manifestations of type 2 diabetes in corneal endothelial cell density, corneal thickness and intraocular pressure. J Biomed Res. 2016;30(1):46–51.
Care D, Suppl SS. (2019) Classification and diagnosis of diabetes: Standards of medical care in diabetesd2019. Diabetes Care. 2019;42(January):S13–28.
Chua J, Chee ML, Chin CWL, et al.(2019) Inter-relationship between ageing, body mass index, diabetes, systemic blood pressure and intraocular pressure in Asians: 6-year longitudinal study. Br J Ophthalmol. 2019;103(2):196-202. doi:10.1136/bjophthalmol-2018-311897
Faralli JA, Filla MS, Peters DM. Role of Fibronectin in Primary Open Angle Glaucoma. Cells. 2019;8(12).
Feke GT, Bex PJ, Taylor CP, Rhee DJ, Turalba A V, Chen TC, et al.(2019) Effect of Brimonidine on Retinal Vascular Autoregulation and Short-term Visual Function in Normal Tension Glaucoma. 2019;158(1):105–12.
Glovaci D, Fan W, Wong ND.(2019) Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr Cardiol Rep. 2019 Mar;21(4):21.
Grzybowski A, Och M, Kanclerz P, Leffler C, Moraes CG.(2020) Primary Open Angle Glaucoma and Vascular Risk Factors: A Review of Population Based Studies from 1990 to 2019. J Clin Med. 2020;9(3):761. Published 2020 Mar 11. doi:10.3390/jcm9030761
Hasslacher C, Kulozik F, Platten I, Lorenzo Bermejo J.(2014) Glycated albumin and HbA1c as predictors of mortality and vascular complications in type 2 diabetes patients with normal and moderately impaired renal function: 5-year results from a 380 patient cohort. J Diabetes Res Clin Metab. 2014;3(1):9.
Hayreh SS.(2007) Neovascular glaucoma. Prog Retin Eye Res. 2007;26(5):470–85.
Hymowitz MB, Chang D, Feinberg EB, Roy S.(2016) Increased intraocular pressure and hyperglycemic level in diabetic patients. PLoS One. 2016;11(3):1–7.
Kang K, Jun S, Shin K, Son D, Yoo B, Kim S, Joe H, Hong S, Cho C, Shin H, Cho Y, Oh J.(2019) Association between Glycated Hemoglobin A1c and Intraocular Pressure in Nondiabetic Subjects. KJFP 2019;9:59-63. https://doi.org/10.21215/kjfp.2019.9.1.59
Khaw PT, Elkington AR.(2004) Glaucoma—1: Diagnosis. Bmj. 2004;328(7431):97.
Song BJ, Aiello LP, Pasquale LR. (2016)Presence and Risk Factors for Glaucoma in Patients with Diabetes. Curr Diab Rep. 2016;16(12).
Mayer-Davis EJ, Kahkoska AR, Jefferies C, Dabelea D, Balde N, Gong CX, et al.(2018) ISPAD Clinical Practice Consensus Guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes. 2018;19(June):7–19.
Nguyen KA, Peer N, De Villiers A, Mukasa B, Matsha TE, Mills EJ, et al.(2019) Glycated haemoglobin threshold for dysglycaemia screening, and application to metabolic syndrome diagnosis in HIV-infected Africans. PLoS One. 2019;14(1):1–11.
Nathan DM, Balkau B, Bonora E, Borch-Johnsen K, Buse JB, Colagiuri S, et al.(2009) International expert committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–34.
Oshitari T, Fujimoto N, Hanawa K, Adachi-Usami E.( 2007) Effect of Chronic Hyperglycemia on Intraocular Pressure in Patients With Diabetes. Am J Ophthalmol. 2007;143(2):363–5.
Patel P, Harris A, Toris C, Tobe L, Lang M, Belamkar A, et al.(2018) Effects of Sex Hormones on Ocular Blood Flow and Intraocular Pressure in Primary Open-angle Glaucoma: A Review. J Glaucoma. 2018;27(12):1037–41.
Pérez-Rico C, Gutiérrez-Ortíz C, González-Mesa A, Zandueta AM, Moreno-Salgueiro A, Germain F.(2015) Effect of diabetes mellitus on Corvis ST measurement process. Acta Ophthalmol. 2015;93(3):e193–8.
Quigley H, Broman AT.(2006) The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–7.
Raihan Esa N, Azwani Mohd Shukri N, Ahmad N, Radzi Hilmi M, Muziman Syah Md Mustafa M, Syahiera Ibrahim N, et al.(2020) Intraocular Pressure: the Effect of Short-term Fasting and Its Association With Fluid and Fat Status. Malaysian J Med Heal Sci. 2020;16(2):2636–9346.
Report A, Consultation WHO. (2011)Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. Diabetes Res Clin Pract. 2011;93(3):299–309.
Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al.(2004) Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–51.
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al.(2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract [Internet]. 2019;157:107843. Available from: https://doi.org/10.1016/j.diabres.2019.107843
Shazly TA, Latina MA.(2009) Neovascular glaucoma: Etiology, diagnosis and prognosis. Semin Ophthalmol. 2009;24(2):113–21.
Takahashi S, Hara K, Sano I, et al.(2020) Systemic factors associated with intraocular pressure among subjects in a health examination program in Japan. PLoS One. 2020;15(6):e0234042. Published 2020 Jun 3. doi:10.1371/journal.pone.0234042
Yang H, Yu X, Sun X.(2018) Neovascular glaucoma: Handling in the future. Taiwan J Ophthalmol. 2018;8(2):60-66. doi:10.4103/tjo.tjo_39_18
Zhao D, Cho J, Kim MH, Friedman DS, Guallar E. (2015)Diabetes, fasting glucose, and the risk of glaucoma: A meta-analysis. Ophthalmology [Internet]. 2015;122(1):72–8. Available from: http://dx.doi.org/10.1016/j.ophtha.2014.07.051