Department of Clinical Dental Sciences, College of Dentistry, Princess Nourah Bint Abdulrahman University, Riyadh Saudi Arabia
Corresponding author email: aalikhulud@gmail.com
Article Publishing History
Received: 06/10/2019
Accepted After Revision: 29/11/2019
The aim of the present communication is to evaluate whether prediabetic state affects peri-implant health status? Several databases were searched from August 1975 up to August 2019 for studies that evaluated the clinical and radiographic peri-implant parameters including plaque index (PI), bleeding on probing (BOP), probing depth (PD), and crestal bone level (CBL) in patients with prediabetes and non-diabetes. The standard mean differences (SMD) of outcomes and 95% confidence intervals (CI) for each variable were calculated using random effect model. Quality assessment and risk of bias were estimated using ROBINS-I and GRADE. Six studies were included. An overall quality assessment showed low to moderate risk of bias. The overall weighted mean difference for PI (SMD=3.42, 95% CI= 0.67 to 6.17, P=0.015), BOP (SMD=6.69, 95% CI= 4.94 to 8.45, P<0.001), PD (SMD=7.77, 95% CI= 4.87 to 10.67, P<0.001) and CBL (SMD=6.87, 95% CI= 0.98 to 12.77, P=0.023) showed statistically significant differences between prediabetes and non-diabetic groups, respectively. The direction of recommendation emerging from this meta-analysis is strong in favour of prediabetes in the deterioration of peri-implant health compared to non-diabetic patients.
Prediabetic State; Peri-implantitis; Dental Implants; Systematic Review
Al-Aali K. A. Does Prediabetic State Affects Dental Implant Health? A Systematic Review and Meta-Analysis. Biosc.Biotech.Res.Comm. 2019;12(4).
Al-Aali K. A. Does Prediabetic State Affects Dental Implant Health? A Systematic Review and Meta-Analysis. Biosc.Biotech.Res.Comm. 2019;12(4). Available from: https://bit.ly/34qytNy
Copyright © Al-Aali This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
According to American Diabetes Association, type-2 diabetes mellitus (T2DM) is defined as impaired blood glucose levels that is associated with either due to deficiency of insulin or simply its altered function, (Association, 2018). Significant amount of research indicates that dental implant therapy could be a viable treatment option if patients maintain their serum glycated haemoglobin (HbA1c) levels, (Ormianer, Block, Matalon, & Kohen, 2018; Vissink, Spijkervet, & Raghoebar, 2018). However, modest amount of data exists that describes the associations between peri-implant health and moderate glycemic conditions including well-controlled diabetes mellitus and prediabetes.
Prediabetic state is described by the elevated blood glucose levels that are nonetheless below the threshold for apparent diabetes, (Association, 2010; Organization, 1999). Prediabetes may be recognized by either of the two conditions such as impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) which are extremely prevalent in the developed countries.(Atlas) Studies have suggested that patients with prediabetes show poor clinical periodontal parameters compared to the non-diabetic counterparts, (Javed et al., 2012; Javed et al., 2014 and Alasqah et al., 2018). This accounts with the persistent hyperglycemia in prediabetic state that establishes an imbalance between destructive periodontal pathogens and the host immune response,(Demmer et al., 2015) which further leads to the formation and accumulation of advanced glycation end-products (AGEs), proinflammatory cytokines, and dysfunction of polymorphonuclear neutrophils, thereby leading to the breakdown of supporting soft tissues and alveolar bone, ( Takeda et al., 2006, Andriankaja & Joshipura, 2014). Similar mechanisms may also be involved in peri-implant health, however there is no agreement on this subject.
Although a significant amount of implant therapy is performed in routine oral health practice, however, it is imperative to ascertain patients who are supposedly at greater risk of oral complications, including dental implant failure due to periimplant diseases, (Mombelli & Cionca, 2006 and Bornstein, Cionca, & Mombelli 2009). Reflecting the rising concerns regarding the high prevalence of prediabetes globally and the increasing amount of patients seeking dental implant treatment, the purpose of this meta-analysis was to gather and summarise all empirical evidence on the potential association between prediabetes and dental implant health and its complications. This systematic review presents the following null hypotheses. Firstly, no significant differences are observed in implant survival rate between patients with prediabetes and those who are non-diabetic. Secondly, no significant differences are observed between these groups with regards to clinical and radiographic peri-implant parameters including peri-implant probing depth (PD), bleeding on probing (BoP), or crestal bone level (CBL) around dental implants.
The aim of the present study was designed to answer the PECOS (Patients; Exposure; Comparators; Outcomes; Study design) question. The focused PECOS clinical question of the present study was: Does prediabetic state (exposure) affects peri-implant clinical parameters (outcomes) considering the outcomes were assessed in retrospective and/or prospective studies (study design)?
MATERIAL AND METHODS
Protocol and eligibility criteria
This systematic review and meta-analysis followed the guidelines described by PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis),(Moher, Liberati, Tetzlaff, Altman, & Group, 2009) and followed the outlines of PECOS,(Schardt, Adams, Owens, Keitz, & Fontelo, 2007). Cross-sectional data gathered in either retrospective, case-control or prospective study design were considered. Clinical or radiographic peri-implant parameters including PI, BOP, PD and CBL reported in human subjects with and without prediabetes (according to American Diabetes Association)(Chamberlain, Rhinehart, Shaefer, & Neuman, 2016) were included. In addition, the present study only considered studies in English language. Studies with missing data on both PD and CBL were excluded. Also, in-vitro, comprehensive reviews, experimental studies, abstracts, case-series were excluded.
Systematic literature search
Main databases (EMBASE, MEDLINE, COHGTR and CENTRAL) were searched between August, 1975 and August, 2019 using the following terms: ((Prediabetic state) OR (prediabetes) OR (impaired fasting glucose) AND ((peri-implant) OR (peri-implantitis) OR (peri-implant diseases) AND (plaque) OR (plaque scores) OR (plaque index) OR (bleeding on probing) OR (radiographs) OR (crestal bone loss) OR (marginal bone loss) OR (peri-implant bone loss). After reading the main titles and abstracts, their eligibility for inclusion in the study were judged. Once the complete list of included articles was gained, their complete texts were downloaded for subsequent data abstraction and assessment. Studies overlooked from electronic search database were subsequently manually searched in the following Web of Science journals including Clinical Implant Dentistry and Related Research, Clinical Oral Implants Research, and Acta Odontologica Scandinavica.
Published studies that satisfied the inclusion criteria were handled for data abstraction. The intent of the project was to comply with the standards set in the PRISMA guidelines. Following this, the details from the included articles were tabulated according to the study designs, level of evidence as described by the Oxford Centre for Evidence-Based Medicine (CEBM) (Howick et al., 2009), patient data, glycemic status, duration of prediabetes, covariates, parameters of peri-implant health, and final conclusions. Data was gathered and summarized according to the PECOS question.
Risk of bias in individual studies
This study evaluated the quality of included studies using the Risk Of Bias In Non-randomised Studies (ROBINS-I) assessment tool.(Sterne et al., 2016) Five important domains that estimates bias includes potential confounders, selection, classification, attrition and reporting bias are assessed in this tool. Each of the sections could be given one of the following ratings: ‘low risk’, ‘moderate risk’, ‘serious risk’, ‘critical risk’, or ‘no information’.
Risk of bias across studies using GRADE approach
Evaluation for the overall quality of evidence was conducted according to the Grades of Recommendation, Assessment, Development and Evaluation (GRADE).(Guyatt, Oxman, Schünemann, Tugwell, & Knottnerus, 2011) These are based on the following scores: ‘high quality’: we are very assertive that the real effect lies close to that of the estimate of the effect, ‘moderate quality’: we are moderately assertive in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different, ‘low quality’: our assurance in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect, and ‘very low quality’: we have very little assurance in the effect estimate: the true effect is likely to be markedly different from the estimate of effect.
Meta-analysis
Literature that reported data on clinical and radiographic peri-implant parameters were processed for data synthesis. I2 and χ2 statistics were applied for estimation of heterogeneity. Depending on the degree of heterogeneity, either random model or fixed models were used in case of heterogeneity being significant (I2 >50%) or being low (I2 ≤50%), respectively,(Borenstein, Hedges, Higgins, & Rothstein, 2010). P-value was set at 0.05 that represented statistical significance. Forest plots were generated explaining standard mean difference (SMD) of outcomes with 95% confidence intervals (CI). Ineligible data for synthesis were described comprehensively.
RESULTS AND DISCUSSION
Selection
Systematic literature search from different databases yielded a total of 66 potential records (break-up shown in Figure 1). After excluding 13 duplicate articles from the search, a total of 53 remained before full-text analysis. A further total of 43 study articles were removed that did not meet the selection criteria. A total of ten studies were included for full-text reading out of which 4 were excluded subsequently (reasons for exclusion described in Figure 1). Finally, 6 articles were included and processed for tabulation of data, (Abduljabbar, Al-Sahaly, Al-Kathami, Afzal, & Vohra, 2017; Al‐Sowygh, Ghani, Sergis, Vohra, & Akram, 2018; Al Amri, Abduljabbar, Al‐Kheraif, Romanos, & Javed, 2017; Alrabiah et al., 2018; Alrabiah et al., 2019; Mokeem et al., 2019).
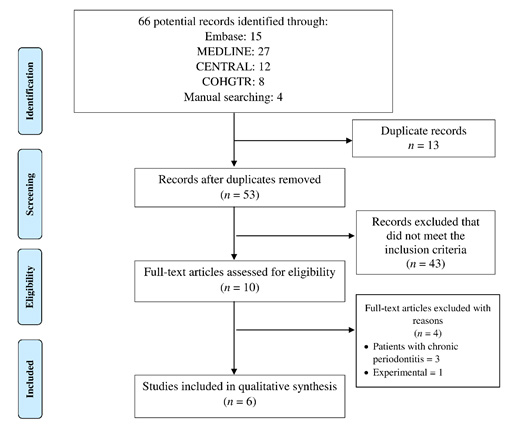 |
Figure 1: PRISMA study flow chart |
General description of the included studies
Table 1 describes the general features of the included studies. Out of a total of six studies, three studies were retrospective,(Al‐Sowygh et al., 2018; Alrabiah et al., 2019; Mokeem et al., 2019). Two studies were case-control,(Abduljabbar et al., 2017; Alrabiah et al., 2018), while one study had a prospective design, (Al Amri et al., 2017). The level of evidence according to CEMB showed four studies had level ‘2b’(Al‐Sowygh et al., 2018; Al Amri et al., 2017; Alrabiah et al., 2019; Mokeem et al., 2019) and two studies had level 3b, (Abduljabbar et al., 2017; Alrabiah et al., 2018).
Table 1. Description of the included studies in chronological order.
| Author et al; Year; Journal name | Study design/Level of evidence* | Demographics
(Total number of patients/implants; mean age in years; male/female ratio; mean HbA1c levels) |
Investigation of glycemic status – duration of prediabetes | Confounding factors adjusted (Yes/No) | Peri-implant evaluation | Final outcome |
| Alrabiah et al.22, 2019; Clinical Implant Dentistry and Related Research | Retrospective/2b | Prediabetes: 39/78; 54.3; 39/0; 6.1%
Non-diabetes: 40/80; 51.2; 40/0; 4.1% |
Serum HbA1c analysis – 5.4 years | No | Plaque index
Bleeding on probing Probing depth Crestal bone loss |
Peri-implant tissue inflammation and crestal bone loss are worse around dental implants in prediabetic patients compared with non-diabetic individuals. |
| Alrabiah et al.23, 2018; Clinical Implant Dentistry and Related Research | Case-control/3b | Prediabetes: 30/42; 52.5; 14/16; 6.1%
Non-diabetes: 30/39; 54.1; 15/15; 4.7% |
Serum HbA1c analysis – 2.7 years | No | Peri-implant crevicular AGE levels
Plaque index Bleeding on probing Probing depth Crestal bone loss |
Clinical and radiographic peri-implant parameters were worse and levels of AGEs were increased in patients with prediabetes compared with non-diabetic individuals. |
| Abduljabbar et al.24, 2017; Acta Odontologica Scandinavica | Case-control/3b | Prediabetes: 45/45; 53.4; NA; 6.1%
Non-diabetes: 42/42; 51.1; NA; 4.5% |
Serum HbA1c analysis – 1.9 years | Yes (periodontal disease) | Plaque index
Bleeding on probing Probing depth Crestal bone loss Number of missing teeth |
Periodontal and peri-implant parameters were worse among patients with prediabetes compared with non-diabetic controls. |
| Mokeem et al.25, 2019; Clinical Implant Dentistry and Related Research | Retrospective/2b | Prediabetes: 22/35; 51.4; 13/9; 6.0%
Non-diabetes: 25/32; 46.2; 17/8; 4.6% |
Serum HbA1c analysis – 3.1 years | Yes (HbA1c, total cholesterol, triglycerides, body mass index) | Plaque index
Bleeding on probing Probing depth Crestal bone loss |
Clinical and radiographic peri-implant parameters were worse in patients with prediabetes compared with non-diabetic individuals. |
| Al Amri et al.26, 2017; Clinical Oral Implants Research | Prospective/2b | Prediabetes: 12/NA; 44.5; 12/0; 6.1%
Non-diabetes: 12/NA; 43.3; 12/0; 4.4% |
Serum HbA1c analysis – NA | No | Plaque index
Bleeding on probing Probing depth HbA1c |
Dental implants inserted in prediabetic and healthy patients have similar success rates and remain clinically and radiographically stable after 1-year follow-up. |
| Al-Sowygh et al.27, 2018; Clinical Implant Dentistry and Related Research | Retrospective/2b | Prediabetes: 25/36; 51.5; 13/12; 6.7%
Non-diabetes: 26/42; 50.1; 13/13; 5.8% |
Serum HbA1c analysis – 10.7 years | No | Peri-implant crevicular AGE levels
Plaque index Bleeding on probing Probing depth Crestal bone loss |
Clinical and radiographic peri-implant parameters were worse and levels of AGEs were increased in patients with prediabetes compared with non-diabetic individuals. |
AGE; advanced glycation end-products, HbA1c; glycated haemoglobin A1c, NA; not available
* Level of evidence of the included studies estimated using Oxford Centre for Evidence-Based Medicine (CEBM).
A total of 173 patients with prediabetes and 175 non-diabetic individuals were included in the studies. The mean age of the patients ranged from 43.3 years to 54.3 years. Percentage of male patients was higher than the females. The total number of implants placed in prediabetic patients were 236, while a total of 235 dental implants were studied in non-diabetic subjects. Mean HbA1c levels in the included data ranged from 6.0% to 6.7%. All investigations related to glycemic status were investigated in serum using HbA1c analyser kits. The overall duration of prediabetic state ranged from 1.9 years to 10.7 years. Only two studies adjusted covariates including periodontal diseases, HbA1c range, total cholesterol, triglycerides, and body mass index, (Abduljabbar et al., 2017; Mokeem et al., 2019).
All studies estimated peri-implant parameters by recording peri-implant PI, BOP, PD and CBL. Two studies, in addition to these parameters, estimated peri-implant crevicular fluid levels of AGEs among their patients, (Al‐Sowygh et al., 2018; Alrabiah et al., 2018).
Clinical data
Data reporting all clinical peri-implant parameters are shown in Table 2. Some studies reported data based on overall mean with standard deviations (SD), (Abduljabbar et al., 2017; Al Amri et al., 2017; Alrabiah et al., 2019). However, other studies reported data as means with range, (Al‐Sowygh et al., 2018; Alrabiah et al., 2018; Mokeem et al., 2019). Plaque index and BOP were reported in percentage mean that ranged from 21.6% to 46.7% and 18.2% to 48.2% in patients with prediabetes, while PI and BOP ranged from 10.6% to 24.4% and 10.8% to 22.6% in patients with prediabetes, respectively. Probing depths ranged from 4.6 mm to 2.2 mm in the prediabetes, while they ranged from 1.3 mm to 2.7 mm in the non-diabetic group, respectively. Crestal bone levels in the prediabetic and non-diabetic ranged from 5.3 mm 1.7 mm and 2.3 mm to 0.7 mm, respectively. Only one study did not report CBL,(Al Amri et al., 2017).
Table 2: Clinical and radiographic peri-implant data of the included studies.
| Author et al. | Plaque index (%) | Bleeding on probing (%) | Probing depth (mm) | Crestal bone levels (mm) |
| Alrabiah et al.22 | Prediabetes: 46.7 ± 4.4
Non-diabetes: 24.4 ± 4.7 |
Prediabetes: 48.2 ± 3.7
Non-diabetes: 22.6 ± 2.4 |
Prediabetes: 4.6 ± 0.2
Non-diabetes: 2.2 ± 0.3 |
Prediabetes:
Mesial – 5.2 ± 0.4 Distal – 5.3 ± 0.2 Non-diabetes: Mesial – 2.3 ± 0.1 Distal – 2.3 ± 0.1 |
| Alrabiah et al.23 | Prediabetes: 22.3 (16.2-25.9)
Non-diabetes: 10.6 (6.4-14.8) |
Prediabetes: 24.7 (16.1-29.8)
Non-diabetes: 13.6 (5.5-15.2) |
Prediabetes: 2.7 (2.1-3.5)
Non-diabetes: 1.3 (0.8-1.9) |
Prediabetes: 2.1 (1.3-3.0)
Non-diabetes: 0.7 (0-1.2) |
| Abduljabbar et al.24 | Prediabetes: 35.5 ± 4.5
Non-diabetes: 19.2 ± 1.5 |
Prediabetes: 36.4 ± 4.1
Non-diabetes: 15.2 ± 0.8 |
Prediabetes: 4.0 ± 0.4
Non-diabetes: 2.1 ± 0.1 |
Prediabetes: 3.4 ± 0.6
Non-diabetes: 1.6 ± 0.2 |
| Mokeem et al.25 | Prediabetes: 24.6 (19.3-29.6)
Non-diabetes: 11.4 (6.4-14.8) |
Prediabetes: 24.7 (16.1-29.8)
Non-diabetes: 13.6 (5.5-15.2) |
Prediabetes: 2.2 (2.0-3.1)
Non-diabetes: 1.8 (0.7-2.1) |
Prediabetes: 1.9 (1.1-2.8)
Non-diabetes: 0.8 (0-1.3) |
| Al Amri et al.26 | Prediabetes: 27.3 ± 7.7
Non-diabetes: 23.2 ± 2.5 |
Prediabetes: 20.1 ± 2.5
Non-diabetes: 11.2 ± 0.4 |
Prediabetes: 5.1 ± 1.4†
Non-diabetes: 2.7 ± 0.5† |
NA |
| Al-Sowygh et al.27 | Prediabetes: 21.6 (14.5-24.7)
Non-diabetes: 12.3 (7.6-15.9) |
Prediabetes: 18.2 (11.4-26.7)
Non-diabetes: 10.8 (6.0-13.1) |
Prediabetes: 2.6 (2.0-2.9)
Non-diabetes: 1.4 (0.7-2.1) |
Prediabetes: 1.7 (1.5-3.1)
Non-diabetes: 0.8 (0-1.1) |
Data represented in percentage of ≥4mm, NA; not available
Quality and evidence profile according to GRADE
Quality assessment of the studies is presented in Table 3 according to ROBINS-I tool,(Sterne et al., 2016). An overall quality assessment showed low to moderate risk of bias, which in the majority of the studies originated from the presence of bias and other covariates in the studies and bias in selection of the reported outcomes. Table 4 demonstrates an overall summary of the various factors used to rate the quality of evidence and strength of recommendations according to GRADE,(Guyatt et al., 2011). Altogether, the strength of approval based on the body of the evidence developing from this study is characterized to be moderate. Given that the effect is large for prediabetic state, the direction of recommendation and suggestions emerging from this meta-analysis is strong in favour of prediabetes in the deterioration of peri-implant health.
Table 3: Risk of bias in non-randomised studies – of interventions (ROBINS-I) tool.
| Author et al. | Bias due to confounding | Bias in selection of participants into the study | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall bias |
| Alrabiah et al.22 | Moderate | Low | Low | Low | Moderate | Moderate |
| Alrabiah et al.23 | Moderate | Low | Low | Low | Moderate | Moderate |
| Abduljabbar et al.24 | Low | Low | Low | Low | Moderate | Low |
| Mokeem et al.25 | Low | Low | Low | Low | Moderate | Low |
| Al Amri et al.26 | Serious | Low | Moderate | Low | Moderate | Moderate |
| Al-Sowygh et al.27 | Moderate | Low | Low | Low | Moderate | Moderate |
Table 4: Summary of findings table on body of the estimated evidence profile (GRADE, 2015) and appraisal of the strength of the recommendation regarding the impact of prediabetes on clinical peri-implant parameters.
| Determinants of quality | Prediabetes |
| Study design | Cross-sectional nature |
| Number of studies n = 6 (Figure 1)
Comparison n = 6 |
6 |
| Risk of bias | Moderate |
| Consistency (Figure 2 and 3) | Consistent |
| Directness | Generalizable |
| Precision | Rather not precise |
| Publication bias (Appendices S1 and S2) | Not for aPDT but for LI |
| Magnitude of the effect | Large |
| Strength of the recommendation based on the body of evidence | Moderate |
| Direction of recommendation | Strong in favour of prediabetes |
Final outcomes and meta-analysis
Based on the qualitative assessment and final conclusions described in the included studies, it was observed that patients with prediabetes show worse clinical and radiographic peri-implant parameters compared with non-diabetic subjects, (Abduljabbar et al., 2017; Al‐Sowygh et al., 2018; Al Amri et al., 2017; Alrabiah et al., 2018; Alrabiah et al., 2019; Mokeem et al., 2019).
Quantitative data in the form of meta-analyses for each variable was conducted. Only those studies presenting data in the form of overall means and SD were included in the meta-analysis. A total of three studies for PI, BOP and PD reported data in range values and not SD,(Al‐Sowygh et al., 2018; Alrabiah et al., 2018; Mokeem et al., 2019). In addition, one study reported their outcomes of PD in percentage of ≥4mm(Al Amri et al., 2017). (Table 2). Therefore, these studies were not considered for meta-analysis and excluded.
Significant heterogeneity was observed for all the parameters including PI, BOP, PD and CBL, therefore a random effect model was used. Considering the effects of prediabetes, significant heterogeneity for PI (χ2=65.26, P<0.0001, I2 =96.94%), BOP (χ2=10.28, P=0.0058, I2 =80.55%), PD (χ2=9.86, P=0.0017, I2 =89.86%) and CBL (χ2=44.31, P<0.0001, I2 =97.74%) was noticed between both the groups. The overall weighted mean difference for PI (SMD=3.42, 95% CI= 0.67 to 6.17, P=0.015, Figure 2A), BOP (SMD=6.69, 95% CI= 4.94 to 8.45, P<0.001, Figure 2B), PD (SMD=7.77, 95% CI= 4.87 to 10.67, P<0.001, Figure 2C) and CBL (SMD=6.87, 95% CI= 0.98 to 12.77, P=0.023, Figure 2D) showed statistically significant differences between prediabetes and non-diabetic groups, respectively.
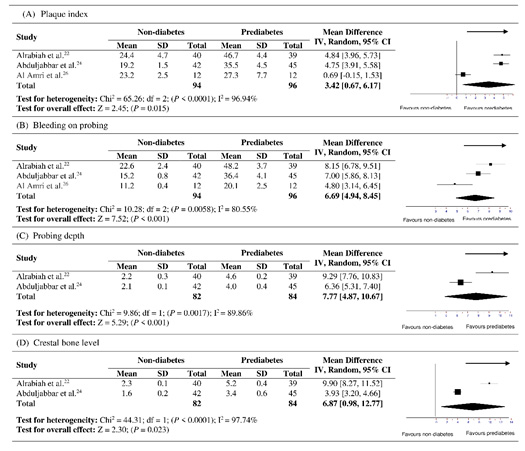 |
Figure 2: Forest plots showing overall effect of prediabetes on periimplant parameters including (A) plaque index, (B) bleeding on probing, (C) probing depth and (D) crestal bone level. |
The present systematic review and meta-analysis was based on the hypothesis that no significant differences are observed in implant survival rate between patients with prediabetes and those who are non-diabetic and no significant differences are observed between these groups with regards to clinical and radiographic peri-implant parameters including peri-implant clinical and radiographic status around dental implants. The null hypothesis was rejected, and all clinical studies showed worse peri-implant inflammatory parameters around dental implants placed in patients with prediabetes compared with non-diabetic controls.
It is well-known that constant hyperglycemia and elevated blood glucose levels lead to non-enzymatic glycosylation of several serum proteins that subsequently leads to the formation and accumulation of AGEs in the body tissues, ( Katz et al., 2005; Joseph Katz, Yoon, Mao, Lamont, & Caudle, 2007). This constant build up also leads in the production of several proinflammatory cytokines that are responsible in the destruction of soft and hard tissues of periodontal and peri-implant structures, (Pertyńska-Marczewska et al., 2004 and Akram, Alqahtani, Alqahtani, Al‐Kheraif, & Javed, 2019). There is impairment in the blood glucose levels in prediabetic state which may consequently lead to the similar proinflammatory destructive pathways. In the present systematic review, it was observed that scores of PD, CBL and peri-implant inflammation were higher in patients with prediabetes compared with non-diabetic individuals. The reason for these increased scores may be associated with raised levels of AGEs as described in the two clinical studies, (Al‐Sowygh et al., 2018; Alrabiah et al., 2018).
Nevertheless, the outcomes of this meta-analysis should be interpreted with caution due to several important factors that may not be found in the included studies and these factors have a profound effect on peri-implant health. For instance, it is well-known that cement retained prostheses showed less CBL as compared to screw-retained, (Lemos, de Souza Batista, et al., 2016). Short dental implants have generally higher risk of failure as proved in a recent meta-analysis, (Lemos, Ferro-Alves, Okamoto, Mendonça, & Pellizzer, 2016). The included studies also described a variation in the mean duration of implants in service. These important factors, yet significant should be considered while giving a definitive conclusion.
Recent data suggests that obesity have a significant impact on the overall survival of dental implants, ( Alkhudhairy, Vohra, Al‐Kheraif, & Akram, 2018 and Alasqah et al., 2019;). These studies demonstrate that chronic systemic inflammation as seen in obesity may show worse peri-implant inflammatory scores compared to non-obese counterparts. In a recent case-control investigation by Vohra et al. revealed that elevated levels of serum c-reactive proteins may lead to worse peri-implant inflammation in different categories of obese individuals depending on their body mass index (BMI), (Vohra, Alkhudhairy, Al‐Kheraif, Akram, & Javed, 2018). It is noted that some of the studies did not report the overall anthropometric assessments including BMI, waist circumference, or even total fat mass. This important missing parameter may have also skewed the results.
The present meta-analysis contains some important limitations that should not be overlooked. For instance, most of the study designs were retrospective. Their questionnaire data (as described in their own studies) reports assessments that relied on recall abilities of the patients. For this reason, due to the inclusion of retrospective cohort data in this meta-analysis does not solely determine causation. Furthermore, the inclusion of studies from the same author groups, (Alrabiah et al., 2018; Alrabiah et al., 2019) and Abduljabbar et al., (2017) may have produced significant bias. Although calibration was performed for clinical measurements, but accuracy of measurements was not calibrated in radiographic assessments which might contribute to geometric errors on the conventional radiographs in the included studies. Moreover, the limited number of studies included does not actually help to translate the impact of prediabetes on peri-implant health. Further well-designed prospective studies with well-designed methods and control of systemic and other local factors should be undertaken in order to establish better understanding and strong conclusions regarding prediabetes and peri-implant inflammation. Within the limitations and the direction of recommendation emerging from this meta-analysis that proves to be strong in favour of prediabetes in the deterioration of peri-implant health compared to non-diabetic patients.
Funding
This research did not receive any funding. Competing interests: None declared
Ethical approval
Not applicable Patient consent: Not applicable.
REFERENCES
Abduljabbar, T., Al-Sahaly, F., Al-Kathami, M., Afzal, S., & Vohra, F. (2017). Comparison of periodontal and peri-implant inflammatory parameters among patients with prediabetes, type 2 diabetes mellitus and non-diabetic controls. Acta odontologica scandinavica, 75(5), 319-324.
Akram, Z., Alqahtani, F., Alqahtani, M., Al‐Kheraif, A. A., & Javed, F. (2019). Levels of advanced glycation end products in gingival crevicular fluid of chronic periodontitis patients with and without type‐2 diabetes mellitus. Journal of periodontology.
Al‐Sowygh, Z. H., Ghani, S. M. A., Sergis, K., Vohra, F., & Akram, Z. (2018). Peri‐implant conditions and levels of advanced glycation end products among patients with different glycemic control. Clinical implant dentistry and related research, 20(3), 345-351.
Al Amri, M. D., Abduljabbar, T. S., Al‐Kheraif, A. A., Romanos, G. E., & Javed, F. (2017). Comparison of clinical and radiographic status around dental implants placed in patients with and without prediabetes: 1‐year follow‐up outcomes. Clinical oral implants research, 28(2), 231-235.
Alasqah, M., Mokeem, S., Alrahlah, A., Al-Hamoudi, N., Abduljabbar, T., Akram, Z., . . . Javed, F. (2018). Periodontal parameters in prediabetes, type 2 diabetes mellitus, and non-diabetic patients. Brazilian oral research, 32.
Alasqah, M. N., Al‐Shibani, N., Al‐Aali, K. A., Qutub, O. A., Abduljabbar, T., & Akram, Z. (2019). Clinical indices and local levels of inflammatory biomarkers in per‐implant health of obese and nonobese individuals. Clinical implant dentistry and related research, 21(1), 80-84.
Alkhudhairy, F., Vohra, F., Al‐Kheraif, A. A., & Akram, Z. (2018). Comparison of clinical and radiographic peri‐implant parameters among obese and non‐obese patients: A 5‐year study. Clinical implant dentistry and related research, 20(5), 756-762.
Alrabiah, M., Al‐Aali, K. A., Al‐Sowygh, Z. H., Binmahfooz, A. M., Mokeem, S. A., & Abduljabbar, T. (2018). Association of advanced glycation end products with peri‐implant inflammation in prediabetes and type 2 diabetes mellitus patients. Clinical implant dentistry and related research, 20(4), 535-540.
Alrabiah, M., Alrahlah, A., Al‐Hamdan, R. S., Al‐Aali, K. A., Labban, N., & Abduljabbar, T. (2019). Survival of adjacent‐dental‐implants in prediabetic and systemically healthy subjects at 5‐years follow‐up. Clinical implant dentistry and related research, 21(2), 232-237.
Andriankaja, O. M., & Joshipura, K. (2014). Potential association between prediabetic conditions and gingival and/or periodontal inflammation. Journal of diabetes investigation, 5(1), 108-114.
Association, A. D. (2010). Diagnosis and classification of diabetes mellitus. Diabetes Care, 33(Supplement 1), S62-S69.
Association, A. D. (2018). 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care, 41(Supplement 1), S13-S27.
Atlas, D. International Diabetes Federation. IDF Diabetes Atlas, 7th edn. Brussels, Belgium: International Diabetes Federation, 2015. In.
Borenstein, M., Hedges, L. V., Higgins, J. P., & Rothstein, H. R. (2010). A basic introduction to fixed‐effect and random‐effects models for meta‐analysis. Research synthesis methods, 1(2), 97-111.
Bornstein, M. M., Cionca, N., & Mombelli, A. (2009). Systemic conditions and treatments as risks for implant therapy. Int J Oral Maxillofac Implants, 24(Suppl), 12-27.
Chamberlain, J. J., Rhinehart, A. S., Shaefer, C. F., & Neuman, A. (2016). Diagnosis and management of diabetes: synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Annals of internal medicine, 164(8), 542-552.
Demmer, R. T., Jacobs Jr, D. R., Singh, R., Zuk, A., Rosenbaum, M., Papapanou, P., & Desvarieux, M. (2015). Periodontal bacteria and prediabetes prevalence in ORIGINS: the oral infections, glucose intolerance, and insulin resistance study. Journal of dental research, 94(9_suppl), 201S-211S.
Guyatt, G. H., Oxman, A. D., Schünemann, H. J., Tugwell, P., & Knottnerus, A. (2011). GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of clinical epidemiology, 64(4), 380-382.
Howick, J., Phillips, B., Ball, C., Sackett, D., Badenoch, D., Straus, S., & Dawes, M. (2009). Oxford Centre for Evidence-based Medicine—levels of evidence (March 2009). Centre for Evidence Based Medicine.
Javed, F., Al-Askar, M., Al-Rasheed, A., Al-Hezaimi, K., Babay, N., & Galindo-Moreno, P. (2012). Comparison of self-perceived oral health, periodontal inflammatory conditions and socioeconomic status in individuals with and without prediabetes. The American journal of the medical sciences, 344(2), 100-104.
Javed, F., Thafeed AlGhamdi, A. S., Mikami, T., Mehmood, A., Ahmed, H. B., Samaranayake, L. P., & Tenenbaum, H. C. (2014). Effect of glycemic control on self‐perceived oral health, periodontal parameters, and alveolar bone loss among patients with prediabetes. Journal of periodontology, 85(2), 234-241.
Katz, J., Bhattacharyya, I., Farkhondeh‐Kish, F., Perez, F., Caudle, R., & Heft, M. (2005). Expression of the receptor of advanced glycation end products in gingival tissues of type 2 diabetes patients with chronic periodontal disease: a study utilizing immunohistochemistry and RT‐PCR. Journal of clinical periodontology, 32(1), 40-44.
Katz, J., Yoon, T. Y., Mao, S., Lamont, R. J., & Caudle, R. M. (2007). Expression of the receptor of advanced glycation end products in the gingival tissue of smokers with generalized periodontal disease and after nornicotine induction in primary gingival epithelial cells. Journal of periodontology, 78(4), 736-741.
Lemos, C. A. A., de Souza Batista, V. E., de Faria Almeida, D. A., Júnior, J. F. S., Verri, F. R., & Pellizzer, E. P. (2016). Evaluation of cement-retained versus screw-retained implant-supported restorations for marginal bone loss: A systematic review and meta-analysis. The Journal of prosthetic dentistry, 115(4), 419-427.
Lemos, C. A. A., Ferro-Alves, M. L., Okamoto, R., Mendonça, M. R., & Pellizzer, E. P. (2016). Short dental implants versus standard dental implants placed in the posterior jaws: A systematic review and meta-analysis. Journal of dentistry, 47, 8-17.
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & Group, P. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine, 6(7), e1000097.
Mokeem, S., Alfadda, S. A., Al‐Shibani, N., Alrabiah, M., Al‐Hamdan, R. S., Vohra, F., & Abduljabbar, T. (2019). Clinical and radiographic peri‐implant variables around short dental implants in type 2 diabetic, prediabetic, and non‐diabetic patients. Clinical implant dentistry and related research, 21(1), 60-65.
Mombelli, A., & Cionca, N. (2006). Systemic diseases affecting osseointegration therapy. Clinical oral implants research, 17(S2), 97-103.
Organization, W. H. (1999). Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1, Diagnosis and classification of diabetes mellitus. Retrieved from
Ormianer, Z., Block, J., Matalon, S., & Kohen, J. (2018). The Effect of Moderately Controlled Type 2 Diabetes on Dental Implant Survival and Peri-implant Bone Loss: A Long-Term Retrospective Study. International Journal of Oral & Maxillofacial Implants, 33(2).
Pertyńska-Marczewska, M., Kiriakidis, S., Wait, R., Beech, J., Feldmann, M., & Paleolog, E. M. (2004). Advanced glycation end products upregulate angiogenic and pro-inflammatory cytokine production in human monocyte/macrophages. Cytokine, 28(1), 35-47.
Schardt, C., Adams, M. B., Owens, T., Keitz, S., & Fontelo, P. (2007). Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC medical informatics and decision making, 7(1), 16.
Sterne, J. A., Hernán, M. A., Reeves, B. C., Savović, J., Berkman, N. D., Viswanathan, M., Boutron, I. (2016). ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Bmj, 355, i4919.
Takeda, M., Ojima, M., Yoshioka, H., Inaba, H., Kogo, M., Shizukuishi, S., . . . Amano, A. (2006). Relationship of serum advanced glycation end products with deterioration of periodontitis in type 2 diabetes patients. Journal of periodontology, 77(1), 15-20.
Vissink, A., Spijkervet, F., & Raghoebar, G. (2018). The medically compromised patient: Are dental implants a feasible option? Oral diseases, 24(1-2), 253-260.
Vohra, F., Alkhudhairy, F., Al‐Kheraif, A. A., Akram, Z., & Javed, F. (2018). Peri‐implant parameters and C‐reactive protein levels among patients with different obesity levels. Clinical implant dentistry and related research, 20(2), 130-136.


