Department of Biology, Faculty of Education, University of Al-Qadisiyah, Al-Diwaniyah, Iraq
Corresponding author Email: Sadiya.anah@qu.edu.iq
Article Publishing History
Received: 27/03/2018
Accepted After Revision: 19/06/2018
A survey of parasites was conducted in 130 snakes collected from five sites of Al-Diwaniyah Province (City Center, Afak, East Hamzah, Al-Badair and Nafer districts of Iraq. The snakes belong to 8 species of two families Colubridae and Biodae, which contain Platyceps ventromaculatus, P. rogers, Malpolon monspesslana, Sapalerosophis clifordi, Dolichophis mesopotamicus, Rhynchocalamus melanocephalu, Eryx jaculus and Natrix tessellate. Eighty-three samples of snakes 63.84% were infected with five species of internal parasites which include two protozoans, Isospora sp. and Cryptosporidium sp, two species of nematodes Kalicephalus sp. and Strongyloides sp. and one species of cestode Oochoristica tandani. Through the site of intestinal parasites in the digestive tract was mostly small intestine, higher percentage of them were detected in other parts of digestive tract. Results also showed that single parasitic infections were most common in comparison to other infections. This is the first survey of intestinal parasites from snakes in Iraq where detailed description of the intestinal parasites are reported for the first time from snakes of Iraq.
Snakes, Reptile, Intestinal Parasites, Iraq
AL-Mayali H. M. H, Anah S. A. Description of Intestinal Parasites Found in Some Snakes of Al-Diwaniyah, Iraq. Biosc.Biotech.Res.Comm. 2018;11(2).
AL-Mayali H. M. H, Anah S. A. Description of Intestinal Parasites Found in Some Snakes of Al-Diwaniyah, Iraq. Biosc.Biotech.Res.Comm. 2018;11(2). Available from: https://bit.ly/30ahPjX
Introduction
Al-Diwaniyah province, (180 KM south of Baghdad) is one of the southern provinces and its territory is part of the plain sedimentary Iraqi, which is characterized by the simple decline from north-west to the south and south-east also show minor differences and other local in the surface of the province because of several factors, most important process of wind sedimentation and can be explained nature by dividing the province. The first part consists of the flood plain, which includes most areas of the province and the area of the shallow and semi-shallow depressions, which the second part represents, the third part which is located in the sand dunes area. Such as the districts of Afak and AL-Badir, and the fourth part, which is represented by the sandy area and covers the southwestern part of the province in the area between the west of the Euphrates River and the Western administrative boundaries of the province, (Al-Janabi & Ghaleb, 1992).
There are about 2,900 species of snakes spread in all countries except the North and South Poles, the Hawaiian Islands, Ireland and New Zealand. The anatomical and physiological behavior of the scale diaphragms in the body is the easiest way to distinguish between these species, (Vitt & Caldwell, 2013 McAllister et al. (2015).
Some of these species are venomous and some other nonvenomous. The poison is mainly used to kill prey or defend itself. Snakes are not at all harmful and many of them are highly beneficial to human beings as they feed on rats and mice and their skin is of great value as it enters in the illegal manufacture of bags and shoes. In addition, the venom of some snakes is used in preparing antidotes for poisoning, (Pasi, 1992; Caswell et al., 2014, Yimming et al. (2016).
Reptiles, including snakes, are exposed to a variety of pathogens, which may be bacterial, fungal, viral, parasitic agents. There are many studies have indicated that snakes are the intermediary or definitive hosts for many of the internal parasites, such as round worms such as Angusticaecum sp, Porocephalus crotali, capillaria sp. as well as intestinal protozoa such as Eimeria sp., Isospora sp., Caryospora sp., Tyzzeria sp, (Klingenberg, 2000 Parc 2008, Rataj et al., 2011).
Cryptosporidium sp. is an intermediate host of Toxoplasma sp. (Duszynski & Upton, 2009), as well as it is a blood parasite similar to Plasmodium, Haemoproteus mesnili and Haemoproteus balli (Telford, 2009; Jacobson, 2007). It is also affected by external parasites, most notably ticks such as Amblyomma sp. and mites like Ophionyssus natricis(Rataj et al., 2011; Pietzsch et al., 2006 McAllister et al. (2015).
According to our literature review, despite of wide distribution of these snakes in Iraq, there is not much comprehensive and adequate published data about intestinal parasites of these snakes. Therefore the current study was conducted to prepare list of intestinal parasites of these snakes in Iraq.
Material and Methods
Examination of Collected Snakes and their Parasites
The dead snakes were kept in the refrigerator at 7° C and dissected within seven days while living species were anesthetized with ether after the removal of the fangs (Fontenot & Font, 1996). Then process of snakes dissection was carried out according to the method of Jacobson, (1978), where the snakes were placed on a large piece of cork designed for this purpose. Then they were opened from abdominal side, starting from annual slit towards the fore front. The digestive tract and its components were then removed by sharp scissors in a sterile Petri dish to look for intestinal worms. It was also examined near the yellow sac around the pancreas adjacent to the stomach as well as the liver and lungs. In case of isolation of intestinal worms, it was washed with water and kept in containers of ethyl alcohol, then added drops of glycerin. For the purpose of clarification and confirmation, use of acetocarmine dye was carried out to pigment tapeworms, trematoda, and acanthocephalus. Nematodes were placed in lactophenol solution and then observed on a clean glass slide using Canada’s balsam (Chaiyabutr & Chanhome, 2002).
For the study of intestinal protozoa a sample of the stool was examined in direct smears with the use of some illustrative pigments such as iodine and Zell-Nelson. All intestinal parasites were examined under low and high magnifications (10x,40x) and necessary measurements were taken using ocular and stage micrometer. Identification of parasites was carried out using characters described by Rataj et al., (2011).
Statistical Analysis
The results were analysed by Completely Randomized Design (CRD) which was adopted as a one-way and two-way laboratory experimental design, as well as a comparison of the averages using a Least Significant Difference (LSD) under probability level of P ≤ 0.05
Results
Of the 130 snakes samples examined,83 snakes, 63.84% were found to be infected with different species of parasites (Table 1) and five species of intestinal parasites were recorded which included two species of protozoans one species of cestoda and two species of nematode. The results show that the distribution of intestinal parasites in digestive tract of snakes, tapeworms was found only in the large and small intestine being 80 and 50 % respectively, while nematodes, Kalicephalus sp. were found in three different places of the gastrointestinal tract, stomach, small and large intestine being 31.82, 52.27 and 20.45% respectively. Strongyloides sp, was found only in the small intestine at a rate of 100%. Isospora sp. was recorded in stomach, small and large intestine being 42.86, 85.71 and 9.52% respectively. Similarly, Cryptosporidium spp. was recorded only in the small and large intestine being 28.57 and 75%, respectively.
| Table 1: Number & percentage of snakes infected with internal parasites. | |||||||||||||||||
| Species of snakes (n=130) | Species of parasites | ||||||||||||||||
| R.melanocephalus
(n=1) |
D.mesopotamicus
(n=15) |
N.tessellata
(n=19) |
S.clifordi
(n=11) |
E.jaculus
(28) |
M.monspessulana
(n=13) |
P.roger s
(n=1) |
P.ventromacultus
(n=42) |
||||||||||
| S.I* | N(%) | S.I* | N(%) | S.I* | N(%) | S.I* | N(%) | S.I* | N(%) | S.I* | N(%) | S.I* | N(%) | S.I* | N(%) | ||
| 0 | 0(0) | 1 | 2(13.33) | 2 | 11(57.89) | 0 | 0(0) | 1 | 2(7.14) | 0 | 0(0) | 0 | 0(0) | 2.8 | 5(11.90) | Oochoristica tandani | |
| 0 | 0(0) | 1 | 2(13.33) | 1.42 | 7(36.84) | 1 | 3(27.27) | 1.66 | 21(75) | 1 | 1(7.69) | 0 | 0(0) | 1.20 | 10(23.81) | Kalicephalus sp. | |
| 0 | (0)0 | 0 | 0(0) | 0 | 0(0) | 0 | 0(0) | 1 | 2(7.14) | 0 | 0(0) | 0 | 0(0) | 1 | 1(2.38) | Strongyloides sp. | |
| 0 | 0(0) | 3.30 | 3(20) | 4 | 2(10.53) | 1.75 | 4(36.36) | 10 | 2(7.14) | 3 | 2(15.38) | 0 | 0(0) | 3.30 | 8(19.05) | Isospora sp. | |
| 0 | 0(0) | 2.50 | 4(26.67) | 2 | 7(36.84) | 1.20 | 5(45.45) | 2 | 3(10.7) | 1.25 | 4(30.77) | 0 | 0(0) | 2.40 | 5(11.90) | Cryptosporidium spp. | |
| To compare the severity infection between parasites species=1.26
To compare the severity infection between snakes species=1.26 |
To compare the infection rate between parasites species=14.40
To compare the infection rate between snakes species=N.S |
LSD(P≤0.05) | |||||||||||||||
| F.calculated:7.03 F.table:2.32
F.calculated:3.32 F.table:2.32 |
F.calculated:4.12 F.table:2.32
F.calculated:2.15 F.table:2.23 |
||||||||||||||||
| *S.I: Severity of infection | |||||||||||||||||
The statistical analysis showed no significant differences in infection rates according to species parasites, while significant differences in infection rates were found according to location of infection, (Table 2). It is clear from (Fig. 12) that the highest incidence of intestinal parasites was in single infections with one species of parasite with 65.62 % dominance , followed by binary infections with 36.14 %, while triple infections (three or more parasites) ranked 7.22 %. Significant differences were found in the types infection at the probability level (P ≤ 0.05).
| Table 2: Number &infection rate of intestinal parasites in digestive tract in snakes. | |||||||
| Site of infection | Number of parasites | Species of parasites | |||||
| Large intestine | Small intestine | stomach | |||||
| % | n | % | n | % | n | ||
| 50 | 10 | 80 | 16 | 0 | 0 | 20 | Oochoristica tandani |
| 31.82 | 14 | 52.27 | 23 | 20.45 | 9 | 44 | Kalicephalus sp. |
| 0 | 0 | 10 | 3 | 0 | 0 | 3 | Strongyloides sp. |
| 42.86 | 9 | 85.71 | 18 | 9.52 | 2 | 21 | Isospora sp. |
| 28.57 | 8 | 75 | 21 | 0 | 0 | 28 | Cryptosporidium spp. |
| To compare the infection rate according to species of parasites =N.S
To compare the infection rate according to site of infection =33.98 |
LSD(P≤0.05) | ||||||
| F.calculated:0.31 F.table:3.83 (to species parasites)
F.calculated:20.91 F.table:4.45 (to site infection) |
|||||||
 |
Figure 1: Some species of snakes |
Isospora sp.(Henyon,1923)
Species of intestinal protozoa were recorded in smears prepared from all snakes except P. roger‘s and Rhynchocalamus melanocephalus. In the isolates from stomach, small and large intestinal ,the immature cysts of Isospora sp. were detected, being oval with one end was having rounded boundary, and the other was thin and had a wall consisting of two thin layers and was with a micropyle at the high end, which was about 19-20 33 x 20 micrometers. The life cycle of this parasite was of direct nature and the host was perhaps infected by mature cysts along with food or water contamination, (Fig.2)
 |
Figure 2: Cysts of Isospora sp. (100x) |
Cryptosporidium spp. (Tyzzer, 1907)
The cyst of Cryptosporidium spp isolated from small and large intestinal of all types of snakes except the two species P. roger’s and R. melanocephalus showed cysts which were spherical or oval-shaped, containing eight spores with double-walled being about 5.1 x 4.5 micrometers and were characterized by having a direct life cycle, (Fig. 3).
 |
Figure 3: Cysts of Cryptosporidium spp.(100x) |
Oochoristica tandani (Luhe, 1898)
This species of cestoda was found in both the large and small intestines of P. ventromaculatus, E.jaculus, N. tessellata and D. mesopotamicus which were tall and thin worms with a length of about 90 mm and a width of 0.77 mm. The head was equipped with four suckers without spines followed by the neck area consisting of 40 immature proglottids followed by mature proglottids, after about 10 pieces of the gravid proglottids, were 18 proglottids and all types of proglottids had length greater than the width by about 4 times. The ovary consisted of 5-4 lobes while number of testes had 37-45 test, both ventral longitudinal excretory ducts and narrow dorsal ducts showed small anastomosing branches (fig. 4, 5 & 6).
 |
Figure 4 |
 |
Figure 5: Immature proglottids |
 |
Figure 6: Mature proglottids Scolex(100x) |
Kalicephalus sp. (Rudolphi, 1819)
This nematode worm had the highest percentage of isolated intestinal worms, it was found in all species of snakes except Platyceps rogers and Rhynchocalamus melanocephalus, and was found in both the large and small intestines as well as stomach, this nematoda female had short, strong worms with a white color that did not exceed 6 mm in length. The mouth was equipped with four pyramid cuticular structures, it had esophagus funnel shaped containing three small teeth and with strong kaitinine lining, excretory pore was near to posterior end, uterine branches were opposed or parallel and were full of eggs with posterior end being conical, (fig. 7,8 & 9).
 |
Figure 7: Anterior end of Kalicephalus sp. female(100x) |
 |
Figure 8: Eggs of Kalicephalus sp. female(100x) |
 |
Figure 9: Posterior end of Kalicephalus sp. female(100x) |
Strongyloides sp. (Grassi, 1879)
This nematode worm was found in the small intestines of only two species of snakes: P. ventromacu- latus and Eryx jaculus. The isolated female length was about 3.5 mm and white color and was covered with a soft layer of cuticle. The oral cavity was small and followed by a cylindrical shaped esophagus. The uterus had a thin wall and was filled with eggs that appeared in the shape of two tubes full of eggs (Fig.10,11 & 12).
 |
Figure 10: Anterior end of Strongyloides sp. female(100x) |
 |
Figure 11: Eggs of two tubes (100x) |
 |
Figure 12: Posterior end of Strongyloides sp. female(100x) |
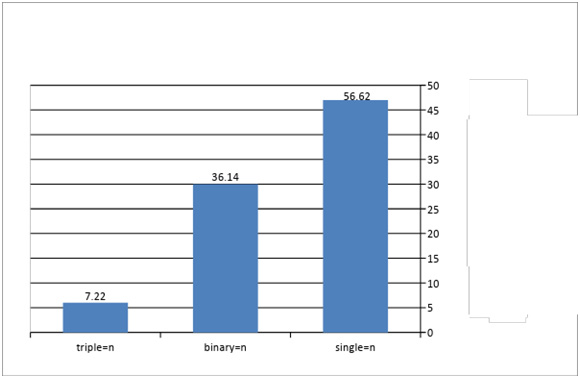 |
Figure 13: Types of infection & percentage of infected snakes with intestinal parasites |
Discussion
The results of the present study indicate that the total infection rates of snakes were 63.84 %, which is lower than that recorded by Chaiyabutr & Chanhome (2002) in Thaland, Santora et al., (2013) in southern Italy and Nasiri et al., (2014) being 75,95 and 73.56 % respectively, and is higher than that recorded by Dusen et al., (2010) in the north-west of Turkey and Rataj et al., (2011) in Scandinavia, which amounted to 27.27 and 47 % respectively. The different recorded ratios (above and below) may be due to the variation of the number of specimens examined, their species, sizes and sources of food, as well as their location and the nature of their livelihood, as well as some of the research was conducted on the captive snake and some on wild species, some of them on aquatic species.
In this study, there were five species of intestinal parasites, the intestinal protozoa was the most widespread, the reason for this was that most of the snakes were subjected to pressure conditions of cages. In the case of infection a of single snake it was put to the cysts of spores with waste, which led to the pollution of cages and water sources, as well as the fact that these parasites had a direct life cycle. A high incidence of nematode infection was observed compared to tapeworms, this is consistent with Chaiyabutr & Chanhome’s study of (2002) in Thailand, Santos et al., (2006) in Iberian Peninsula and Dusen et al., (2010) in the north-west of Turkey and Ribas et al., (2010) in the north-east of Spain and of Fontenot & Font’s (1996) in south-eastern Louisiana in aquatic vipers, which pointed to a higher incidence of tapeworms compared to nematodes.
This is due to the availability of intermediate hosts which play a major role in the incidence of tapeworms. Also observed was that the number of intestinal parasites located in the intestines is much higher than the numbers present in the stomach and large intestine may be due to the fact that the intestinal environment suitable for the parasites because of the integration of the physiological characteristics of the food in addition to its presence in a soluble and ready for absorption and this is consistent with Santora et al., (2013) and Dusen (2010).
The results of the current study showed that the incidence of one species of parasite was the highest percentage compared with binary and triple infections. The reason for this may be due to the living and environmental competition between the parasites of the host.
The results of the study indicate that snakes of medium size are most susceptible to parasitic infections, reaching the highest level within the group which ranged between 50 – 100 cm. This result is consistent with the reports of Santora et al., (2013) and Capizzi et al., (2008). This may be due to the fact that medium-sized snakes are more active than small and large species and therefore have a wide food diversity, Small snakes are always inactive and poorly nourished. While few infection in large snakes due to having evolution immune system (Santora et al., 2013; Capizzi et al., 2008; Poulin, 2007). Parasitic infection occurs under the influence of a biological agents which include host, parasite and carrier, such as host species, genus and feeding nature (Osgood & Schall, 2004).
This study indicated only one species of tapeworm was found, which was found in the small and large intestine. It was recorded in only four species of snakes: Platyceps ventromaculatus, Eryx jaculus, Natrix tessellata and D. mesopotamicus, being 11.9, 7.14, 57.89 and .3, respectively. It is worth mentioning this species is not only in snakes, as it was previously recorded in Iraq by Al-Hashimi (2006) in Al-Anbar in his study of Iraqi reptiles. It has been isolated by King & Babero (1974) in Australian Kangaroo Dipodomys ssp. in Nevada by Bursey et al., (1996) in the Australian lizards called Moloch horridus and Rataj et al., (2011) in Scandinavia from lizards and Bursey et al. (1996) pointed to the presence of more than 74 species, which is concerned with the infection of different species of reptiles, Their life cycle is indirect and requires beetles and some insects as an intermediate host to complete their life cycle. While Kalicephalus sp. was one of the most common nematodes recorded during the current study, it was found in the stomach, small and large intestine and has been isolated from most species of snakes and the highest percentage was in the Eryx jaculus, which was 75% and the lowest in Malpolon monspesslana, which amounted to 7.69%, while Strongyloides sp. was found only in two species of snakes, Platyceps ventromaculatus and Eryx jaculus with a percentage of 2.38 and 7.14 respectively. This species was universally recorded in snakes by Fontenot & Font (1996) in South-East Louisiana in group of aquatic snakes at a rate of 4 % and Rataj et al., (2011) in Scandinavia snakes and turtles at a rate 3.7 %.
Protozoa included two species such as Isospora sp. and Cryptosporidium spp Isospora spp which were recorded in most species of snakes. The highest percentage was the S. clifordi, which was 36.36 % and the infection was obtained by swallowing the cysts containing spores with contaminated water and food, which is globally registered in snakes by Asmundsson et al., (2001) in Ecuador and Chaiyabutr & Chanhome (2002) in Thailand and McAllister et al. (2015) in Oklahoma.
Cryptosporidium spp. was recorded in six species of snakes and highest percentage was registered in S. clifordi, which was % 45.45. This finding agrees with that of Brower & Cranfield (2001) who have shown similar data in snakes of Animal Farm Zoo in Baltimore, Maryland USA. Our present study of the histological changes caused by the parasite considerably agrees with that of Kuroki et al., (2008) in Japan and Rataj et al., (2011) in Scandinavia and Yimming et al., (2016) in Thailand. This protozoa was recorded in 57 different species of reptiles, including 40 species of snakes, 15 species of lizards, and 2 turtles (Xiao et al.,2004). The incidence of species without apparent disease in mammals and birds (Ramirez et al., 2004) with the exception of C. serpentis, which leads to clear pathological symptoms such as abdominal bloating, low weight and lethargy of infected animals,This species is concerned with reptiles only (Fayer et al., 1997). The infection with Cryptosporidium is obtained by swallowing cysts containing spores with water and food or through contaminated soils, (Rosenthal,1997).
Refrences
AL-Hashimi S . F. A (2006) Parasitic worms of alimentary canal of some reptile species in AL-Ramadi city Msc. Thesis Edu. Univ AL-Anbar: 77Pp.
Al-Janabi S.H. & Ghaleb S. A. (1992). Geography of Iraq. Publishing books for printing and publishing, University of Mosul.Pp 77.
Asmundsson, IM; Upton, SJ & Freed, PS. (2001) Five new species of coccidian (Apicom plexa: Eimeriidae) from colubrid snakes of Ecudor. J. Parasitol., 87(5):1077-1081.
Brower, AL. & Cranfield, MR. (2001). Cryptosporidium sp. associated enteritis without gastritis in rough green snakes (Opheodrys aestivus) and a common garter snake (Thamnophis sirtalis) J of Zoo. Wild. Med 32(1):101-105.
Bursey, C.; Goldberg, S.R. & Woolery, D.N. (1996). Oochoristica piankai sp.n. (cestoda: Linstow – iidae ) and helminths Moloch horridus (Sauria: Agamidae) from Australia J Helminthol. Soc.Wash., 63(2): 215-221.
Capizzi, D.; Capula, M.; Rugiero, L. & Luiselli, L (2008). Dietary patterns of two sympatric Mediterranean snakes (Hierophis viridiflavus and Zamenis longissimus) along a gradient of habitat alteration Herpetological Journal 18:141-146.
Casewell N.R. Wagstaff, S.C. Wuster, W.; Cook D.A. Bolton F.M. King, S.L. Pla D. Sanz, L. (2014). Medically important difference in snake venom composition are dictated by distinct postgenomic mechanisms. Alistair Reid Venom Research unit and Bioinformatics Unit Pnas,111(25):9205-9210.
Chaiyabutr, N & Chanhome, L. (2002). Parasites in snakes of Thailand – Bulletin of the Maryland Herpetological Society., 38(2):39-50.
Dusen, S. Ugurtas, I.H. & AL-Tunel, F.N. (2010). Nematode parasites of the smooth snake, Coronella austriaca and the Aesculapian snake, Zamenis longissimus (Ophidia: Colubridae), collected from North Western Turkey. North–Western Journal of Zoology 6(1):86-89.
Duszynski, D.W. & Upton, S.J. (2009). The biology of the coccidi (Apio complexa) of snake of the World: A scholarly Handbook for Identification and Treatment Crest Space Publishing.PP.422.
Fayer, R.; Speer, C.A. & Dubey, J.P. (1997). The general biology of Cryptosporidium and cryptospo-ridiosis. CRC Press, Boca Rotan, Florida. Pp:1-41.
Fontenot, L.W. & Font, W.F. (1996). Helminth parasites of four species of aquatic snakes from two habitats in Southeastern Louisiana. J. Helminth Soc of Washington., 63(1):66-75
Jacobson E.R. (2007) Parasites and parasitic disease of reptiles. In infection disease and pathology of reptiles Jacobson (ed).CRC Press, Boca Raton Florida Pp : 571-666.
Jacobson, E.R. (1978). Reptile Necropsy Protocol.The Journal of Zoo Animal., 9(1):7-13.
King, S.R. & Babero, A.B. (1974). Helminth of Kangaroo Rats (Dipodomys ssp.) in Nevada with repots of other warm parasites from these hosts. J. Helminthol. Soc. Wash., 41(2):241-249.
Klingenberg, R. (2000). Diagnosing parasites in old world chameleons Exotic DVM., 1:7-21.
Kuroki, T.; Lzumiyama, S.; Yagita, K.; Une, Y.; Hayashidani, H.;Kuroo, M.; Mori, A.; Moriguchi, H.; Toriba, M.; Ishibashi, T. & Endo, T. (2008). Occurrence of Cryptos-poridium sp. in snakes in Japan. Parasitol. Res.,103(4):801-805.
McAllister CT. Seville, RS & Connior, MB. (2015) A new species of Isospora (Apicomple-xa: Eimeriidae) from eastern coach whip coluber Flagellum flagellum from Oklahoma., Acta. Parasitol., 60(3)466-470.
Nasiri, V. Mobedi, I. Dalimi, A. Mirakabadi, A.Z. Ghaffarifar, F. Teymurzadeh, S. Karimi, G.; Abdoli, A. & Paykari, H. (2014) A description of parasites from snakes. Exper. Parasitol 147:7-15.
Osgood S.M. & Schall J.J. (2004). Gametocyte six of ratio of a malaria parasite: response to experimental manipulation of parasite clonal Diversity Parasitol.,128:23-29.
Parc, J (2008) An overview of pentastomiasis in Reptiles and other vertebrates J. of Exotic Pet Medicine (17):285-294.
Pietzsch Quest R. Hillyard PD. Modlock JM. And Leach S. (2006). Importation of exotic ticks into the united kingdom via the international trade in reptiles Exp. Appl. Acarol., 38:59- 65.
Poulin (2007) Evolutionary ecology of parasites Princeton Princeton university Press. PP.332.
Psi, M.H. (1992) Snakes translated by Abdel Halim Kamel. Dar Al Ma’arif .Cairo. The sixth edition. Pp 60.
Ramirez, NE. Ward, LA & Sreevatsan S. (2004). A review of the biology and epidemiology of creptosporidiosis in humans and animals Microbes Infect 6:773-785.
Rataj A.V. Knific R.L. Vlahovic K. Mavri, U. and Dovc A (2011). Parasites in pet reptiles Acta Verterinaria Scandinavica., 53(33):20 PP.
Ribas A. Lopez S. & Roca V (2010) Helminths from snakes in northeast Spain Bul. Asoc. Herpetol. Esp 21:44-46.
Rosenthal KL. (1997). Practical exotic animal medicine (The compendium collection). New Jersey: Veterinary Learning System.
Santora M. Aznar F Mattiucci, S. Kinsella, J.M Pellegrino F. Cipriani P & Nascettti, G. (2013) Parasite assemblages in the Western Whip snake Hierophis viridiflavus carbonarius (Colubridae) from Southern Italy. J. Helminthology., 87:277-285.
Santos X. Freiria F.M. Pleguezuelos T.M. & Roca V. (2006).First helminthological data on Iberian vipers: Helminth communities and host parasite relationship. Acta Parasitologica 51(2):130-135.
Telford, S. R. (2009) Hemoparasites of the Reptilia: Color atlas and text. CRC Press Taylor and Francis group Boca Raton Florida. PP.394.
Vitt, L.J. and Caldwell J.P. (2013) Herpetology an introductory biology of amphibian and reptiles Academic Press Pp 555-575.
Xiao, L. Ryan, UM. Graczyk, TK. Limor, J. (2004). Genetic diversity of Cryptosporidium sp. in captive reptiles APP. Envion Microb 70:891-899.
Yimming B. Pattanatanang K. Sanyathitiseree K. Kamyingkird (2016) Molecular identification of Cryptosporidium species from pet Snakes in Thailand. Korean. J. Parasitol 54(4):423-429.


