Biodiversity Research Lab, Shri Shivaji Science College, Amravati, Maharashtra, India.
Corresponding author email: pratik.chaudhary15@yahoo.in gajuwagh252424@rediffmail.com
Article Publishing History
Received: 05/04/2024
Accepted After Revision: 29/06/2024
Birds are useful bioindicators and provide conducive dispersal pathways and sufficient cover for migrating birds. As there is a lack of data regarding riverine avian diversity, the present study was carried out from November 2022 to December 2023. The study was done along the rivers flowing through the Melghat landscape in the district of Amravati India. The presence of birds in the area was recorded by a line transect and point count method using binoculars and DSLR cameras. A total of 245 birds belonging to 54 families were recorded. Out of which, 72 species from 20 families of water birds were recorded in the riverine zone and 173 species from 34 families of forest birds were recorded in the riverine zone. The majority of the observed species belonged to the Anatidae family followed by Ardeidae and Scolopacidae. Similarly, the Corvidae, Muscicapidae, and Sylviidae families show the maximum number of forest birds. According to the IUCN status, 87% of species associated with water are classified as least concern (LC), 10% as near-threatening (NT), only 3% are vulnerable. Similarly, for forest recorded birds, it is categorized as 98% species of least concern (LC), and only 2% are categorized as near-threatened (NT). Maximum species diversity was recorded with the forest bird associated in the riverine zone (D = 0.991 and H = 4.903), and minimum was recorded with the water bird associated in the riverine zone (D = 0.973 and H = 3.961). The study showed riverine avian diversity and threats in rivers.
Diversity, Maharashtra, Melghat, Near threatened, Riverine birds.
Pratik C, Wagh G, Kuralkar V. Diversity of Riverine Birds in Melghat Landscape, Maharashtra India. Biosc.Biotech.Res.Comm. 2024;17(2).
Pratik C, Wagh G, Kuralkar V. Diversity of Riverine Birds in Melghat Landscape, Maharashtra India. Biosc.Biotech.Res.Comm. 2024;17(2). Available from: <a href=”https://shorturl.at/0mYWR“>https://shorturl.at/0mYWR</a>
INTRODUCTION
Riverine ecosystems are crucial habitats for a wide range of species, includes birds. It supports a disproportionately large fraction of its biodiversity while also acted as significant corridors for the movement of plants, animals, and nutrients (Naiman et. al., 1993; Strayer and Dudgeon, 2010). Riparian zones also provide conducive dispersal pathways and sufficient cover for migrating birds, thereby often supported a higher diversity of bird species (Sinha et. al., 2019). The phenomenon of rivers drying up is a global environmental challenge that has far-reaching implications for ecosystems, communities, and water security. Across the world, numerous rivers are experiencing reduced flow and, in some cases, complete drying. This alarming trend is attributed to a combination of natural and anthropogenic factors, posing serious threats to biodiversity, livelihoods, and the availability of freshwater resources.
Various studies have been conducted on the impact of such human actions on the river flow regime (Adib et al., 2016;; Kousali et al., 2022, Adib 2022). Choi et al (2005) examined the effects of the Hapchon dam on regime change in the Hwang River flow in South Korea. They found that the dam construction caused significant downstream changes in the river path. Humans exploiting rivers will reduce discharges and cross-sectional flow, depth, and even flow velocities, dramatically impacting river-dependent habitats (Jiao et al. 2019). That’s why understanding the present status of riverine birds is crucial for assessing the impact of changing river conditions on avian biodiversity. Riverine birds are highly dependent on healthy river ecosystems for their survival, as rivers provide critical habitats for nesting, feeding, and breeding.
India hosts 1353 species of birds out of the 10721 total birds in the world, constitutes 13% of the total bird population, and thus is an area of high avian diversity. The bird fauna of India represents 114 families out of the total 249 families in the world. The inventory of birds in the state of Maharashtra comprises 556 species (Mahabal et al., 2005). More than 577 species have been reported from Maharashtra State (Kasambe 2016). Similarly, in Vidarbha, there are a total of 417 species, and in Amravati, there are 392 bird species (Wadatkar et al., 2016, Praveen & Jayapal 2023).
Documentation of avian diversity in Melghat has been done for the last 100 years. A preliminary checklist of 33 species of birds was prepared by R.T. Jenkin (then DFO, Melghat) and published in 1925 (Nelson 1925). Later, V. B. Sawarkar, then Director of Melghat Tiger Reserve, prepared and published the first comprehensive checklist of the birds of Melghat, which included 252 species. Malabar Pied Hornbill was not listed in the checklist of birds of MTR until 2003. (Wagh et al., 2003). Thereafter, some important sightings occurred, like the Critically Endangered Forest Owlet (Rithe 2003) and the Malabar Pied Hornbill (Kasambe & Wadatkar 2006). Previously, a total of 263 bird species were recorded in the overall Melghat Tiger Reserve (Mahabal, Anil 2005).
Then, in addition to the checklist of birds in Melghat, which went up to 265 species (Wadatkar et al., 2012), Later on, blue-tailed bee eaters breeding by the Tapti River at the boundary of Melghat were observed by Wadatkar et al. (2014). (Wagh et al., 2015) proposed that the preferential route of dispersal for Malabar Pied Hornbill from the Himalayas to the Western Ghats is through the Satpuda Hills in Central India. Nesting of River Lapwings was first recorded in the Tapi River near the Melghat Tiger Reserve (Wagh et al., 2020). The River Lapwing’s population in the Vidarbha area is constrained to just a few large rivers; however, the species is presently at risk of extinction.
The first ever bird survey of Melghat Tiger Reserve reported a total of 340 bird species (January 2023). During our regular bird watching and surveys being conducted for the River Lapwing and Malabar Pied Hornbill projects, we sighted some species of birds that were not listed in the published checklists of MTR and Amravati district 2016. The results of this study shed light on the importance of conservation and protection of riverine ecosystems for the conservation of avian biodiversity.
MATERIAL AND METHODS
Study Area: The Melghat region is a part of the Satpuda Range of Hills in the Amravati district of central India. This area has dry deciduous forests dominated by teak and bamboo, with excellent tiger habitat. The study area is within latitudes 21°0ʹ15ʺ to 21°0ʹ45ʺ N and longitudes 76°00ʹ57ʺ to 77°00ʹ30ʺ E at elevations of 312 to 1178 m MSL. It is the largest of the three project tiger programmes in the state. The yearly average temperature is 42.7°C, and the annual rainfall is 1000 mm. The reserve is a catchment area for five major rivers: the Sipna, Gadga, Khandu, Khapra, and Dolar, all of which are tributaries of the Tapti River, which flows through the northern part of Melghat Tiger Reserve and forms the boundary of the Melghat landscape together with the Gawilghur ridge of the Satpura Range. (Fig.1,Table 1)
Table 1: The coordinates of birding station in the rivers of Melghat Landscape.
| Rivers | Survey
Stations |
GPS Coordinates |
|
Tapi River |
Chethar | 21.6023°N & 76.9085°E |
| Kutanga | 21.71468°N & 77.0840°E | |
| Rangubeli | 21.71775°N & 77.1401°E | |
|
Sipna River |
Semadoh | 21.4944°N & 77.3122°E |
| Kolkas | 21.5021°N & 77.1748°E | |
| Harisal | 21.5236°N & 77.1248°E | |
|
Gadga River |
Dhakna | 21.4338°N & 77.0509°E |
| Kalamkhar | 21.52823°N & 76.813°E | |
| Aamner fort | 21.52814°N & 76.784°E |
Figure. 1 Map Showing the Rivers of Melghat Landscape with Surveyed Stations, Maharashtra.
Survey Methods: Selected locations in the research area will be surveyed over the duration of a study period. Several surveys will be carried out in the summer (March–June) and after the rainy season (July–October) in 2023. A number of trips will be made to the Sipna River at Semadoh, Kolkas, and Harisal. Similarly, surveys of the Tapti river at the specified locations (Chethar, Kutanga, Rangubeli) and Gadga river locations (Dhakna, Kalamkhar, Aamner Fort) and other nearby potential rivers are needed to better understand the status and distribution of river birds.
A survey has not been conducted on the Khandu River yet. Line transects and point count methods will be used in the riverine ecosystem to determine the diversity and distribution of riverine birds within the river basin. This methodology also helps to determine their abundance in a given research region. Visual scanning is a method of spotting birds primarily based on their visual characteristics and aids in locating their nest during the breeding season. This approach is based on the researcher being able to identify birds visually while recording birds along a predetermined route. For the purpose of searching for riverine birds, visual scanning is the process of scouting riverbanks, mudflats, and sandy areas. Binoculars and spot scopes were used to record observations, and Nikon DSLR cameras equipped with zoom lenses as well as video cameras were used to capture images and videos.
Table 2: Water Birds recorded in riverine habitat of the Melghat landscape.
| Common Name | Scientific Name | Family | ST | IUCN
status |
| Lesser Whistling Duck | Dendro cygnajavanica | DENDROCYGNDIAE (1) | R | LC |
| Northern Pintail | Anas acuta | ANATIDAE (12) | W | LC |
| Common Teal | Anas crecca | W | LC | |
| Red-crested Pochard | Rhedonessa rufina | W | LC | |
| Common Pochard | Aythya ferina | W | VU | |
| Indian Spot-billed Duck | Anas poecilorhyncha | R | LC | |
| Gadwall | Mareca strepera | W | LC | |
| Garganey | Anas querquedula | W | LC | |
| Northern Shoveller | Anas clypeata | W | LC | |
| Eurasian Wigeon | Anas penelope | W | LC | |
| Ruddy (Brahminy) Duck | Tadorna ferruginea | W | LC | |
| Comb Duck (Knob-billed) | Sarkidiornis melanotos | R | LC | |
| Cotton Pigmy goose | Nettapus coromandelianus | R | LC | |
| Common Kingfisher | Alcedo atthis | ALCEDINIDAE (1) | R | LC |
| White-throated Kingfisher | Halcyon smyrnensis | HALCYONIDAE (3) | R | LC |
| Black- Capped Kingfisher | Halcyon pileata | R | LC | |
| Stork-billed Kingfisher | Halcyon capensis | R | LC | |
| Pied Kingfisher | Ceryle rudis | CERYLIDAE (1) | R | LC |
| White-breasted Waterhen | Amanrornis phoenicurus | RALLIDAE (4) | R | LC |
| Purple Swamphen | Porphyrio porphyrio | R | LC | |
| Common Moorhen | Gallinula chloropus | R | LC | |
| Common Coot | Fulica atra | R | LC | |
| Black-tailed Godwit | Limosa limosa | SCOLOPACIDAE (10) | W | NT |
| Pintail Snipe | Gallinago stenura | W | LC | |
| Common Snipe | Gallinago gallinago | W | LC | |
| Common Greenshank | Tringa nebularia | W | LC | |
| Spotted Redshank | Tringa erythropus | W | LC | |
| Green Sandpiper | Tringa ochropus | W | LC | |
| Common Sandpiper | Actitis hypoleucos | W | LC | |
| Wood Sandpiper | Tringa glareola | W | LC | |
| Marsh Sandpiper | Tringa stagnatilis | W | LC | |
| Little Stint | Calidris minuta | W | LC | |
| Temminck’s Stint | Calidris temminckii | W | LC | |
| Greater-painted Snipe | Rostratula benghalensis | ROSTRATULIDAE (1) | R | LC |
| Indian Stone-Curlew | Burhinus indicus | BRUHINIDAE (2) | R | LC |
| Great Stone Curlew | Esacu srecurvirostris | R | NT | |
| Black-winged Stilt | Himantopus himantopus | CHARADRIIDAE (6) | RM | LC |
| Little-ringed Plover | Charadrius dubius | W | LC | |
| Kentish Plover | Charadrius alexandrinus | BM | LC | |
| Yellow-wattled Lapwing | Vanellus malabaricus | R | LC | |
| River Lapwing | Vanellus duvaucelii | R | NT | |
| Red-wattled Lapwing | Vanellus indicus | R | LC |
| Small Pratincole | Glareola lactea | GLAREOLIDAE (1) | R | LC |
| River Tern | Sterna aurantia | STERNIDAE(2) | RM | NT |
| Little Tern | Sterna albifrons | BM | LC | |
| Little Grebe | Tachybaptus ruficollis | PODICIPEDIDAE (1) | R | LC |
| Darter | Achinga melanogaster | ANHINGIDAE(1) | R | NT |
| Little Cormorant | Phalacrocorax niger | PHALACROCORACIDAE (3) | R | LC |
| Indian Cormorant | Phalacrocorax fuscicollis | R | LC | |
| Great Cormorant | Phalacrocorax carbo | R | LC | |
| Little Egret | Egretta garzetta | ARDEIDAE (10) | R | LC |
| Great Egret | Casmerodius albus | R | LC | |
| Intermediate Egret | Mesophoyx intermedia | R | LC | |
| Cattle Egret | Bubulcus ibis | R | LC | |
| Grey Heron | Ardea cinerea | R | LC | |
| Purple Heron | Ardea purpurea | R | LC | |
| Indian Pond Heron | Ardeola grayii | R | LC | |
| Little Green Heron | Butorides striatus | R | LC | |
| Yellow Bittern | Ixobrychus sinensis | R | LC | |
| Black Bittern | Ixobrychus flavicollis | R | LC | |
| Black-headed Ibis | Threskiornis melanocephalus | PHOENICOPTERIDAE (2) | R | NT |
| Red-naped Ibis | Pseudibis papillosa | R | LC | |
| Glossy Ibis | Plegadis falcinellus |
THRESKIORNITHIDAE(1) |
R | LC |
| Painted Stork | Myeteria leucocephala | CICONIIDAE (4) | RM | NT |
| Asian Openbill | Anastomus oscitans | W | LC | |
| Asian Woolly-necked Stork | Ciconia episcopus | R | VU | |
| Black Stork | Ciconia nigra | W | LC | |
| White Wagtail | Motacilla alba | PASSERIDAE (6) | W | LC |
| White-browed Wagtail | Motacilla maderaspatensis | R | LC | |
| Citrine Wagtail | Motacilla citreola | W | LC | |
| Yellow Wagtail | Motacilla flava | W | LC | |
| Grey Wagtail | Mptacilla cinereal | W | LC | |
| R- Widespread Resident, W- Widespread Winter Visitor, PV- Passage visitors, RM- Resident Migrant, BM- Breeding Migrant, V- Vagrant or irregular visitors. IUCN’s list of Threatened species (2023), categorized as Least Concerned (LC), Near Threatened (NT) and Vulnerable (VU). | ||||
Figure 2: Family-wise water birds recorded in rivers of Melghat Landscape.
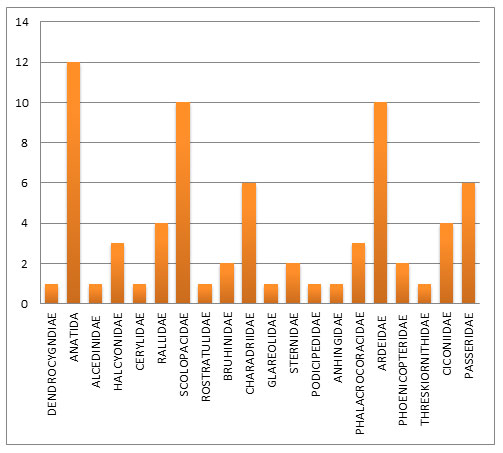
Figure 3: Family-wise forest birds recorded in riverine zone of Melghat Landscape.
The Garmin GPS was also used to record the latitude and longitude of the broadcast spots. Field survey protocol: The study was carried out during the most active periods, i.e., early morning to mid-morning (6 a.m. to 10 a.m.) and late afternoon to evening (3 p.m. to 6 p.m.). The survey was conducted in the study area with the help of field assistants and local forest staff. To gather more information about the riverine birds in the study area, interviews were performed with locals, tribes, local forest employees, birdwatchers, fishermen, and nature guides. Data sheets and avian science forums were also used, along with field guides for the identification of river birds recorded in water and forest.
Table 3: Forest birds recorded in riverine habitat of the Melghat landscape.
| Common Name | Scientific Name | Family | ST | IUCN
status |
| Grey Francolin | Francolinus pondicerianus | PHASIANIDAE (12) | R | LC |
| Painted Francolin | Francolinus pictus | R | LC | |
| Common Quail | Coturnix coturnix | W | LC | |
| Jungle Bush Quail | Perdicula asiatica | R | LC | |
| Rain Quail | Coturnix coromandelica | R | LC | |
| Barred Buttonquail | Turnix suscitator | R | LC | |
| Rock bush Quail | Perdicula argoondah | R | LC | |
| Yellow legged Button Quail | Turnix tanki | R | LC | |
| Red Spurfowl | Galloperdix spadicea | R | LC | |
| Grey Junglefowl | Gallus sonneratti | R | LC | |
| Red Jungle fowl | Gallus gallus | R | LC | |
| Indian Peafowl | Pavo cristatus | R | LC | |
| Eurasian Wryneck | Jynx torquilla | PICIDAE (8) | W | LC |
| Lesser Yellownape | Picus chlroplus | R | LC | |
| Yellow-crowned Woodpecker | Dendrocopos mahrattensis | R | LC | |
| Golden-rumped Flameback | Dinopium benghalense | R | LC | |
| Common Flame Black Woodpecker | Dryocopus javensis | R | LC | |
| White-naped Woodpecker | Chrysocolaptes festivus | R | LC | |
| Brown-pigmy Woodpecker | Yungipicus nanus | R | LC | |
| Lesser yellownape | Picus chlorolophus | R | LC | |
| Brown-headed Barbet | Megalaima zeylanica | MEGALAIMIDAE (2) | R | LC |
| Coppersmith Barbet | Megalaima haemacephala | R | LC | |
| Indian Grey Hornbill | Ocyceros birostris | BUCEROTIDAE(2) | R | LC |
| Malabar Pied Hornbill | Anthracoceros coronatus | R | NT | |
| Common Hoopoe | Upupa epops | UPUPIDAE (1) | R | LC |
| Common Blackbird | Turdus merula | TURDIDAE(1) | R | LC |
| Indian Roller | Coracias benghalensis | CORACIIDAE(2) | R | LC |
| European Roller | Coracioas garrulus | W | NT | |
| Green Bee-eater | Merops orientalis | MEROPIDAE (2) | R | LC |
| Blue -tailed Bee eater | Merops philippinus | R | LC | |
| Pied Cuckoo | Clamator jacobinus | CUCULIDAE (6) | BM | LC |
| Common Hawk Cuckoo | Hierococcyx varius | BM | LC | |
| Indian Cuckoo | Cuculus micropterus | R | LC | |
| Grey-bellied Cuckoo | Cacomantis passerinus | R | LC | |
| Asian Koel | Eudynamys scolopaceus | R | LC | |
| Sirkeer Malkoha | Phaenicophaeus leschenaultii | R | LC | |
| Southern Coucal | Centropus sinensis | CENTROPODIDAE | R | LC |
| Alexandrine Parakeet | Psittacula eupatria | PSITTACIDAE (3) | R | NT |
| Rose-ringed Parakeet | Psittacula krameri | R | LC | |
| Plum-headed Parakeet | Psittacula cyanocephala | R | LC |
| Little Swift | Apus affinis | APODIDAE (2) | R | LC |
| Asian Palm Swift | Cypsiurus balasiensis | R | LC | |
| Crested Tree Swift | Hemiprocne coronata | HEMIPROCNIDAE(1) | R | LC |
| Common Barn Owl | Tyto alba | TYTONIDAE (1) | R | LC |
| Eurasian Eagle Owl | Bubo bubo | STRIGIDAE (8) | R | LC |
| Eurasian Scops Owl | Otus scopus | R | LC | |
| Spotted Owlet | Athene brama | R | LC | |
| Collared Scops Owl | Otus scops | R | LC | |
| Jungle Owlet | Glaucidium radiatum | R | LC | |
| Brown Fish- Owl | Ketupa zeylonensis | R | LC | |
| Mottled Wood Owl | Strix ocellata | R | LC | |
| Forest Owlet | Heteroglaux blewitti | R | EN | |
| Indian Nightjar | Caprimulgus asiaticus | CAPRIMULGIDAE (3) | R | LC |
| Indian Jungle Nightjar | Caprimulgus indicus | R | LC | |
| Savanna Nightjar | Caprimulgus affinis | R | LC | |
| Rock Pigeon | Columba livia | COLUMBIDAE (7) | R | LC |
| Yellow-footed Green Pigeon | Treronphoenicoptera | R | LC | |
| Eurasian Collard-Dove | Streptopeliadecaocto | R | LC | |
| Red Collard-Dove | Streptopelia tranquebarica | R | LC | |
| Spotted Dove | Spilopelia chinensis | R | LC | |
| Laughing Dove | Spilopelia senegalensis | R | LC | |
| Oriental Turtle Dove | Streptopelia orientalis | R | LC | |
| Black-shouldered Kite | Elanus axillaris | ACCIPITRIDAE (12) | R | LC |
| Shikra | Accipiter badius | R | LC | |
| Eurasian Sparrow Hawk | Accipiter nisus | W | LC | |
| Eurasian Marsh Harrier | Circus aeruginosus | W | LC | |
| Pallid Harrier | Circus macrourus | W | LC | |
| Short-toed Snake Eagle | Circaetus gallicus | R | LC | |
| Pallas’s Fish Eagle | Haliaeetus leucoryphus | R | NT | |
| Changeable Hawk-Eagle | Spizhaetus cirrhatus | R | LC | |
| Black Eagle | Ictinaetus malayensis | R | LC | |
| Crested Serpent Eagle | Spilornis cheela | R | LC | |
| Oriental Honey Buzzard | Pernis ptilorhynchus | R | LC | |
| White-eyed Buzzard | Butastur teesa | R | LC | |
| Common Kestrel | Falco tinnunculus | FALCONIDAE (2) | W | LC |
| Lesser Kestrel | Falco naumanni | PV | LC | |
| Indian pitta | Pitta brachyura | PITTIDAEZ(1) | R | LC |
| Blue- winged Leafbird | Chloropsis cochinchinensis | IRENIDAE(2) | R | LC |
| Golden Fronted Leafbird | Chloropsis aurifrons | R | LC | |
| Bay-backed Shrike | Lanius vittatus | LANIIDAE (2) | R | LC |
| Long-tailed Shrike | Lanius schach | R | LC | |
| Rufous Treepie | Dendrocitta vagabunda | CORVIDAE (18) | R | LC |
| House Crow | Corvus splendens | R | LC | |
| Large-billed (Jungle) Crow | Corvus macrorhynchos | R | LC | |
| Eurasian Golden Oriole | Oriolus oriolus | R | LC | |
| Black-hooded Oriole | Oriolus xanthornus | R | LC | |
| Large Cuckoo-Shrike | Coracina macei | R | LC | |
| Black headed Cuckoo-Shrike | Coracina melanoptera | R | LC | |
| White-bellied Minivet | Pericrocotus erythropygius | R | LC | |
| Small Minivet | Pericrocotus cinnamomeus | R | LC |
| Black Drongo | Dicrurus macrocercus | R | LC | |
| Ashy Drongo | Dicrurus leucophaeus | R | LC | |
| White-bellied Drongo | Dicrurus caerulescens | R | LC | |
| Greater Racket-tailed Drongo | Dicrurus paradiseus | R | LC | |
| White-browed Fantail | Rhipidura aureola | R | LC | |
| White-throated Fantail | Rhipidura albicollis | R | LC | |
| Asian Paradise-flycatcher | Terpsiphone paradisi | R | LC | |
| Common Woodshrike | Tephrodornis pondicerianus | R | LC | |
| Common Iora | Aegithina tiphia | R | LC | |
| Oriental Magpie Robin | Copsychus saularis | MUSCICAPIDAE (17) | R | LC |
| Indian Robin | Saxicoloides fulicatus | R | LC | |
| Orange-headed Thrush | Zoothera citrina | R | LC | |
| Blue Rock Thrush | Monticola solitaries | W | LC | |
| Malabar Whistling Thrush | Myophonus horsfieldii | R | LC | |
| Eurasian Blackbird | Turdus merula nigropileus | R | LC | |
| Red-throated Flycatcher | Ficedula parva | W | LC | |
| Ultramarine Flycatcher | Ficedula superciliaris | W | LC | |
| Tickell’s Blue Flycatcher | Cyornis tickelliae | W | LC | |
| Verditer Flycatcher | Eumyis thalassina | W | LC | |
| Grey-headed Canary Flycatcher | Culicicapa ceylonensis | W | LC | |
| Black-naped Monarch | Hypothymis azurea | W | LC | |
| Bluethroat | Luscinia svecica | W | LC | |
| Black Redstart | Phoenicurus ochruros | W | LC | |
| Indian Chat | Cercomela fusca | R | LC | |
| Common Stonechat | Saxicola torquata | W | LC | |
| Pied Bushchat | Saxicola caprata | R | LC | |
| Brahminy Starling | Sturnia pagodarum | STURNIDAE (5) | R | LC |
| Rosy Starling | Sturnia roseus | W | LC | |
| Asian Pied Starling | Gracupica contra | R | LC | |
| Common Myna | Acridotheres tristis | R | LC | |
| Chestnut-tailed Starling | Sturnia malabarica | W | LC | |
| Chestnut-bellied Nuthatch | Sitta castanea | SITTIDAEM(2) | R | LC |
| Velvet – fronted Nuthatch | Sitta frontalis | R | LC | |
| Great Tit | Parus major | PARIDAE(2) | R | LC |
| Black-lored Tit | Parus xanthogenys | R | LC | |
| Dusky Craig Martin | Hirundo concolor | HIRUNDINIDAE (6) | R | LC |
| Plain Martin | Riperia paludicola | R | LC | |
| Barn Swallow | Hirundo rustica | W | LC | |
| Wire-tailed Swallow | Hirundo smithii | R | LC | |
| Red-rumped Swallow | Hirundo daurica | R | LC | |
| Streak-throated Swallow | Hirundo fluvicola | R | LC | |
| Red-vented Bulbul | Pycnonotus cafer | PYCNONOTIDAE (2) | R | LC |
| Red -whiskered Bulbul | Pycnonotus jocosus | R | LC | |
| Zitting Cisticola | Cisticola juncidis | CISTICOLIDAE (1) | R | LC |
| Jungle Prinia | Prinia sylvatica | SYLVIIDAE (17) | R | LC |
| Plain Prinia | Prinia inornate | R | LC | |
| Ashy Prinia | Prinia socialis | R | LC |
| Grey-breasted Prinia | Prinia hodgsonii | R | LC | ||
| Oriental White-eye | Zosterops palpebrosus | R | LC | ||
| Blyth’s Reed Warbler | Acrocephalus dumetorum | W | LC | ||
| Lesser Whitethroat | Sylvia curruca | W | LC | ||
| Clamorous Reed Warbler | Acrocephalus stentoreus | W | LC | ||
| Booted Warbler | Hippolais caligata | W | LC | ||
| Greenish Warbler | Phyloscopus trochiloides | W | LC | ||
| Sulphur-bellied Warbler | Phylloscoppus griseolus | W | LC | ||
| Tawny-bellied Babbler | Dumetia hyperythra | R | LC | ||
| Common Tailor Bird | Orthotomus sutorius | R | LC | ||
| Yellow-eyed Babbler | Chrysomma sinense | R | LC | ||
| Large Grey Babbler | Turdoides malcolmi | R | LC | ||
| Jungle Babbler | Turdoides striatus | R | LC | ||
| Common Babbler | Turdoides caudatus | R | LC | ||
| Indian Bush Lark | Mirafra erythroptera | ALAUDIDAE (6) | R | LC | |
| Ashy-crowned Sparrow Lark | Eremopterix griseus | R | LC | ||
| Sykes’s Lark | Galerida deva | R | LC | ||
| Singing Bushlark | Mirafra cantillllans | W | LC | ||
| Rufous-tailed Lark | Ammomanes phoenicura | R | LC | ||
| Greater Short-toed Lark | Calandrella brachydactyla | W | LC | ||
| Purple-rumped Sunbird | Leptocomazeylonica | NECTARINIDAE (3) | R | LC | |
| Purple Sunbird | Cinnyris asiaticus | R | LC | ||
| Thick-billed Flowerpecker | Dicaeum agile | R | LC | ||
| Paddyfield Pipit | Anthus rufulus | PASSERIDAE (13) | W | LC | |
| Tawny Pipit | Anthus campestris | W | LC | ||
| Tree pipit | Anthus trivialis | W | LC | ||
| House Sparrow | Passer domesticus | R | LC | ||
| Chestnut-shouldered Petronia | Petronia xanthocollis | R | LC | ||
| Baya Weaver | Ploceus philippinus | R | LC | ||
| Red Avadavat | Amandava amandava | R | LC | ||
| Indian Silverbill | Euodice malabarica | R | LC | ||
| Scaly-breasted Munia | Lonchura punctulata | R | LC | ||
| Crested Bunting | Melophus lathami | R | LC | ||
| Black-headed Bunting | Emberiza melanocephala | W | LC | ||
| Red-headed Bunting | Emberiza bruniceps | W | LC | ||
| Grey-necked Bunting | Emberiza buchanani | W | LC | ||
| R– Widespread Resident, W– Widespread Winter Visitor, PV– Passage visitors, RM– Resident Migrant, BM- Breeding Migrant, V– Vagrant or irregular visitors, from Melghat Landscape.
IUCN’s list of Threatened species (2023), categorized as Least Concerned (LC), Near Threatened (NT) and Vulnerable (VU). |
|||||
Statistical Analysis: Biological diversity indices were calculated to compare riverine sites. Various types of total species diversity indices, including Simpson’s diversity index (-D) was used to estimate the biodiversity using the equation: D = ∑ ni (ni-1)/ N (N-1), Where D = Simpson’s Index of Dominance ni = total number of individuals of a particular species N = the total number of individuals of all species (Simpson, 1949). Similarly, Shannon diversity index was determined by H´= – ∑ (Pi) (ln Pi), in which Pi = Proportion of total species belonging to ith species.The diversity indices were calculated using the software PAST version 4.03. (Table 4).
Table 4: Summary of Diversity indices of water birds and forest birds recorded in rivers of Melghat Landscape.
| Observations | Water birds
recorded in river |
Forest birds
recorded in river |
| Species numbers | 72 | 173 |
| Total Individuals | 861 | 2229 |
| Simpson Diversity Index [D] | 0.973 | 0.991 |
| Shannon Diversity Index [H] | 3.961 | 4.903 |
| Menhinick Index | 2.42 | 3.604 |
| Margalef Index | 10.36 | 21.93 |
| Evenness | 0.7396 | 0.7921 |
| Berger-Parker | 0.06504 | 0.03146 |
| Dominance | 0.0247 | 0.0009 |
| Fisher alpha | 18.35 | 42.83 |
Figure 4: Percentage of resident (R) and migratory (M) Water and Forest birds in study area.
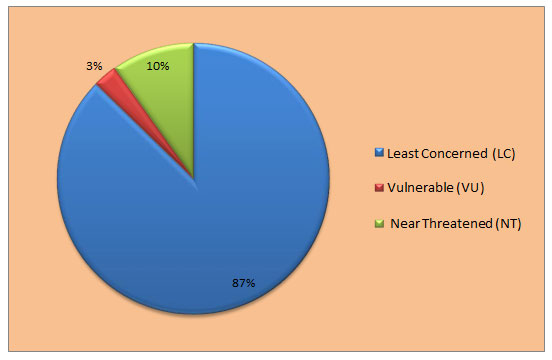
Figure 5: IUCN status of Water birds recorded in rivers in Melghat Landscape.
RESULTS AND DISCUSSION
In the course of an extensive survey conducted to determine the diversity of riverine birds in the riverine ecosystems of the Melghat Landscape, several key observations were made. The results of the study are as follows: total 245 species of riverine birds belonging to 54 families were recorded. In the riverine ecosystem of the Melghat landscape, a total of 861 individuals from water-recorded bird species were reported, and from forest-recorded birds, 2229 individuals were reported. The study area is richly diversified, with flowing, clean rivers all over the Melghat landscape (Table 4).
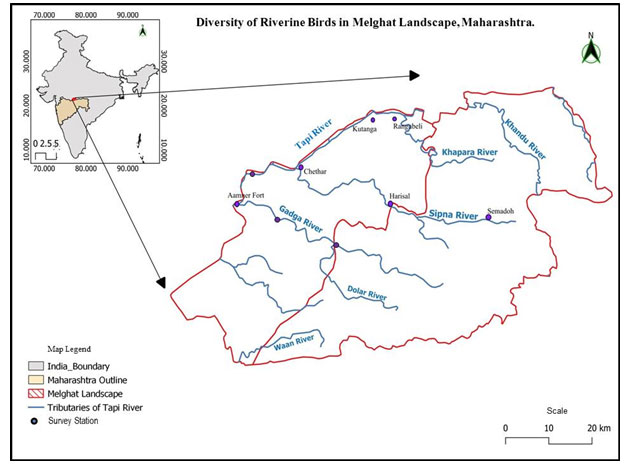
Out of which, 72 species from 20 families of water birds were recorded in the riverine ecosystem, and 173 species from 34 families of forest birds were recorded in the riverine ecosystem. Out of the 54 families of birds observed in the course of the study, the majority belonged to the Ardeidae family, followed by the Sylviidae, Phasianidae, Anatidae, Accipitridae, Corvidae, and Charadriidae families, which belong to forest birds (Fig 2 and 3).
A maximum of 12 species were recorded from the Anatidae family of birds in riverine ecosystems, including the Common Teal, Red-crested Pochard, Common Pochard, Indian Spot-billed Duck, Gadwall, Northern Shoveller, Eurasian Wigeon, Ruddy (Brahminy) Duck, Comb Duck (Knob-billed), and Cotton Pigmy Goose. While 10 species from the Scolopacidae family include Black-tailed Godwit, Pintail Snipe, Common Snipe, Common Greenshank, Spotted Redshank, Green Sandpiper, Common Sandpiper, Wood Sandpiper, Little Stint, Temminck’s Stint, Also, a maximum abundance of 10 species were recorded from the Ardeidae family, including the Little Egret, Great Egret, Intermediate Egret, Cattle Egret, Grey Heron, Purple Heron, Striated Heron, Indian Pond Heron, Yellow Bittern, and Black Bittern, while the Charadriidae family includes the Red-Wattled Lapwing, River Lapwing, Black-Winged Stilt, Little-Ringed Plover, and Kentish Plover, as well as only one member from the Rostratulidae family, which is the Greater-painted Snipe.
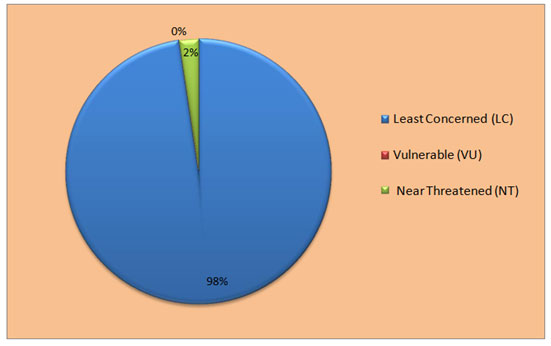
We also noted the conservation status of recorded birds according to the latest updates on the IUCN’s list of threatened species (2023), categorised as least concerned (LC), near threatened (NT), and vulnerable (VU), and the red lists of Bird Life International (Tables 2, 3, and Fig. 5 and 6). The IUCN Red List (2023) classified 87% of species as of least concern (LC) and 10% as near-threatening (NT): River Lapwing, Black-tailed Godwit, Great Stone Curlew, River Tern, Darter, Black-headed Ibis, and Painted Stork, where the 2 species that come in the vulnerable category are Asian Woolly-necked Stork and Common Pochard from water recorded birds in the riverine ecosystem of the Melghat landscape. Similarly, for forest recorded birds, it is categorised as 98% species of least concern (LC), and only Malabar Pied Hornbill, European Roller, Alexandrine Parakeet, and Pallas’s Fish Eagle are categorised as near-threatened (NT). The study area habitat serves as a suitable habitat for more diversity and species richness in avian fauna.
Figure 6: IUCN status of forest birds recorded in rivers in Melghat Landscape.
Observations over different seasons highlighted variations in the composition and abundance of riverine bird species. Certain species were found to be migratory, emphasising the seasonal dynamics and the significance of the Melghat Landscape as a stopover or breeding ground for these birds. Birds migrate by rivers for several compellation reasons. Firstly, rivers serve as natural navigational guides, offering a clear and linear path. Their flow and direction act as visual cues and help birds stay on course during long migrations. This makes the journey more efficient and minimizes the risk of getting lost.

Secondly, rivers are abundant sources of food, provided migratory birds with a consistent and easily accessible food supply. Fish, insects, and other aquatic organisms thrive in and around rivers, allowed birds to replenish their energy reserves during stopovers. Additionally, the riparian habitats along riverbanks offer suitable resting and roosting sites for birds. Resting is crucial during migration to conserve energy, and these areas provide shelter and safety, ensured the birds are well-prepared for the next leg of their journey. Lastly, rivers also provide a readily available source of water, essential for birds to drink and bathe in. Migratory birds often pause at rivers to quench their thirst and maintain their plumage, further contributing to their overall well-being during migration.
Plate: 1 Significant Birds Sightings in the riverine habitat of Melghat Landscape.



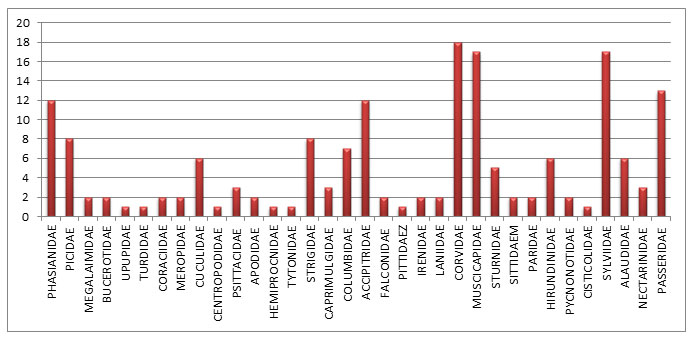
In the riverine survey of Melghat rivers, all the recorded species were categorised according to their presence in the study area. Where Widespread Resident (R) constitutes 40 species that account for 56%, Widespread Winter Visitor (W) includes 27 species that account for 37%, Breeding Migrant (BM) includes Kentish Plover, Little Tern, and Resident Migrant (RM), 3 species that are Painted Stork, Black-Winged Stilt, and River Tern from the water recorded birds of the riverine ecosystem of Melghat landscape (Fig.4). Similarly, Widespread Resident (R) comprises 136 species that account for 79%, Widespread Winter Visitor (W) includes 34 species that account for 20%, Breeding Migrant (BM) includes Pied Cuckoo, Common Hawk Cuckoo, and Passage Visitors (PV) have Lesser Kestrel from the forest recorded birds of the riverine ecosystem of Melghat landscape (Fig.4).
Indicator bird species were chosen according to their ecological affinity for one of the four major wetland habitat types (marshes, wet meadows, shrub swamps, and treed swamps), their sensitivity to hydrological conditions (depth of surface water and fluctuations of water level) (Weller, 1999), and their nesting strategy (i.e., on ground near shoreline, on floating vegetation, or attached to vegetation above water or on ground) (Gibbs et al., 1991; Steen and Gibbs, 2002). The analyses presented here are restricted to a limited selection of indicator species distributed according to the four major wetland habitat types, riverine habitat types, the vulnerability of their nests, and the nature of the statistical relationship with hydrological variables, (Sinha et. al 2019).
Riverine birds are birds that are found in and around rivers, streams, and other bodies of freshwater. Some indicator species of riverine birds that are located as indicator species include the Green Sandpiper, River Tern, River Lapwing, Malabar Whistling Thrush, Pied Kingfisher, Stork Billed Kingfisher, Little Egret, Indian Cormorant, etc. These species were chosen based on their association with riverine habitats, sensitivity to changes in hydrological conditions, and nesting strategies.
The survey revealed a rich diversity of riverine birds in the Melghat Landscape, included various species such as egrets, kingfishers, herons, ducks, and waders. The presence of multiple species indicated a healthy and diverse avian community dependent on riverine ecosystems. The diversity index, species evenness, and species abundance were studied. In the water bird recorded with riverine ecosystem study area, various diversity indices, as mentioned, showed the result like Simpson Diversity Index is 0.973, Shannon Diversity Index is 3.961, Menhinick Index is 2.42, Margalef Index is 10.36, Berger-Parker is 0.06504, and evenness is 0.7396. Similarly, in forest birds recorded in riverine ecosystem areas, the Simpson Diversity Index is 0.991, the Shannon Diversity Index is 4.903, the Menhinick Index is 3.604, the Margalef Index is 21.93, the Berger-Parker is 0.03146, and evenness is 0.7921. Whereas abundance on the river of Melghat landscape reservoir is 3086 (Table 4).
The consistent presence of both the Pied kingfisher and the White-throated kingfisher across all riverine habitats in the Melghat landscape highlights the ecological adaptability and widespread distribution of these avian species in the region. The presence of the Black-capped kingfisher exclusively observed along the Dolar River underscores its ecological significance and uniqueness. The population of the Black-capped Kingfisher in this specific location is experiencing a decline. The potential sites for the River Lapwing as a hotspot are Chethar, Kutanga, and Rangubeli, and similar sites for the Stork-billed Kingfisher are Kolkas, Chaurakund, Rangubeli, Semadoh, and Harisal.
The observations of this survey contributed to significant bird sightings like River Lapwing and Stork-billed Kingfisher from the Tapi River and Sipna River, which had significant results at Rangubeli, Kutanga, Chethar, Amner Fort, Semadoh, and Harisal, respectively. The Forest Owlet (Heteroglaux blewitti), a critically endangered species of Owl that was thought to be extinct for over a century, was observed at Churni Nala Chaurakund, which is ultimately part of the Sipna River. The distribution of River Lapwing (Vanellus duvaucelii) is confined to the Tapi River; it is not observed in other rivers of the Melghat landscape.
The presence of the Green Sandpiper in the riverine habitats of the Tapti and Sipna rivers indicates the significance of these water bodies as suitable environments for this particular bird species. Likewise, Malabar Pied Hornbill, Painted Stork, Black Stork, Asian Woolly-necked Stork, Eurasian Eagle Owl, Brown Fish-Owl, Great Stone Curlew, Black-headed Ibis, Glossy Ibis, European Roller, Brown Crake, etc. contributed to the scientific understanding of riverine birds in the Melghat Landscape but also provided a foundation for informed conservation actions. This study has established the framework for focused conservation measures aimed at preserving the high biodiversity of riverine habitats in the Melghat Landscape by extensively documenting the condition and variety of these birds.
Primary threats to riverine ecosystems include direct and indirect threats, such as sand mining and illegal fishing with explosive material and feral dog movements which can disrupt critical nesting and foraging sites for riverine birds. Anthropogenic activities, such as water extraction for agriculture can alter water levels, ecological parameters, and seasonal events, such as seasonal crop framing in river banks, affecting their survival and health.
CONCLUSION
The study revealed that the Melghat landscape is a unique habitat for a diverse range of riverine birds. The high diversity of 245 riverine bird species in the study area highlights the importance of the riparian zones as a crucial element of the natural system. The majority of the observed species belonged to the Ardeidae family, with the maximum number of Little Egrets, Little Cormorant, and River Tern as it is an indicator species of riverine ecosystems. These are opportunistic feeders and consume a variety of aquatic organisms, such as fish, amphibians, crustaceans, and insects. Their feeding activities can help to regulate the population of prey species and thus maintain the balance of the riverine ecosystem, which is consistent due to clean water in riverine systems.
These findings suggest that the Tapi River is a crucial habitat for these near-threatened species, River Lapwing, and its distribution is restricted to the only Tapti River, which is a large and flowing river. Such a flowing river serves as a lifeline for riperine birds. The Stork-billed Kingfisher and Black-capped Kingfisher are important in Melghat for their roles in maintaining ecological balance by controlling fish and insect populations, serving as indicators of healthy riparian ecosystems, and contributing to the region’s biodiversity and ecotourism appeal.
ACKNOWLEDGEMENTS
The authors extend heartfelt gratitude to the Institute Innovation Cell, HRD and Principal Dr. G.V. Korpe, Shri Shivaji Science College, Amravati, for generous fu to the corresponding authornding and unwavering support. The authors also acknowledge the CCF, field director and staff of MTR for granting permission for this research.
Conflict of Interest: Authors declare no conflict of interest.
Data Availability: Data will be available on reasonable request, made to the corresponding author.
REFERENCES
Ali, S., & Ripley, S. D. (1987). The compact handbook of the birds of India and Pakistan together with those of Bangladesh, Nepal, Bhutan and Sri Lanka (2nd ed). Oxford University Press.
Ashwin L, Gajanan W, Amol R, Pratik C. Diversity of Avian species in Upper Wardha Reservoir Amravati, Maharashtra. Biosc.Biotech.Res.Comm. 2023;16(3). Available from: <a href=”http://surl.li/lskqm“>http://surl.li/lskqm</a>
Bharos, A. & Verma, Faiz, Naidu, & Ravi. (2020). First report of summer nesting avian species at River Mahanadi (Chhattisgarh segment), Chhattisgarh, India. Jageshwar and Bux, 9, 367–373.
Birdlife International. (2020). Vanellus duvaucelii. IUCN Red List of Threatened Species, 2016:.http://doi.org/10.2305/IUCN.UK.2020
Grimmett, R., Inskipp, C., & Inskipp, T. (1999). Birds of the Indian subcontinent. Oxford University Press.
Kasambe, R., Wagh, G., Mahajan, A., Wadatkar, J., & Dhurve, M. (2012). Recent sighting records of Grey-headed Lapwing (Vanellus cinereus) in Maharashtra. Newsletter for Birdwatchers, 52(6), 90–91+One illustration on back cover.
Kayet, N., Chakrabarty, A., Pathak, K., Sahoo, S., Dutta, T., & Hatai, B. K. (2020). Comparative analysis of multi-criteria probabilistic FR and AHP models for forest fire risk (FFR) mapping in Melghat Tiger Reserve (MTR) forest. Journal of Forestry Research, 31(2), 565–579. https://doi.org/10.1007/s11676-018-0826-z
Kumar, V., & Mishra, H. (2020). Foraging behavior in River Lapwing, Vanellus duvaucelii (Lesson, 1826) (Charadriiformes: Charadriidae): Differences in technique, prey, and habitat. Journal of Asia-Pacific Biodiversity, 14. https://doi.org/10.1016/j.japb.2020.09.011
Jayadevan, P., & Jayapal. (2023). Rajah. Taxonomic Updates to the Checklists of Birds of India and the South Asian Region, 2023. Indian BIRDS. 18, 131–134.
Mahabal, Anil (2005) Aves. In : Fauna of Melghat Tiger Reserve, Conservation Area Series, 24 : 115-163. Publ. by Director, Zool. Surv. India, Kolkata.
Rasmussen, P. C., & Anderton, J. C. (2012). Birds of South Asia. The Ripley Guide. Michigan State University and Lynx Edicions. MI and Barcelona, 1 and 2(2)^nd edition. National Museum of Natural History – Smithsonian institution.
Sinha, A., Chatterjee, N., Ormerod, S. J., Adhikari, B. S., & Krishnamurthy, R. (2019). River birds as potential indicators of local- and catchment-scale influences on Himalayan river ecosystems. Ecosystems and People, 15(1), 90–101. https://doi.org/10.1080/26395916.2019.1591508
Wadatkar, J., Kasambe, R., Wagh, G., Abhang, N., & Morey, K. (2016). Checklist of Birds of Amravati district. Wildlife and Environment Conservation Soc. Amravati, 1–22.
Wadatkar, J. S., Wagh, G. A., Dudhe, N. S., & Thakre, A. V. (2012). Additions to the checklist of birds of Melghat Tiger Reserve. Mistnet, 13(2), 6–7.
Wadatkar, Jayant & Wagh, Gajanan & Kadu, G. (2014). Breeding record of Blue – tailed Bee eater (Merops philippinus) in Tapti River , Melghat Tiger Reserve, India. Newsletter for Birdwatchers. 54. 51.
Wagh, G. A., & Prathmesh, T. D. (2020). On the diversity and abundance of avian species from grassland and wetland areas of an industrial area of tropical Maharashtra. Biosc. Biotech.Res.Comm, 13(2).
Wagh, G. A. (2019). Wetlands and Water birds of Amravati District (1st ed) (pp. 1–61). Wildlife and Environment Conservation Society.
Wagh, Gajanan & Wadatkar, Jayant & Kasambe, Raju. (2015). Status and distribution of Malabar Pied Hornbill anthracoceros coronatus in melghat tiger reserve, maharashtra. International Journal of Plant , Animal and Environmental Sciences (IJPAES). 5. 60-69.
Raju, Qureshi, Akhtar, H., Borode, & Nikhil. (2020). Wagh, Gajanan and Wadatkar, Jayant and Kasambe. River Lapwing Vanellus Duvaucelii Breeding by the Tapi River, 24, 12–14.
Zargari, A., Salarijazi, M., Ghorbani, K., & Ahmad Dehghani, A. (2023). Effect of dam construction on changes in river’s environmental flow (case study: Gorganrood river in the south of the Caspian Sea). Applied Water Science, 13(11), 212.
https://www.magicalmelghat.in/public/website/pdf/Cover-Check-List-of-Birds-2023-Final-July.pdf


