1Department of Zoology, N. S. S. College, Nemmara, Palakkad, Kerala, India
2Division of Molecular Biology, Department of Zoology, University of Calicut, Kerala, India
Corresponding author email: sreedevisd@gmail.com
Article Publishing History
Received: 29/10/2020
Accepted After Revision: 23/12/2020
Mangroves are coastal ecosystems, found in tropical and subtropical regions around the world. Life in mangroves requires special adaptations to survive due to high moisture, high salinity, and hypoxia conditions which breed many kinds of novel organisms. Such complex ecosystems harbour diverse groups of microorganisms including bacteria, actinomycetes, fungi etc. The present study focuses on the distribution and hydrolytic enzyme potential of culturable bacteria from mangroves of Northern Kerala with respect to changes in seasonal and physico chemical characteristics. Sediment samples were collected from 8 locations in 5 districts along the northern coast of Kerala, during monsoon and post monsoon seasons of 2018-19. pH of the sediments varied between 6.4 – 7, temperature between 20-29°C and organic matter between 0.2%± SD to 4.1%± SD.
The colony forming unit per gram (CFU/g) of bacterial isolates showed a radical variation between the seasons. 66% of the isolates showed various hydrolytic enzyme activities during monsoon while 87% in post monsoon. The current study demonstrates the seasonal variation in microbial count and their enzyme activity related to a seasonal shift in availability of substrates. Monsoon season shows increase in number and were as post monsoon shows the hydrolytic potential of bacteria. Mangroves provide a rich resource for the discovery of potential bacterial species capable to produce various extracellular enzymes that could be used for human life, agriculture, industry and bioremediation etc. The study shows the influence of rainfall in distribution and bioactivity of bacteria in the unique mangrove ecosystem.
Mangrove, Bacteria, Population, Hydrolytic Potential, Organic Matter
Paul T, Sebastian C. D, Kutty S. N. Distribution and Hydrolytic Potential of Bacteria During Monsoon and Post Monsoon Seasons in the Mangrove Sediments of North Kerala. Biosc.Biotech.Res.Comm. 2020;13(4).
Paul T, Sebastian C. D, Kutty S. N. Distribution and Hydrolytic Potential of Bacteria During Monsoon and Post Monsoon Seasons in the Mangrove Sediments of North Kerala. Biosc.Biotech.Res.Comm. 2020;13(4). Available from: https://bit.ly/35N0Au3
Copyright © Paul et al., This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Mangroves are amongst the most productive marine ecosystems on earth, providing a habitat for many species and key goods and services for human beings. Mangroves, sea grass meadows and salt marshes collectively termed “Blue forests” are counted among the most vulnerable and productive coastal ecosystems on the planet. Mangrove forests evolved where two most contrasting environments meet – the interface between the land and sea. Mangroves acts as a natural barrier against sea level rise and coastal flooding, apart from providing numerous other ecosystem services such as carbon sequestration, climate regulation (Brander et al., 2012; Barbier, 2016; Himes et al., 2018).
Kerala was once blessed with these amazing ecosystems covering about 700 km2 till 1957, but it is now in a drastically declined state reducing to less than 17 km2. Despite the breadth and quantity of services that mangrove ecosystems provide, that are being degraded at an alarming rate, the continuous threat is from the consequences of rapid urbanization and population change. India contains nearly 3.3 percent of this mangrove habitat (MEA, 2005; Tallis et al., 2012; Ghosh, 2019).
Sediment microorganisms play important roles in the mangrove ecosystem and make essential contributions to its productivity. Bacteria create mutualistic relationships with mangrove flora. The bacteria provide services such as N-fixation while the mangroves trees provide root exudates, stimulating microbial growth activity. There is also competition among the microorganisms because of the limited amount of nutrients available in mangroves. All of these things together make mangrove microbes highly efficient nutrient cyclers. Bacteria plays important role in nitrogen fixation, sulphate reduction, phosphate solubilization and methanogenesis (Liang et al., 2007; Ghosh, 2019).
Decomposition of mangrove vegetation is carried out by organisms such as crabs, fungi, bacteria, protozoa, and microalgae. Crabs relocate and macerate the fallen leaves while the other microorganisms decompose the leftover material through the use of enzymes such as cellulase, pectinase, protease, and amylase. Most of soil organic matter is derived from the vegetation that is partly disposed on the soil surface as an organic layer (litter) and partly distributed into the soil (Klein, 2000; Regina and Tarazona, 2001; Liang et al., 2007). The main factors that control the organic matter transformation process are: the quantity and quality of litter material components, the physical and chemical environment, and the decomposition organisms (Swift et al., 1979). Among the soil organisms, the bacteria and fungi present the highest values of biomass and respiratory metabolism, and have greater participation in the organic matter decomposition process (Persson et al., 1980; Liang et al., 2007).
Bacteria represent the major group, responsible for 25 to 30% of the total soil microbial biomass. Increase in the bacterial community during the summer have been attributed to increase in air temperature (Chhonkar and Tarafdar, 1984); however, decreases fungi and increases on bacteria relative quantities, respectively, were observed with increasing soil fertility. The soil microbial enzyme activity is affected by edaphic and climatic factors (Jha et al., 1992; Pennanen et al., 1999). Microbes constitute the largest pool of metabolic pathways on earth with potential biotechnological and environmental implications. In 1988, Alongi reported that in tropical mangroves, 91% of the total microbial biomass is bacteria and fungi, another 7% is algae, and 2% is protozoa. Microbial diversity of mangrove ecosystems provides information on their ecological role and unique biotechnological potential in the field of agriculture, industry, medicine, and pharmaceuticals (Lageiro et al., 2007).
In India, particularly in Kerala, there is lack of studies about the mangrove microbes, despite its vital role in this peculiar ecosystem. The present study attempts to investigate the distribution and hydrolytic enzyme potential of culturable bacterial isolates from the mangroves of Northern Kerala during the monsoon and post monsoon seasons of 2018-19. Currently Kannur and Kasaragod districts in Kerala are having the maximum coverage of mangroves (Mohandas et al., 2014).
MATERIAL AND METHODS
Sediment samples collected from the mangroves of the 5 districts along North Kerala coast from 8 sites (Fig. 1), Chandragiri (KGD) – 12°05’32” N 75°13’39” E, Edat (EDT) – 12°05’32” N 75°13’39” E, Pazhayangadi (PYD )- 12°02’72” N 75°29’31” E, Valapattanam (VPT) – 11°93’45” N 75°35’35” E, Elathur (ELR) – 11°19’43” N 75°45’2” E, Kadalundi (KDI) – 11°07’43” N 75°49’48” E, Ponnani (PON) – 10°47’1” N 75°55’3” E and Chettuva (CTV) – 11°1’41” N 75°52’6” E.
Figure 1: Map of Kerala showing the sampling sites of mangrove sediment
Scale bar 1mm = 1 Km
Samples were collected from same spot during two periods of the year viz., monsoon (June – September, 2018) and post monsoon (November – February, 2018-19). Sub surface (0-15 cm) samples (approximately 10-20 gm) were collected using hand core method and transferred aseptically into sterile polythene bags, transported in ice boxes and processed within 4 hrs of collection. A total of five sub-samples collected from each location and pooled for the analysis. A fraction of the sediment sample was stored at –20°C for biochemical analysis. Temperature and pH of the sediment were measured during the time of collection using portable digital pH meter and mercury thermometer respectively. Organic matter was analyzed in triplicates using modified Walkley and Black method. The collected soil samples were serial diluted (10-1 to 10-3) and plated to nutrient agar medium employing spread plate method. The plates incubated at 28 ± 2° C for 24 hours. The developed colonies were purified and transferred to nutrient agar slants for further analysis (Trivedi, 1986).
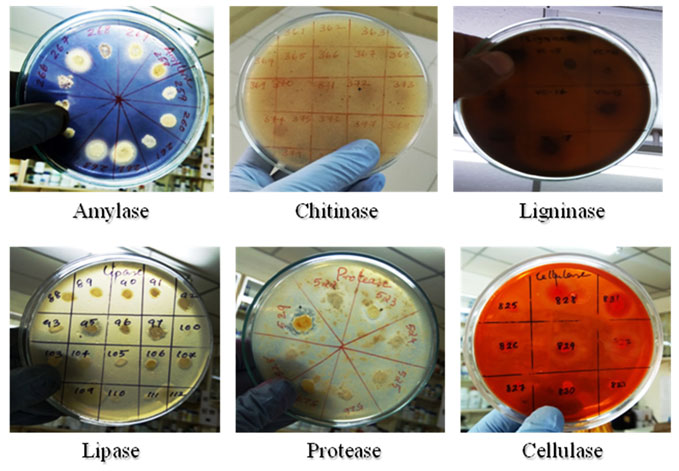
The bacterial isolates were screened for their capacity for the production of hydrolytic enzymes viz. protease, amylase, lipase, cellulase, ligninase, DNase and laccase as per the standard methods. Nutrient agar supplemented with casein (2%), starch (1%) and tributyrin (1%) were used for detection of protease, amylase and lipase respectively. DNase agar, Cellulase agar, agar supplemented with 0.01% guiacol and Crawford’s agar supplemented with 0.5% tannic acid were used for the detection of DNase, cellulose, laccase and ligninase activity respectively. The plates were spot inoculated and incubated at 28 ± 2°C overnight. Formation of clearance/halo zone or brown colour around the colonies indicates positive result.
RESULTS AND DISCUSSION
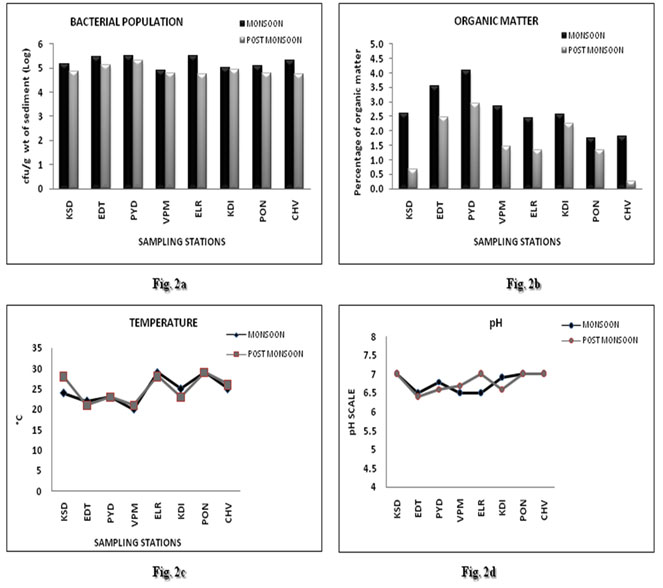
A total of 253 bacterial isolates were obtained, studied and stored during the collection period in which 153 isolates were obtained during monsoon and 100 isolates during post monsoon. The colony forming units per gram (CFU/g) of the diluted sediment sample from each of the duplicate plates were calculated and the average number was taken into consideration. The CFU/g of bacteria in mangrove sediments showed significant difference between the sampling periods (Fig. 2a). During monsoon, maximum CFU/g was found in the mangrove sediments from Pazhayangadi (310000) while those from Valapattanam showed the minimum CFU/g (78000). The trend was slightly different during post monsoon period where the sediments from Pazhayangadi (208000) showed high while those of Chettuva showed low bacterial count (54000). In all mangrove stations CFU/g decreased from monsoon to post monsoon (Vidya and Sebastian, 2020).
The physico chemical parameters of the sediments like pH and temperature of the mangrove sediments were recorded from sampling sites during the time of collection. The organic matter content was determined later in the laboratory. The total organic matter content in the sediment varied between 0.2% ± SD to 4.1% ± SD (Fig. 2b), temperature between 20-29 °C (Fig. 2c) and pH between 6.4 -7 (Fig. 2d), during the period of study. Organic matter content was high in Pazhayangadi both in monsoon (4.1% ± SD) and post monsoon (3% ± SD) and low in Ponnani (1.7% ± SD) during monsoon. But, in post monsoon, Chettuva (0.2% ± SD) showed lower values. Generally, the organic matter was found to higher in the monsoon than in post monsoon (Vidya and Sebastian, 2020).
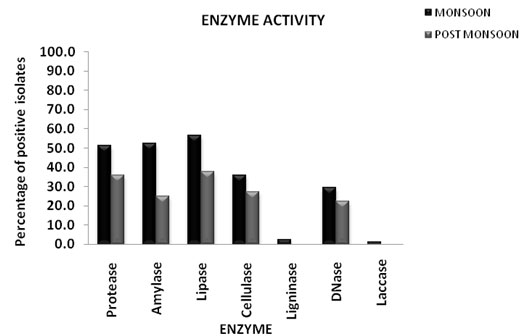
The hydrolytic enzyme potential of bacterial isolates during the study period showed considerable variations between the monsoon and post monsoon season (Fig. 3). The number of isolates showing enzymatic potential in each season is calculated and expressed as percentage. The results showed that the number of bacterial isolates having hydrolytic activity increased during post monsoon compared to monsoon season. 66% of the isolates showed various hydrolytic enzyme activities during monsoon and 87% in post monsoon. Significant changes were found in the pattern of enzyme activity showed by the bacterial isolates between two seasons.
The order of the enzyme activity showed by the bacterial isolates during monsoon was found to be lipase> amylase>protease>cellulase>DNase>ligninase>laccase and that of post monsoon lipase>protease> cellulase>amylase> DNase (Fig. 4). Lipase and protease enzyme activity were shown by maximum number of isolates while ligninase and laccase activity by minimum number of isolates during the study period. There was no ligninase and laccase producing isolates obtained during post monsoon while 2.6% isolates showed ligninase and 1.3% isolates showed laccase activity (Vidya and Sebastian, 2020).
The present study showed increase in the CFU/g of cultivable bacteria from the sediments of all the 8 mangrove stations during monsoon when compared to post monsoon period. The percentage of organic matter in the mangrove sediments from all the sites studied showed increasing pattern from monsoon to post monsoon. The increase in the moisture content and organic matter might be the reason for enhancement of bacterial count in monsoon season. Several studies showed that microbial population varied based on moisture and temperature changes among seasons and are the main factors related to microbial abundance and distribution (Kutty and Philip, 2008; Luo et al., 2019; Vidya and Sebastian, 2020). The reported relationship between soil physicochemical properties and microbial communities varies among studies. In some ecosystems, soil microbes grow rapidly via energy and nutrition consumption (Huang et al., 2005; Huang et al., 2013).
Organic matter content is high in Pazhayangadi both in monsoon and post monsoon, it may be due to the climate and sediment changes of mangrove station. In fact, the factors that trigger rapid propagation of microbes most likely are high moisture, warm temperature, appropriate pH and outside carbon input. Some of the microbes consume carbon and nitrogen sources from outside, some likely get nutrition in soil, and some of them get energy from metabolites of other microbial groups. Most importantly, several key microbial organisms living in the habitat even effect the whole interaction between microbial community dynamics and soil chemical properties. It is hardly conclusive because the function of many key sediment microbes remains unclear. In this regard, extensive studies are needed for understanding the interrelationship between microbial distribution and diversity along with all components of the microbial community and the physico chemical properties (Luo et al., 2019).
The number of bacterial isolates showing various enzymatic activities was higher during post monsoon season. Previous studies have demonstrated a seasonal variation in microbial enzyme activity related to a seasonal shift in availability of substrates and a seasonal variation in soil temperature and moisture (Kaiser et al., 2010). Seasonal and experimentally induced alterations in resource availability and abiotic factors, however, have also been shown to induce changes in microbial community composition and hydrolytic enzyme potential (Lauber et al., 2008; Kaiser et al., 2010; Fierer et al., 2012). In the present study also, considerable variations were observed in microbial populations and enzyme activity during the two seasons and also between the sampling stations, which substantiate with the studies of Vidya and Sebastian (2020).
Majority of the bacterial isolates from the mangrove sediments studied were lipolytic and proteolytic. This shows the presence of lipid and protein substances and its metabolic processes in the sediments. The extracellular enzymes produced by bacteria have variety of applications in industrial processes. Many studies reported that microorganisms possess distinct enzyme production ability to degrade complex hydrocarbon mixers such as crude oil from industrial waste water, fresh water and marine environments (Silva et al., 2015; Safitri et al., 2015). Bacteria are the most dynamic agents in petroleum degradation and they perform as primary degraders of spilled oil in various environments (Wang and Shao, 2012). Microbial enzymes have applications in the detergent, food, flavor, pharmaceutical, agrochemical, chemical and cosmetic industries. Enzymes are also use effectively in bioremediation of environments contaminated with hazardous substances (Dash et al., 2013; Willsey and Wargo, 2015; Raveendran et al., 2018). Since a good number of mangrove bacterial isolates during our study showed considerable amount of enzyme activities, they can be further optimized for the large-scale production (Raveendran et al., 2018).
Due to unique environment, mangrove inhabitants develop adaptations for their growth and survival. Mangrove bacteria also show unique enzymatic properties (Gomes et al., 2011). This needs research attention and must be explored for the discovery of novel bioactive compounds. The current study focuses on the bacterial richness in the mangrove habitat and their hydrolytic enzyme potential. The study attempts to highlight the importance of mangrove ecosystem and the preservation. The findings from our study suggest that monsoon season harbored more bacterial species in mangrove sediments compared to other seasons. This is due to the environmental and physico chemical characteristic changes in the habitat. Further, the promising bacterial isolates showing higher enzymatic potential will be identified and optimized for future applications (Gomes et al., 2011; Raveendran et al., 2018).
Figure 2: Variations in the population of cultivable bacterial colonies (CFU/g) and physico-chemical parameters of sediment (Total organic matter, Temperature and pH) from different mangrove sites during monsoon and post monsoon seasons Sampling stations: KSD – Kasaragod, EDT – Edat, PYD – Pazhayangadi, VPM – Valapattanam, ELR – Elathur, KDI-Kadalundi, PON – Ponnani, CHV-Chettuva
Figure 3: Culturable bacterial isolates from mangrove sediments showing various enzyme activities
Figure 4: Percentage of bacterial isolates showing various enzyme activities during monsoon and post monsoon
CONCLUSION
The present study focuses on bacterial isolates from the mangrove sediments of Northern Kerala, their enzymatic potential and sediment ecology during two seasons of the year viz monsoon and post monsoon. The number of cultivable bacteria and their hydrolytic enzyme production were found to be significantly affected by the moisture content and the organic matter present in the sediment. As the mangroves are unique ecosystem with extreme characteristics, more attention has to be given so as to explore novel bioactive compounds which can be applied in industries and also in the management of mangrove ecosystem. The present study thus provides light to the need for extensive studies in mangrove habitats for the conservation and management of this unique ecosystem.
ACKNOWLEDGEMENTS
The authors are grateful to Kerala State Council for Science, Technology and Environment for the financial assistance.
Conflict of Interest: The authors declare that they have no conflicts of interest.
REFERENCES
Alongi DM (1988) Bacterial productivity and microbial biomass in tropical mangrove sediments, Microbial Ecology, Vol 15 Pages 59–79.
Barbier EB (2016) The protective service of mangrove ecosystems: a review of valuation methods, Marine Pollution Bulletin, Vol 109 Pages 676–681.
Brander LM, Wagtendonk JA, Hussain SS, McVittie A, Verburg PH, de Groot RS and van der Ploeg S (2012) Ecosystem service values for mangroves in Southeast Asia: a meta-analysis and value transfer application, Ecosystem Services, Vol 1 Pages 62–69.
Chhonkar PK and Tarafdar JC (1984) Accumulation of phosphatases in soils, Journal of the Indian Society of Soil Science, Vol 32 No 2 Pages 266-272.
Dash HR, Mangwani N, Chakraborty J, Kumari S and Das S (2013) Marine bacteria: potential candidates for enhanced bioremediation, Applied Microbiology and Biotechnology, Vol 97 No 2 Pages 561-571.
Fierer N, Lauber CL, Ramirez KS, Zaneveld J, Bradford MA and Knight R (2012) Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients, Multidisciplinary Journal of Microbial Ecology, Vol 6 No 5 Pages 1007-17.
Ghosh S (2019) On the frontline of disasters, mangroves at the receiving end of development and climate change Mongabay series, News & inspirations from nature’s front line in INDIA.
Gomes NC, Cleary DF, Calado R and Costa R (2011) Mangrove bacterial richness, Communicative and Integrative Biology, Vol 4 No 4 Pages 419-423.
Himes-Cornell A, Grose SO and Pendleton L (2018) Mangrove Ecosystem Service Values and Methodological Approaches to Valuation: Where Do We Stand? Frontiers in Marine Science, Vol 5 Pages 376.
Huang PM, Wang MK and Chiu CY (2005) Soil mineral-organic matter-microbe interactions: impacts on biogeochemical processes and biodiversity in soils, Pedobiogia, Vol 49 Pages 609–635.
Huang PM, Wang SL, Tzou YM, Huang Y, Weng B, Zhuang S and Wang MK (2013) Physicochemical and biological interfacial interactions: impacts on soil ecosystem and biodiversity, Environmental Earth Sciences, Vol 28 Pages 2199–2209.
Jha DK, Sharma GD and MishraI RR (1992) Soil microbial population numbers and enzyme activities in relation to altitude and forest degradation, Soil Biology and Biochemistry, Vol 24 Pages 761-767.
Kaiser C, Koranda M, Kitzler B, Fuchslueger L, Schnecker J, Schweiger P, Rasche F, Zechmeister-Boltenstern S, Sessitsch A and Richter A (2010) Below ground carbon allocation by trees drives seasonal patterns of extracellular enzyme activities by altering microbial community composition in a beech forest soil, New Phytologist, Vol 187 No 3 Pages 843-58.
Klein DA (2000) The rhizosphere. In: Encyclopedia of microbiology Pp 117-126 (Edited by) J Lederberg. Academic Press, San Diego.
Kutty SN and Philip R (2008) Marine yeasts — A review, Yeast, Vol 25 No 7 Pages 465-483.
Lageiro MM, Moura MJ, Reis A and Ferreira MJC (2007) Microbial proteases application in leather industry, Journal of Biotechnology, Vol 131 Pages S239–S240.
Lauber CL, Strickland MS, Bradford MA and Fierer N (2008) The influence of soil properties on the structure of bacterial and fungal communities across land-use types, Soil Biology & Biochemistry, Vol 40 Pages 2407–2415.
Liang J, Chen Y, Lan C, Tam N, Zan Q and Huang L (2007) Recovery of novel bacterial diversity from mangrove sediment, Marine Biology, Vol 150 Pages 739-747.
Luo X, Wang MK, Hu G and Weng B (2019) Seasonal change in microbial diversity and its relationship with soil chemical properties in an orchard, PloS one, Vol 14 No 12 e0215556.
Millennium Ecosystem Assessment (2005) Millennium Ecosystem Assessment. World Resources Institute Washington DC.
Mohandas M, Lekshmy S and Radhakrishnan T (2014) Kerala mangroves–Pastures of estuaries–Their present status and challenges, International Journal of Scientific Research, Vol 3 No 11 Pages 2804-2809.
Pennanen T, Liski J, Baath E, Kitunen V, Uotila J, Westman C J and Fritze H (1999) Structure of the microbial communities in coniferous forest soils in relation to site fertility and stand development stage, Microbial Ecology, Vol 38 No 2 Pages 168-179.
Persson T, Baath E, Clarholm M, Lundkvist H, Soderstrom BE and Sohlenius B (1980) Trophic structure, biomass dynamics and carbon metabolism of soil organisms in a Scots pine forest, Ecological Bulletins, Pages 419-459.
Raveendran S, Parameswaran B, Beevi Ummalyma S, Abraham A, Mathew AK, Madhavan A, and Pandey A (2018) Applications of microbial enzymes in food industry, Food Technology and Biotechnology, Vol 56 No 1 Pages 16-30.
Regina SI and Tarazona T (2001) Nutrient pools to the soil through organic matter and through fall under a Scots pine plantation in the Sierra de la Demanda Spain, European Journal of Soil Biology, Vol 37 Pages 125-133.
Safitri R, Priadie B, Miranti M and Astuti AW (2015) Ability of bacterial consortium: Bacillus coagulans, Bacilus licheniformis, Bacillus pumilus, Bacillus subtilis, Nitrosomonas sp. and Pseudomonas putida in bioremediation of waste water in Cisirung waste water treatment plant, AgroLife Scientific Journal, Vol 4 No 4 Pages 146-152.
Silva DSP, Cavalcanti DL, Melo EJV, Santos PNF, Luz ELP, Gusmao NB and Sousa QMDFV (2015) Bio-removal of diesel oil through a microbial consortium isolated from a polluted environment, International Biodeterioration and Biodegradation, Vol 97 Pages 85-89.
Swift MJ, Heal OW and Anderson JM (1979) Decomposition in terrestrial ecosystems. 372Pp, Blackwell Scientific Publications Oxford.
Tallis H, Lester SE, Ruckelshaus M, Plummer M, McLeod K, Guerry A, Andelman S, Caldwell MR, Conte M, Copps S, Fox D, Fujita R, Gaines SD, Gelfenbaum G, Gold B, Kareiva P, Kim C, Lee K and White C (2012) New metrics for managing and sustaining the ocean’s bounty, Marine Pollution, Vol 36 Pages 303–306.
Trivedy RK and Goel PK (1986) Chemical and biological method for water pollution studies, Environment Publication (Karad, India), Vol 6 Pages 10-12.
Vidya P and Sebastian CD (2020) A study on the distribution and hydrolytic enzyme potential of yeasts in the mangrove sediments of Northern Kerala, Indian Journal of Microbiology, Vol 7 No 2 Pages 161–167.
Wang W and Shao Z (2012) Genes involved in alkane degradation in the Alcanivorax hongdengensis strain A-11-3, Applied Microbiology and Biotechnology, Vol 94 No 2 Pages 437-448.
Willsey GG and Wargo MJ (2015) Extracellular lipase and protease production from a model drinking water bacterial community is functionally robust to absence of individual members, Plos one, Vol 10 No 11 e0143617.