University Institute of Engineering and Technology, Kurukshetra
University, Kurukshetra Haryana, India
Corresponding author email: agupta2015@kuk.ac.in
Article Publishing History
Received: 25/11/2021
Accepted After Revision: 25/03/2022
The present study proposed differential effect of solvents on extraction, pharmacognostic evaluation and antioxidant activity of Long pepper (Piper longum) fruit extract. The bio-analytes present in P. longum fruit were extracted by dissolving powder fruit in water, ethanol, methanol, acetone, and ethyl acetate. Further, the extracts were dried using rotavapor at 55 °C and used to assess the phytochemical analysis where total phenolic compounds (TPCs) were evaluated using Folin-Ciocalteu Assay. Additionally, ascorbic acid content was measured, and Fourier Transform Infrared Spectroscopy (FTIR) analysis was performed to predict the presence of various compounds based on their surface functional groups. Further, antioxidant efficiency was determined by considering DPPH radical scavenging activity.
The quantitative measurements of the TPC and ascorbic acid reveal the presence of the pharmacognostic compounds in P. longum, which is also supported by FTIR analysis. The FC assay test confirmed the highest polyphenols (181.0 ± 8.69 mg GAE/g) in ethyl acetate extract, while methanolic extract showed the maximum ascorbic acid content (1.51 ± 0.067 mg AE/g). Methanolic and ethyl acetate-based extraction process showed great results than other solvents as they showed maximum DPPH radical scavenging activity (% degradation), 81.57 %, followed by ethyl acetate (79.35%). The obtained results announced P. longum extract can be used as a raw material in pharmaceutical industries and local levels to improve health conditions.
Antioxidant Activity, Ascorbic Acid, Extraction, P. longum, Total Phenolic Compounds.
Chauhan S, Mittal A. Differential Effects of Solvents on Extraction, Pharmacognostic Evaluation and Antioxidant Activity of Long Pepper Piper longum Fruit Extract. Biosc.Biotech.Res.Comm. 2022;15(1).
Chauhan S, Mittal A. Differential Effects of Solvents on Extraction, Pharmacognostic Evaluation and
Antioxidant Activity of Long Pepper Piper longum Fruit Extract. Biosc.Biotech.Res.Comm. 2022;15(1). Available from: <a href=”https://bit.ly/3hHH4Em“>https://bit.ly/3hHH4Em</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Tremendous development in the technologies and improved medical facilities result in an increase in the population with a great pace where highly healthy and nutritive food is required. Many plants and their extracts are being used for several applications at the industrial and household level since ancient times, i.e., medicinal plants, spices, herbs, and nutritive plants (Goyal et al. 2018; Giannenas et al. 2020). Along with the aforementioned applications, various medicinal plants have been used to get several benefits, i.e., nutrients, medicine and preservatives (Deekshith et al. 2021; Ahmad et al. 2021). Additionally, spices and medicinal plants have been reported as potential antimicrobial (antibacterial, antifungal) and antioxidant agents, which increased their demand in medical biotechnology, promising many beneficial outcomes against newly emerging harmful microbes and diseases (Cenobio-Galindo et al. 2019; Szymandera-Buszka et al. 2020).
Medicinal plants are a rich and combined source of beneficial compounds, i.e., antioxidants, proteins, vitamins, polyphenols etc. (Khan et al. 2010; Chikatipalli 2021). Various studies have been performed to analyze the nutritive and medicinal potential of many spices, herbs, medicinal plant parts and their extracts. Various analytical techniques can be used to evaluate the presence of the aforementioned compounds in the plant raw (Boukhatem and Setzer 2020; Fitzgerald et al. 2020). Directly or indirectly, medicinal plants are a vast part of animal’s life and human being in the whole world. Medicinal plants are highly used in INDIA in the form of food, spices, juice and preservatives daily, which increased the demand for some optimal study to find out the potential of spices and their most delicate use (Olalere et al. 2017; Srikacha and Ratananikom 2020; Beya et al. 2021).
Indian spices are not just limited to taste, colour and aroma, but it also has potential in therapeutic properties, which increased their uses in pharmaceutical industries (Kalra et al. 2021). Now Indian spices are used worldwide for food and medicinal purposes.Indian Ayurveda of medicine also enlightened the properties of Indian spices, their varieties, their benefits, and their potential use in treating or preventing various diseases (Chauhan et al. 2015). Piperine, an alkaloid, is a highly used spice in medicine and homemade “nukshas” for health purposes. Piperine, Piper longum also known as Indian long pepper or pippali, is highly used to treat various diseases, i.e., indigestion, bronchial disorders, snake venom, and breathing problems. Additionally, it has also shown beneficial results in infertility, menstrual problems and pregnancy (Kumar et al. 2011; Chauhan et al. 2019; Tiwariet al. 2020). Additionally, a variety of compounds increase the availability of both reducing and capping agents in P. longum, which might increase its demand in nanotechnology and material science for the synthesis of nanoparticles for various applications, i.e., wastewater treatment, drug delivery system, antimicrobial activity, implants, and chemical conversion (Jamila et al. 2020;Favre et al. 2021; Schnabel et al. 2021; Cheraghipour et al. 2021).
As mentioned, these spices and other food items are highly rich for small but highly beneficial tracing compounds, but optimum use and combinations had not been evaluated. Hence, optimising the composition of spices and their constituents should be done to get the maximum possible results. To accomplish the present demand, we have successfully extracted the bioanalytes present in the selected Piper longum in different solvents, i.e., water, ethanol, methanol, acetone, and ethyl acetate. Further, the dried extracts were characterized using FTIR spectroscopy and the phytochemical analysis studies, i.e., Total phenolic compounds (TPCs), ascorbic acid equivalent and DPPHradical scavenging activity, performed to evaluate the constituents present in the P. longum and their potential activity.
MATERIAL AND METHODS
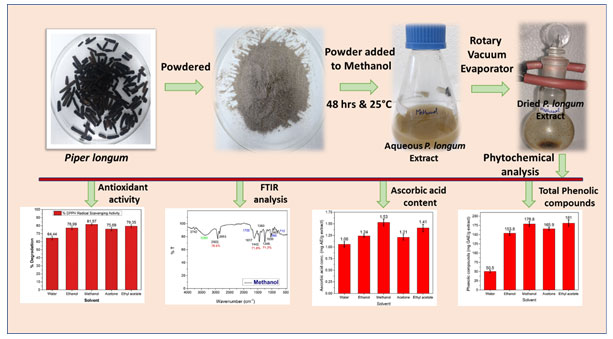
The dehydrated fruits of Piper longum were procured from the local shop of Kurukshetra. The purchased fruits were washed under tap water followed by distilled water and kept at 40°C for 72 hrs. Further, washed fruits were precast into powder form by grinding and stored for further analysis. Morphological appearance of Piper longum present in literature, and images of seeds used to identify the fruit (Kumar et al. 2011; Murphin Kumar et al. 2020). All the chemicals were purchased from Himedia, INDIA.For extraction, 10g of P. longum powder was macerate to 100 ml solvents (methanol, ethanol, acetone, ethyl acetate, and water) at room temperature for 48 hours by frequent mixing.Further, all the extracts were centrifuged at 5000 rpm, and the supernatant was filtered using Whatman no.1 filter paper. Then, the filtrate was dried using a rotary vacuum evaporator (Heidolph, VE-11) and stored at 4°C for further analysis. The schematic representation of the extraction process and phytochemical analysis is shown in Fig. 5(Du et al. 2012; Cheraghipour et al. 2021).
Polyphenols (Folin ciocalteu) assay used with some modification to Deng et al. (2013) to detect the total phenolic compounds present in each extracted sample. Further, 0.2 ml of prepared solution, 4 ml of sodium bicarbonate solution (2% w/v) and 5.6 ml of DI water were mixed and added with 0.2 ml of 50% (v/v) Folin-Ciocalteu reagent and incubated for 15 minutes at RT after proper mixing. Further, the resultant mixer was analysed for absorbance value at 750 nm in UV-Vis Spectrophotometer and concentration was calculated by using a standard curve (Deng et al. 2013; Golder et al. 2021).
The ascorbic acid concentration was measured using Chelli and Golder (2018) method. 200 µl of extract (10 mg/ml) was mixed with 1800 µl DCPIP followed by measurement of absorbance at 604 nm after 20 minutes.
A calibration curve was plotted between various ascorbic acid concentrations and their absorbance after incubation. Further, the linear fitting was performed for the standard curve equation. The obtained equation was used to calculate the AA concentration of unknown samples by using their absorbance value (Chelli and Golder 2018). The obtained extracts were analyzed by using Fourier-transform infrared spectroscopy (FTIR) under KBr mode (PerkinElmer, spectrum two). FTIR spectrums were compared with the standard library and literature to assign the functional groups and their expected compounds. Additionally, % transmission or absorbance values for a specific peak (peak intensity) can be used to compare the fraction of specific compounds present in two different samples. In this case, high absorbance or low % transmission defines more percentage of a specific compound in solution (Ravi and Pandey 2019).
In-vitro DPPH radical scavenging activity was investigated using DPPH as an indicator of antioxidant activity. For this, 250µM DPPH stock solution was prepared by diluting with 9.9 mg DPPH with 100 ml ethanol (96% v/v). The control solution was prepared by adding DI to the DPPH stock solution maintaining the ratio (1:1). The sample solution was prepared by adding 0.5 ml Bio-extract to 1.5 ml DPPH solution, and blank was prepared by adding ethanol to DI and incubated at 25℃ for 30 minutes for homogenization. Further, the absorbance value was measured at 517 nm using a UV-vis spectrometer (Jirankalgikar et al. 2014, More and Makola 2020). The scavenging activity was examined as % DPPH radical activity by using the expression given below:
![]()
Where, As defined the absorbance value of sample (extract) and As defined the absorbance value of control solution.
RESULTS AND DISCUSSION
Determination of total phenolic compounds (TPCs): Polyphenols (Folin Ciocalteu) assay was used to detect the TPCs present in each extracted sample. A standard curve was plotted by using various gallic acid concentrations and their absorbance after incubation. Further, the linear fitting was performed for the standard curve equation. The obtained calibration curve and calibration equation is given in Fig. 1 (A) and eq. (2). The obtained calibration curve equation is as follows:
y= 0.00465x + 0.04717 (2)
Where y defines the absorbance value and x defines the concentration of the gallic acid (gallic acid equivalent).
Figure 1: (A) Calibration curve of gallic acid and (B)Total phenolic compounds
concentration present in each extract (mg GAE/g extract)
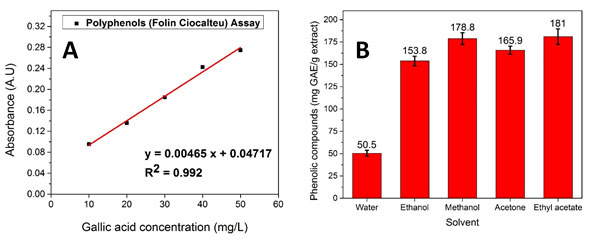
Further, the gallic acid equivalent of extract samples were calculated by using a calibration equation with respect to their absorbance value. The number of phenolic compounds obtained in each sample of bio-extract is stated as mg GAE/g Extract. The obtained gallic equivalent values and absorbance of all the samples are given in Table 1 and Fig. 1 (B). The obtained data confirmed that P. longum is a rich source of polyphenols containing up to 181.0 8.7mg polyphenols per g of dry extract. Additionally, ethyl acetate showed the best extraction of the polyphenol with 181.0 8.7 mg GAE/g while water showed the least extraction with 50.5 3.3 mg GAE/g(Alara et al. 2021).
Furthermore, methanol (178.8 6.6 mg/g) showed better extraction of polyphenols than acetone (165.9 4.5 mg/g) and ethanol (153.8 5.4 mg/g). The FTIR pattern also confirms the presence of maximum total phenolic compounds in ethyl acetate-based extract. An increase in the extraction of phenolic compounds in organic solvent than water might be attributed to the high solubility of polyphenols due to the suitable polarity of organic solvents than water (Alara et al. 2021). Methanolic extract of Knoxia sumatrensis leaves showed similar with 55.0 ± 1.3 mg GAE/g total phenolic compounds, whereas ethyl acetate extract of X. granatum leaves showed ethyl acetate extracts with 28.36 ± 0.50 mg GAE/g TPC in the aqueous state (Loganathan et al. 2021; Darmadi et. al. 2021).
Determination of ascorbic acid equivalent (AE): The standard curve was plotted between known ascorbic acid concentrations and their absorbance, as shown in Fig. 2 (A). AE content was calculated from its absorbance value by using the standard curve equation as follows:
y = 0.00232x (3)
Where y defines the absorbance value, and x defines the ascorbic acid equivalent (AE) concentration (Darmadi et. al. 2021).
Figure 2: (A) Standard curve of ascorbic acid and (B) Ascorbic acid concentration present in each extract
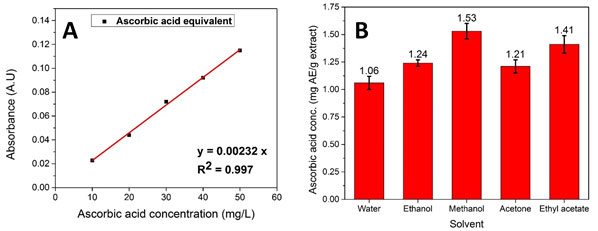
The amount of ascorbic acid obtained in each bio-extract sample is expressed as mg AE/g dry extract. The obtained ascorbic acid concentration and absorbance of all samples are given in Fig. 2 (B) and Table 1.The obtained data confirm that P. longum also contains ascorbic acid but in very less amount. Additionally, the methanolic extract showed the highest ascorbic acid concentration (1.53 0.07mg/g), which attributed to the high solubility of the ascorbic acid in methanol than others. Unlikely, the water showed the least extraction of the ascorbic acid (1.01 0.06 mg/g) and ethyl acetate (1.41 0.08mg/g) showed better extraction than acetone (1.21 0.06 mg/g) and ethanol (1.24 0.03 mg/g). Hence, ascorbic acid has better solubility in methanol than others which might be due to appropriate polarity and partition coefficient. Pleurotus floridanus extract showed a higher 17.54 mg/g ascorbic acid content and osage orange’s extract showed around 12 mg/g ascorbic acid, which claimed presence of less amount of ascorbic acid (vitamin C) in spices than citric acid fruits (Bains et. al. 2020; Dadayan et. al. 2021).
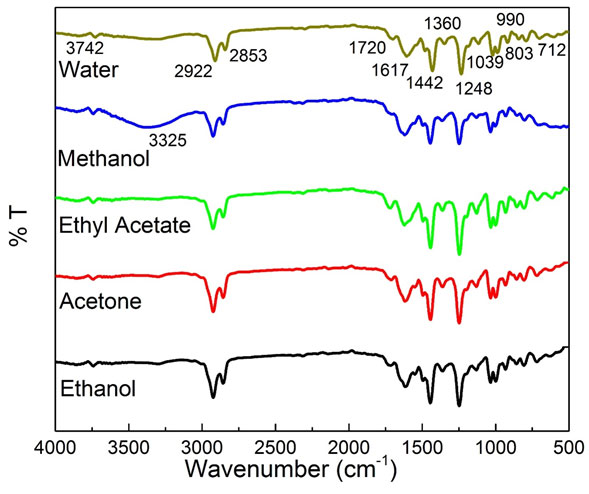
Analysis of compounds present in extracts by FTIR: All the bioextract samples were analysed by using FTIR spectroscopy to study the corresponding functional groups. The FTIR spectrum for all the bio-extract samples and their corresponding functional groups are given inFig. 3.The small peak obtained at 3742 and a broad peak at 3325 cm-1 (in methanol solvent extraction) correspond to the O-H group of flavonoids/tannins and tannins/phenols/polyphenols, respectively. Further, a peak around 2922 cm-1 and 2853 cm-1 represents the O-H stretching vibration of polyphenols and C–H stretching vibration of aromatic compounds, respectively. The peak present at 1720 cm−1 is allocated to the C=O stretching vibration of the carboxylic group of hemicellulose. Peaks at 1617, 1442, and 1360 cm-1 claimed the C=O group of ketone/aldehyde functional groups, C-H bending mode of lignin/carboxylic acid, and CH2/CH3 deformation due to the presence of glycosides/carbohydrates, respectively. Further, peaks of 1248, 1039, and 990 cm-1 are linked to C-O bonding of polyphenols/phenols, OH-CH stretching in sugar and polysaccharides, and β-linkage of the polysaccharide, respectively (Brangule et al. 2020).
Lastly, small peaks at 803 and 712 cm-1 correspond to the C-H chain of carbohydrate or fatty acid chain (Purkayastha et al. 2012; Trifunschi et al. 2015; Oliveira et al. 2016; Monisha and Vimala 2018; Ravi and Pandey 2019). Conclusively, all the extracts showed the same peaks, but the % transmittance of these peaks vary due to the different extraction power of solvents. However, a broad peak is clearly visible in methanol (represented by blue), corresponding to the O-H group of tannins, phenols, and polyphenols, which claimed the high level of phenolic compounds present in methanol solvent-based extract than others. Additionally, % transmittance value of ethyl acetate showed the least transmission than others which confirmed the presence of the high amount of analytes in the extract (Brangule et al. 2020; Manasa et al. 2020).
Figure 3: FTIR spectrum of P. longum bio-analytes extracted in water, ethanol, methanol, acetone, and ethyl acetate.
In vitro antioxidant activity: The antioxidant activity of all the extracted samples was examined. The obtained results are shown in Table 1 and Fig. 4. The obtained data were fitted to equation 3 to calculate % DPPH degradation. The obtained data confirmed that the methanolic extract showed maximum DPPH radical activity (% degradation), 81.57 1.1 %, while water extract showed the least DPPH radical activity (64.44 2.3 %) (Espinozaet al. 2020).
Figure 4: DPPH scavenging activity (% degradation) of each extract
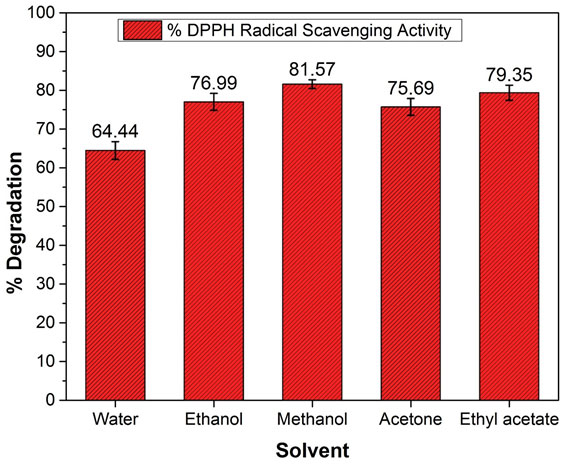
Additionally, ethyl acetate (79.35 1.9%) showed better DPPH radical scavenging activity than acetone (75.69 2.2 %) and ethanol (76.95 2.1 %).The obtained result confirmed that methanol and ethyl acetate are the better solvents for extraction of DPPH radical scavengers than other solvents, which might be attributed to the presence of scavenger compounds in methanolic solvent. The obtained results are well supported by total phenolic compound and ascorbic acid content, which might influence enhanced DPPH scavenging activity. Similar results have been reported where ethyl acetate extract of Mangifera indica leaves showed 79.73 % DPPH inhibition, and methanolic extract of Conyza bonariensis L. leave showed 47.83% inhibition (Ibrahimet al. 2020; Espinozaet al. 2020).
Table 1. Total phenolic compounds concentration, ascorbic acid concentration and antioxidant activity of each extract
| Solvent | Gallic acid equivalent (mg GAE/g) | Ascorbic Acid Concentration (mg AE/g extract) ???? | % Degradation (Antioxidant activity) |
| Water | 50.53 3.29 | 1.01 0.063 | 64.44 2.30 |
| Ethanol | 153.76 5.41 | 1.25 0.028 | 76.95 2.13 |
| Methanol | 178.85 6.57 | 1.51 0.067 | 81.57 1.12 |
| Acetone | 165.94 4.48 | 1.21 0.058 | 75.69 2.18 |
| Ethyl Acetate | 181.0 8.69 | 1.38 0.076 | 79.35 1.93 |
Figure 5: Schematic representation of extraction of the bio-analytes and their analysis.
CONCLUSION
The findings of the study claimed the excellent DPPH radical scavenging activity of bio-analytes present in Piper longum extracted by using different solvents. Additionally, the obtained results confirmed the presence of polyphenols and ascorbic acid, which can be used for other applications. Methanolic and ethyl acetate-based extraction system showed great results than other solvents as they showed maximum DPPH radical scavenging activity (% degradation), 81.57 %, followed by ethyl acetate (79.35%).Variation in phytochemicals quantity and quality with change in the solvent, rectify the optimization of solvent for extraction to contract maximum benefits. Hence, Piper longum and other spices bio-analyte’s composition can be stimulated in the future for various health benefits with appropriate practice.
ACKNOWLEDGEMENTS
This study was financially supported by UIET, KUK which provides all necessary equipment and facilities to conduct this research work. The authors also acknowledge the Centre for the Environment, IIT Guwahati for FTIR analysis.
Conflict of Interests: Authors declare no conflicts of interests to disclose.
Data Availability Statement: The database generated and /or analysed during the current study are not publicly available due to privacy, but are available from the corresponding author on reasonable request.
REFERENCES
Alara OR Abdurahman NH and Ukaegbu CI (2021). Extraction of phenolic compounds: a review. Curr. Res. Nutr. Food Sci., 4, 200-214.
Ahmad S Zahiruddin S Parveen B et al. (2021). Indian medicinal plants and formulations and their potential against COVID-19–preclinical and clinical research. Front. Pharmacol., 11, 2470.
Bains A Chawla P Tripathi A et al. (2020). A comparative study of antimicrobial and anti-inflammatory efficiency of modified solvent evaporated and vacuum oven dried bioactive components of Pleurotus floridanus. J. Food Sci. Technol., 26, 1-10.
Beya MM Netzel ME Sultanbawa Y et al. (2021). Plant-based phenolic molecules as natural preservatives in comminuted meats: A review. Antioxidants, 10(2), 263.
Boukhatem MN and Setzer WN (2020). Aromatic herbs, medicinal plant-derived essential oils, and phytochemical extracts as potential therapies for coronaviruses: future perspectives. Plants, 9, 800.
Brangule A Šukele R and Bandere D (2020). Herbal Medicine Characterization Perspectives Using Advanced FTIR Sample Techniques–Diffuse Reflectance (DRIFT) and Photoacoustic Spectroscopy (PAS). Front. Plant Sci., 11, 356
Chauhan A Semwal DK Mishra SP et al. (2015). Ayurvedic research and methodology: Present status and future strategies. Ayu, 36, 364.
Chauhan N Uniyal P Chauhan R et al. (2019). In vitro antibacterial effects of Piper longum fruit extracts on human pathogens and phytochemical analysis. Int. j. res. anal., 232-288.
Chelli VR and Golder AK (2018). Ag-doping on ZnO support mediated by bio-analytes rich in ascorbic acid for photocatalytic degradation of dipyrone drug. Chemosphere, 208, 149-158.
Cheraghipour K Beiranvand M Zivdari M et al. (2021). In vitro potential effect of Pipper longum methanolic extract against protoscolices of hydatid cysts. Exp. Parasitol., 221, 108051.
Chikatipalli R Saravana KK and Bannoth CSK (2021). Pharmacognostic evaluation and free radical scavenging activity of Bombax ceiba leaf extracts. Int. J. Green Pharm., 15 (1), 59-65.
Dadayan AS Stepanyan LA Sargsyan TH et al. (2021). Quantitative analysis of biologically active substances and the investigation of antioxidant and antimicrobial activities of some extracts of Osage orange fruits. Pharmacia, 68, 731.
Darmadi J Batubara RR Himawan S et al. (2021). Evaluation of Indonesian mangrove Xylocarpus granatum leaves ethyl acetate extract as potential anticancer drug. Sci Rep, 11(1), 1-8.
Cenobio-Galindo ADJ Pimentel-González DJ Razo-Rodríguez OED et al. (2019). Antioxidant and antibacterial activities of a starch film with bioextracts microencapsulated from cactus fruits (Opuntia oligacantha). Food Sci. Biotechnol., 28, 1553-1561.
Deekshith C Jois M Radcliffe J and Thomas J (2021). Effects of culinary herbs and spices on obesity: A systematic literature review of clinical trials. J. Funct. Foods, 81, 104449.
Deng GF Lin X Xu XR et al. (2013). Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Food, 5, 260-266.
Du XF Song JJ Hong S et al. (2012). Ethanol extract of Piper longum L. attenuates gentamicin-induced hair cell loss in neonatal cochlea cultures. Die Pharmazie-Int. J. Pharm. Sci. Res., 67, 559-563.
Espinoza RV Peñarreta J Quijano-Avilés M et al. (2020). Antioxidant activity and GC-MS profile of Conyza bonariensis L. leaves extract and fractions. Rev. Fac. Nac. Agron. Medellín, 73 (3), 9305-13.
Favre LC López-Fernández MP Ferreira CDS et al. (2021). The antioxidant and antiglycation activities of selected spices and other edible plant materials and their decay in sugar-protein systems under thermal stress. Food Chem., 22, 131199.
Fitzgerald M Heinrich M and Booker A (2020). Medicinal plant analysis: A historical and regional discussion of emergent complex techniques. Front. Pharmacol., 10, 1480.
Giannenas I Sidiropoulou E Bonos Eet al. (2020). The history of herbs, medicinal and aromatic plants, and their extracts: Past, current situation and future perspectives, Feed Additives. Elsevier, pp. 1-18.
Goyal PK Verma SK and Sharma AK (2018). Optimization of extraction protocol of Parmelia perlata and its validation for protective effects against oxalate-induced renal injury in NRK-52E cells. J. Herb. Med., 12, 79-87.
Ibrahim Y Busari M Yusuf R et al. (2020). In vitro Antioxidant Activities of Ethanol, Ethyl Acetate and n-Hexane Extracts of Mangifera indica Leaves. Tanz. J. Sci., 46 (3), 628-35.
Jamila N Khan N Bibi A et al. (2020). Piper longum catkin extract mediated synthesis of Ag, Cu, and Ni nanoparticles and their applications as biological and environmental remediation agents. Arab. J. Chem., 13, 6425-6436.
Jirankalgikar N Nariya P and De S (2014). In vitro antioxidant activity evaluation and HPTLC profile of Cow dung. Int. J. Green Pharm., 8 (3), 158–162.
Kalra R Conlan XA Areche C et al. (2021). Metabolite Profiling of the Indian Food Spice Lichen, Pseudevernia furfuracea Combined with Optimised Extraction Methodology to Obtain Bioactive Phenolic Compounds. Front. Pharmacol., 12.
Khan MA Naidu MA and Akbar Z (2010). In-vitro antimicrobial activity of fruits extract of Embelia ribes Burm. Int. j. pharm. biol. sci. arch., 1, 267-270.
Kumar S Kamboj J and Sharma S (2011). Overview for various aspects of the health benefits of Piper longum linn. fruit. J. Acupunct. Meridian Stud., 4, 134-140.
Loganathan S Selvam K Sivasakthi V et al. (2021). Phytochemical and Pharmacological Evaluation of Methanolic Extract of Knoxia sumatrensis Leaves. J. Herbs Spices Med. Plants, 27(2), 200-17.
Manasa V Chaudhari SR and Tumaney AW (2020). Spice fixed oils as a new source of γ-oryzanol: nutraceutical characterization of fixed oils from selected spices. RSC Adv. 10 (72), 43975-84.
Monisha SI and Vimala JR (2018). Extraction, Identification and Pharmacological Evaluation of Phyto-Active Compound in Manilkara hexandra(Roxb.) Dubard Stem Bark. Biosci. Biotechnol. Res. Asia, 15, 687-698.
More GK Makola RT (2020). In-vitro analysis of free radical scavenging activities and suppression of LPS-induced ROS production in macrophage cells by Solanum sisymbriifolium extracts. Sci., 10, 1-9.
Murphin Kumar PS Al-Muhtaseb AAH Kumar G et al. (2020). Piper longum Extract-Mediated Green Synthesis of Porous Cu2O: Mo Microspheres and Their Superior Performance as Active Anode Material in Lithium-Ion Batteries. ACS Sustain. Chem. Eng., 8, 14557-14567.
Olalere OA Abdurahman NH Alara OR et al. (2017). Parametric optimization of microwave reflux extraction of spice oleoresin from white pepper (Piper nigrum). Anal. Sci. Technol., 8, 1-8.
Oliveira RN Mancini MC Oliveira FC et al. (2016). FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Matéria (Rio de Janeiro), 21, 767-779.
Purkayastha MD Kalita D Das VK et al. (2012). Effects of L-ascorbic acid addition on micro-filtered coconut water: Preliminary quality prediction study using 1H-NMR, FTIR and GC-MS. Innov. Food Sci. Emerg. Technol., 13, 184-199.
Ravi and Pandey LM (2019). Enhanced adsorption capacity of designed bentonite and alginate beads for the effective removal of methylene blue. Appl. Clay Sci., 169, 102-111.
Schnabel A Athmer B Manke K et al. (2021). Identification and characterization of piperine synthase from black pepper, Piper nigrum L. Commun. Biol., 4 (1), 1-10.
Srikacha, N and Khakhanang R (2020). Antibacterial activity of plant extracts in different solvents against pathogenic bacteria: An in vitro experimentJ. Acute Dis. 9 (5), 223.
Szymandera-Buszka K Waszkowiak K Jędrusek-Golińska A et al. (2020). Sensory analysis in assessing the possibility of using ethanol extracts of spices to develop new meat products. Foods, 9, 209.
Tiwari A Mahadik KR and Gabhe SY (2020). Piperine: A comprehensive review of methods of isolation, purification, and biological properties. Medicine in Drug Discovery, 7, 100027.
Trifunschi S Munteanu MF Agotici V et al. (2015). Determination of flavonoid and polyphenol compounds in Viscum album and Allium sativum extracts. Int. Curr. Pharm. J., 4, 382-385.


