1Drug Bioassay-Cell Culture Laboratory, Pharmacognosy Department, National Research
Centre, Pharmaceutical and Drug Industries Division, Dokki, Giza, Egypt
2Faculty of Biotechnology, Modern Science and Art University,
3Medical Sciences Department, Faculty of Dentistry, The British University in Egypt, Cairo, Egypt,
4Department of Biological Science, Faculty of Science, P.O. Box 80200, King Abdulaziz University, Jeddah, 21589, Saudi Arabia.
Corresponding author email: abatawi@kau.edu.sa
Article Publishing History
Received: 13/03/2021
Accepted After Revision: 12/06/2021
In nature, several drugs could be discovered as it is generous in phytochemicals that could be used as remedies. In fact, various cytotoxic drugs used against cancer arose from plants. Cancer is caused by genetic and epigenetic alterations that make the cells divide uncontrollably, ignoring the cell’s normal machinery. In this study, seven methanol plants extracts from leaves, flowers, and branches of Albizia procera. White siris is the common name of such a tall, rapid-growing tree with an open canopy where all parts of the plant are reported to show anti-cancer activity. The bark and leaves of Ailanthus altissima which is commonly known as the tree of heaven in the family Simaroubaceae, were tested on three types of human cancer cell line: colorectal cancer (HCT-116) which is the third most frequent cancer, breast cancer (MCF-7) which is the most prevalent cancer among women, and hepatocellular carcinoma (HEPG-2) which is the third leading cause of death related to cancer worldwide.
The investigation done evaluated the cytotoxicity of the extracts in a 2D cell culture, on the three cell lines, and a 3D cell culture on HCT-116, using MTT and Acid phosphatase assays respectively. The results reported one active extract on HCT-116 with an IC50 of 0.23 µg/ml in the 2D system and percentage inhibition of 72.0% using 100 µg/mL of the extract, accompanied by a regression in the proliferative peripherals in the 3D system. Regarding the MCF-7, four extracts were considered active having an IC50 of 2.53, 4.16, 11.8, and 13.4 µg/ml. Ultimately, the HEPG-2 cell line responded to two extracts, one having a percentage inhibition reaching 92% at 25 µg/ml, and the other having a percentage inhibition of 98.1% at 50 µg/ml.
HCT-116, MCF-7, HEPG-2, Albizia procera, Ailanthus altissima, MTT
El-Hallouty S. M, Afifi A. G, El-Zohiry D. A, Batawi A. H. Cytotoxic Study of Albizia procera and Ailanthus altissima Extracts on Human Tumor Cell Lines. Biosc.Biotech.Res.Comm. 2021;14(2).
El-Hallouty S. M, Afifi A. G, El-Zohiry D. A, Batawi A. H. Cytotoxic Study of Albizia procera and Ailanthus altissima Extracts on Human Tumor Cell Lines. Biosc.Biotech.Res.Comm. 2021;14(2). Available from: <a href=”https://bit.ly/3bWXZQY“>https://bit.ly/3bWXZQY</a>
Copyright © El-Hallouty et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Cancer is one of the most dreaded diseases of the 20th century and spreading further with the continuance and increasing incidence in the 21st century. The situation is so alarming that every fourth person is having a lifetime risk of cancer (Roy and Saikia, 2016). The evolution of the normal cell to a malignant one involves processes by which genes involved in normal homeostatic mechanisms that control proliferation and cell death suffer mutational damage which results in the activation of genes stimulating proliferation or protection against cell death, the oncogenes, and the inactivation of genes which would normally inhibit proliferation, the tumor suppressor genes.
Finally, having overcome normal controls on cell birth and cell death, an aspiring cancer cell faces two new challenges: it must overcome replicative senescence and become immortal and it must obtain adequate supplies of nutrients and oxygen to maintain this high rate of proliferation (Bertram, 2000). Three types of cancer were investigated in this research: breast cancer, colorectal cancer, and hepatocellular carcinoma.
Breast cancer is one of the most common cancers and >10.5 new breast cancer cases per 100,000 individuals occur worldwide each year (Khoobchandani et al., 2009) while colorectal cancer, the third most commonly diagnosed cancer in the world, has a higher predominance in developed countries (Des Guetz et al., 2010, Adelstein et al., 2011, Ibrahim et al., 2014). The third type this study is subjecting to investigation is hepatocellular carcinoma (HCC) which is one of the most devastating malignancies worldwide (Maluccio and Covey, 2012, Wong et al., 2017). Despite recent advances in the diagnosis and treatment including hepatectomy and liver transplantation, the prognosis of patients with remains poor due to the high rates of recurrence and early metastasis (Omata et al., 2017).
Despite progress in anticancer therapeutics, there are few efficient drugs with low toxicity available to treat cancer. Numerous plants have been previously used in cancer therapy (da Rocha et al., 2001). Throughout the centuries, certain plant extracts have been tested for antitumor potential (Ho et al., 2002.). In addition, plant products demonstrate fewer side effects compared with chemical drugs. There has been an increasing interest in identifying and isolating natural compounds from medicinal plants with an aim to develop novel anticancer drugs (Bishayee, 2012).
Albizia procera belongs to the angiosperms, from the family of Febaceae, a subfamily of Mimosoideae, and genus Albizia. Albizia procera is a native tree that is mostly found in America, Asia, Pakistan, India, and Australia. The common name of Albizia procera is white siris and is a medicinal plant, used for the stomachache, during pregnancy and healing of wounds (Rukunga and Waterman, 1996) but it exhibited cytotoxicity against HEPG2 cell line (Melek et al., 2007). It also possesses antioxidant properties and can be involved in medicinal plant category (Sivakrishnan and KottaiMuthu, 2014). Ailanthus altissima (Synonyms: Ailanthus cacodendron (Ehrh.) and Ailanthus glandulosa Desf.)
belongs to Simaroubaceae family, native to China and Japan and has a peanut or cashew-like fragrance (Albouchi et al., 2013, Albright et al., 2010). Extract of A. altissima possess antimicrobial and antifungal activities (Khan, 2017) and considered as an anticancer agent (Wang et al., 2018). Ailanthus altissima is an example of a plant that has been used in tumor therapy (Efferth et al., 2007). The antitumor effect that has enabled the use of this plant in the treatment of colonic, cervical, and rectal cancer has been previously described by Wang et al., (2013).
The potential cytotoxicity of ethanol extract and its derived fractions chloroform, ethyl acetate, butanol, and aqueous) of Adenosma bracteosum Bonati. (A. bracteosum) on human large cell lung carcinoma (NCI-H460) and hepatocellular carcinoma (HepG2). Among these fractions, the chloroform showed significant activity in the inhibition of proliferation of both cancerous cells because of the presence of bioactive compoundsIn 3D models, the cultured cells form 3 dimensional spheres where the cells become arranged in numerous layers with the peripheral cells proliferating the most, and the core containing hypoxic and quiescent cells as they receive fewer growth factors, oxygen, and nutrients from the medium (Edmondson et al., 2014). This layout highly mimics the structure of the tumor masses, enabling a realistic a cell-to-cell and cell-ECM interactions where the cells are able to receive signals from their environment (Cawkill and Eaglestone, 2007, Lee et al., 2008).
The purpose of this research is to determine the cytotoxic effect of 7 methanol plant extracts from Albizia procera and Ailanthus altissima on 3 different human cancer cell lines including HCT-116, MCF-7, and HepG-2; as well as to examine the safety of the potential cytotoxic extracts on normal human cell line (BJ-1) and test the cytotoxicity on the 3D model of (HCT-116).
MATERIAL AND METHODS
Plant material : Amount of the attainable wild and cultivated plants, Albizia procera and Ailanthus altissima were collected randomly from diverse habitat in Egypt. Voucher specimens were prepared and deposited in the Herbarium of the Pharmacognosy Department, National Research Centre, Egypt, and taxonomical nomenclature was performed ( Boulos , 2002, 2005).
Preparation of extracts: The collected plants were separated into different parts. Albizia procera, leaves, flowers, and bark and Ailanthus altissima, leaves and bark samples were dried in solar ovens at 45°C and then ground. Sufficient weight of each plant powder was exhaustively extracted with 95% methanol. The methanol extracts were filtered, evaporated under vacuum at 35°C, freeze-dried and stored at -20 °C. The freeze-dried plant extracts were deposited at the Extract Bank of the In-Vitro Bioassay Laboratory, National Research Center, Egypt.
Cell culture: HCT116 human colon carcinoma cell line was obtained from ATCC and was maintained in McCoy’s 5A modified medium/10% FBS while hTERT-RPE1 cell line was obtained from CLONTECH. The human retinal epithelial cell line, hTERT-RPE1, is an immortalized cell line that stably expresses human telomerase reverse transcriptase (hTERT). It was maintained in DMEM: F12 Medium/10% FCS. Both cell lines were incubated at 37°C in 5% CO2 and 95% humidity. Cells were sub-cultured using trypsin-versene 0.15%.
Cytotoxicity bioassay on monolayers: After 24 h of seeding 10000 cells per well (in 96-well plates), a 100-ppm final concentration of the test extracts were added in triplicates. The cells were treated for 48hrs. 1 μM staurosporine was used as positive control and 0.5% DMSO was used as a negative control. Cytotoxicity was ascertained using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay as described by Mosmann in 1983. Cytotoxicity was calculated according to the following equation:
As/ Ac x100 (El-Mostafa et al., 2014).
Where: As: absorbance of sample at 595 nm, Ac: absorbance of negative control.
Cytotoxicity bioassay on HCT116 multicellular spheroids: Spheroids were generated as previously described (Fayad et al., 2011, Awad et al., 2016). FBS was filtered through a 0.45 μm filter, to remove any particulates that cause deformation in the spheroids. 10 000 cells of HCT116 was added to each well of poly-HEMA-coated round bottom 96-well plates. The wells were overfilled by adding 170 μl media to obtain convex curvature. Plasticine spacers were placed in the four corners of plates to prevent the lids from touching the surface of the media. The plates were inverted and incubated for 24 h on a rotary shaker. Plates were flipped back to the normal position to allow the formed aggregates to settle down in the bottom of the wells. The excess medium was removed using a syringe needle connected to a pump and plates were incubated for more 4 days. By this time, mature spheroids were generated (~500 μm in diameter).
Before treatment, the media was changed and were adjusted to 200 μl. Test extracts were added in triplicates to a final concentration of 100 ppm and were incubated for 120 h. 1 μM final concentration staurosporine was used as positive control and 1 μl DMSO was used as a negative control. At the end of incubation, cytotoxicity was determined using the acid phosphatase method (Friedrich et al., 2009). After washing twice with 250 μl PBS buffer, 100 μl of 0.1M sodium acetate, 0,1% Triton X-100, p-nitrophenyl phosphate (Pierce Biotechnology Inc, Rockford, IL) were added to each well and incubated for 1.5 hours at 37°C. After incubation, 10 μl 1N NaOH stop solution was added to each well and absorbance was read at 405 nm. Cytotoxicity was calculated according to the following equation:
(1-(av(x))/ (av (NC))] *100.
Where: Av: average, X: absorbance of sample, NC: absorbance of negative control.
RESULTS AND DISCUSSION
Albizia procera (extract 1), leaves (extract 2), flowers (extract 3), and bark (extract 4) and Ailanthus altissima (extract 5), leaves (extract 6) and bark (extract 7) were obtained and their activities as antitumor agents were determined. The data were collected from the MTT assays that were done on the 3 cell lines HCT-116, MCF-7, and HEPG-2, using 7 methanol plant extracts from A. procera and A. altissima. The calculated percentage inhibition of the different concentrations was used to determine the IC50 and IC90 values of the 7 extracts by performing probity analysis using the SPSS program. One extract from Albizia procera which showed high cytotoxic effects was evaluated for its safety on normal cells using the cell line BJ-1.
Extracts 1, 2, and 3 showed low cytotoxic effects in the 4 concentrations (100, 50, 25, and 12.5 µg/ml). The other 4 extracts exhibited a higher cytotoxic effect, especially the extract 4 which exerted an exceptional cytotoxicity on the cell line HCT-116 and showing a high percentage inhibition of 99.6% with a low concentration of 6.25 µg/ml. However, the 3 remaining extracts showed low cytotoxicity in low concentrations (25 and 12.5 µg/ml) (Table 1, Figure 1). The IC50 and IC90 values of the 7 extracts on HCT-116 were determined (Table 2) to evaluate the efficacy of the potential drugs on colorectal carcinoma. Only extract 4 is considered as very active and have an IC50 value less than 20.
Table 1. Mean percentage inhibition of the 7 extracts on HCT-116 cell line.
| Methanol extract | Mean percentage inhibition | |||||||
| 100
μg/ml |
50
μg/ml |
25
μg/ml |
12.5
μg/ml |
6.25
μg/ml |
3.125
μg/ml |
1.56
μg/ml |
0.78
μg/ml |
|
| 1 | 49.2 | 33.4 | 15.0 | 0.64 | N/A | N/A | N/A | N/A |
| 2 | 34.6 | 33.4 | 23.4 | 11.5 | N/A | N/A | N/A | N/A |
| 3 | 41.2 | 28.3 | 16.8 | 3.8 | N/A | N/A | N/A | N/A |
| 4 | 99.1 | 99.7 | 100 | 99.2 | 99.6 | 67.4 | 29.9 | 7.2 |
| 5 | 89.4 | 75 | 48.6 | 40.0 | N/A | N/A | N/A | N/A |
| 6 | 83.7 | 41.9 | 27.4 | 23.9 | N/A | N/A | N/A | N/A |
| 7 | 99.3 | 98.7 | 42.2 | 14.0 | N/A | N/A | N/A | N/A |
Figure 1: Percentage inhibition of the 7 plant extracts with different concentrations on HCT-116 cell line.
Albizia procera (extract 1) , leaves (extract 2), flowers (extract 3), and bark (extract 4) and Ailanthus altissima (extract 5), leaves (extract 6) and bark (extract 7)
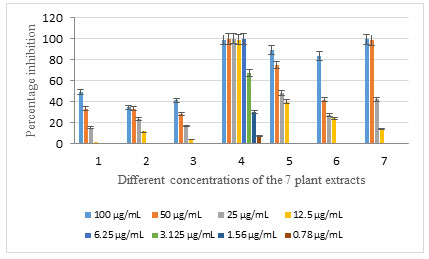
Table 2. IC50 and IC90 values of the 7 extracts on HCT-116 cell line.
| Methanol extract | IC50 (µg/ml) | IC90 (µg/ml) |
| 1 | 93.3 | 167.8 |
| 2 | 146.9 | 338.7 |
| 3 | 109.8 | 207.4 |
| 4 | 0.23 | 21.6 |
| 5 | 23.7 | 94.9 |
| 6 | 54.1 | 119.2 |
| 7 | 27.5 | 48.2 |
Albizia procera (extract 1) , leaves (extract 2), flowers (extract 3), and bark (extract 4) and Ailanthus altissima (extract 5), leaves (extract 6) and bark (extract 7).
On the MCF-7 cell line, extracts 1, 2, 3, 5, 6, and 7 exhibited high to moderate percentage inhibition in the two concentrations 100 and 50 µg/ml, and moderate to low percentage inhibition in the two concentrations 25 and 12.5 µg/ml (Table 3, Figure 2). On the other hand, the methanol extract 4 showed high to moderate percentage inhibition down to the concentration 3.125 µg/ml with a percentage inhibition of 72.2%. The percentage inhibition was low in the two concentrations 1.56 and 0.78 µg/ml only, regarding this extract (Table 3, Figure 2). Regarding the HEPG-2 cell line, extracts 1, 2, 3, 5, and 6 had a minimal or negligible percentage inhibition in the 4 concentrations; while the extract 4 had a high percentage inhibition down to the concentration 25 μg/ml, and extract 7 showed a high percentage inhibition in the two highest concentrations 100 and 50 μg/ml ( Figure 3).
The IC50 and IC90 values of the 7 extracts on MCF-7 were determined (Table 4) to evaluate the efficacy of the potential drugs on breast cancer. Extracts 4, 5, 6, and 7 had an IC50 less than 20 showing high efficacy against the breast cancer cell line. The IC50 and IC90 values of the extracts against the cell line HEPG-2 could not be calculated due to their low cytotoxicity and percentage inhibition against this cell line. The extract 4 might have needed a secondary screening to be able to calculate these values.The highly cytotoxic extract 4 was evaluated for its safety on the cell line BJ-1, and showed a mean percentage inhibition of 4.3% with the concentration 100 μg/ml.
Table 3. Mean percentage inhibition of the 7 extracts on MCF-7 cell line
| Methanol extract | Mean percentage inhibition (%) | |||||||
| 100
μg/ml |
50 μg/ml | 25 μg/ml | 12.5 μg/ml | 6.25 μg/ml | 3.125 μg/ml | 1.56 μg/ml | 0.78 μg/ml | |
| 1 | 95.2 | 72.5 | 31.3 | 12.5 | N/A | N/A | N/A | N/A |
| 2 | 84.4 | 53.8 | 21.7 | 10.8 | N/A | N/A | N/A | N/A |
| 3 | 74.8 | 60.8 | 27.1 | 11.6 | N/A | N/A | N/A | N/A |
| 4 | 99.1 | 99.3 | 99.8 | 96.5 | 80.0 | 72.2 | 32.5 | 1.93 |
| 5 | 96.0 | 89.0 | 71.3 | 50.2 | N/A | N/A | N/A | N/A |
| 6 | 92.6 | 80.0 | 64.1 | 43.8 | N/A | N/A | N/A | N/A |
| 7 | 97.5 | 98.9 | 75.2 | 35.8 | N/A | N/A | N/A | N/A |
Albizia procera (extract 1) , leaves (extract 2), flowers (extract 3), and bark (extract 4) and Ailanthus altissima (extract 5), leaves (extract 6) and bark (extract 7)
Figure 2: Percentage inhibition of the 7 plant extracts with different concentrations on MCF-7 cell line
Albizia procera (extract 1) , leaves (extract 2), flowers (extract 3), and bark (extract 4) and Ailanthus altissima (extract 5), leaves (extract 6) and bark (extract 7)
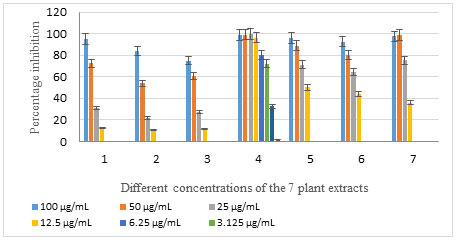
Table 4. IC50 and IC90 values of the 7 extracts on MCF-7 cell line.
| Methanol extract | IC50 (µg/mL) | IC90 (µg/mL) |
| 1 | 39.9 | 77.9 |
| 2 | 54.7 | 105.4 |
| 3 | 55.8 | 121.02 |
| 4 | 2.53 | 8.26 |
| 5 | 4.16 | 63.8 |
| 6 | 11.8 | 82.1 |
| 7 | 13.4 | 47.9 |
Albizia procera (extract 1) , leaves (extract 2), flowers (extract 3), and bark (extract 4) and Ailanthus altissima (extract 5), leaves (extract 6) and bark (extract 7)
Figure 3: Percentage inhibition of the 7 plant extracts with different concentrations on HEPG-2 cell line
Albizia procera (extract 1) , leaves (extract 2), flowers (extract 3), and bark (extract 4) and Ailanthus altissima (extract 5), leaves (extract 6) and bark (extract 7)
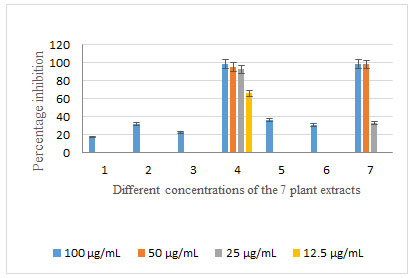
Table 5. Mean percentage inhibition of the 7 extracts on HEPG-2 cell line
| Methanol extracts | Mean percentage inhibition (%) | |||
| 100 μg/ml | 50
μg/ml |
25
μg/ml |
12.5
μg/ml |
|
| 1 | 17.5 | 0 | 0 | 0 |
| 2 | 31.7 | 0 | 0 | 0 |
| 3 | 22.6 | 0 | 0 | 0 |
| 4 | 98.9 | 95.3 | 92.6 | 66.2 |
| 5 | 36.8 | 0.8 | 0 | 0 |
| 6 | 31.1 | 0 | 0 | 0 |
| 7 | 98.5 | 98.1 | 33.1 | 0 |
Albizia procera (extract 1) , leaves (extract 2), flowers (extract 3), and bark (extract 4) and Ailanthus altissima (extract 5), leaves (extract 6) and bark (extract 7)
Spheroids of HCT-116 cells were studied under the microscope before and after treatment with a concentration of 100 μg/ml of the 7 examined extracts, as well as their percentage inhibition were calculated after measuring the absorbance of the plate at 405 nm. Before treatment, the spheroids had a length that was around 600 μm (Figure 4b). The positive control, using the chemotherapeutic drug cisplatin, showed a reduction in the spheroid length of approximately 100 μm (Figure 4b). On the other hand, the negative control exhibited a growth in the spheroid length of about 200 μm, with the core and the proliferative peripherals observed (Figures 4c ).
The wells treated cells with extracts 1 (Figure 4d) and extract 2 (Figure 5e) showed no inhibitory or cytotoxic effect and an increase in the spheroid’s length of around 160 and 70 μm respectively. Figure 5 e showed HCT-116 spheroid treated with extract 2 (668.85 μm) while Figure 5f showed HCT-116 spheroid treated with extract 3 (660.59 μm). Similarly, Figure 5g showed HCT-116 spheroid treated with extract 4 (590.37 μm) and Figure 5 h- HCT-116 spheroid treated with extract 5 (577.03 μm). Figure 6i showed HCT-116 spheroid treated with extract 6 (609.80 μm), and Figure 6j showed HCT-116 spheroid treated with extract 7 (672.20 μm).
It is clear that, extracts 3, 5, 6 and 7 showed insignificant cytotoxic effects on the HCT-116 cells cultured in 3D model. On the other hand, extract 4 showed a decrease in the diameter of the spheroid, as well as a discontinuation in the proliferation of the peripherals of the spheroid was remarkable. Regarding the mean percentage inhibition of the 7 extracts on the HCT-116 spheroids, extracts 1 and 2 had no cytotoxic effects at all, while extracts 3, 5, 6, and 7 had differential cytotoxic effects; however, none of them showed high cytotoxicity. The only extract that displayed an increased cytotoxicity is extract 4 (Table 6).
Figure 4: a- HCT-116 spheroid before treatment (604.73 μm), b- HCT-116 spheroid treated with cisplatin (510.20 μm), c- HCT-116 negative control (783.23 μm), and d- HCT-116 spheroid treated with extract 1 (760.69 μm).
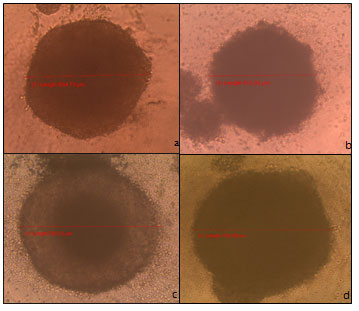
Figure 5 : e- HCT-116 spheroid treated with extract 2 (668.85 μm), f- HCT-116 spheroid treated with extract 3 (660.59 μm), g- HCT-116 spheroid treated with extract 4 (590.37 μm), and h-HCT-116 spheroid treated with extract 5 (577.03 μm).
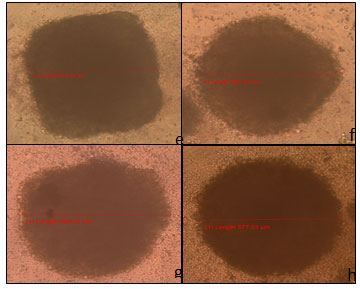
Figure 6 : i- HCT-116 spheroid treated with extract 6 (609.80 μm), and j- HCT-116 spheroid treated with extract 7 (672.20 μm).
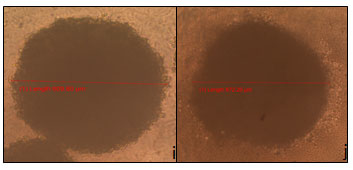
Table 6. Mean percentage inhibition of the 7 extracts (100 μg/ml) on HCT-116 spheroids
| Methanol extract | Mean percentage inhibition (%) |
| 1 | 0 |
| 2 | 0 |
| 3 | 14.1 |
| 4 | 72.0 |
| 5 | 33.2 |
| 6 | 17.35 |
| 7 | 54.9 |
Albizia procera (extract 1) , leaves (extract 2), flowers (extract 3), and bark (extract 4) and Ailanthus altissima (extract 5), leaves (extract 6) and bark (extract 7)
According to the World Health Organization (2010), cancer is a disease that causes about 13% of total deaths worldwide. Chemotherapeutic or cytotoxic drugs can largely be discovered and developed from natural sources. In fact, drugs against infectious diseases could be discovered as well from natural products (Fayad et al, 2017). Plants and nature comprise an extensive variety of phytochemicals and chemical structures that offer several biological activities and hence could be used as remedies. Indeed, almost 60% of the currently prescribed drugs against malignant tumors originated and were developed from natural products (Newman et al., 2007). It has been vividly documented that different research projects have investigated the cytotoxic effects of Albizia procera, Ailanthus altissima, and other plants as well, and they have found that they have active cytotoxic effects on a variety of cell lines.
The saponins which are isolated from Albizia procera’s bark exhibited cytotoxic effects on the HEPG-2 cell line with an IC50 of 9.13 μg/ml (Melek et al., 2007). However, another study that was done on the bark of Albizia procera included the isolation of triterpene glycosides with N-acetyl glucosamine units which displayed no significant cytotoxic effects on any of the cell lines MCF-7, HEPG-2, HT-29, or A-549. Additionally, experiments performed on oleanane-type saponins that were purified from the leaves of Albizia anthelmintica showed potent cytotoxic effects on HCT-116 and HEPG-2 cell lines with respective IC50 values of 4.75 and 3.60 μg/ml (Al-Sayed and Esmat, 2016).
An additional study was conducted on a β-carboline alkaloid, called 9-hydroxycanthin-6-one, was isolated from the bark of Ailanthus altissima and showed cytotoxic activity against ovarian cancer by increasing the intracellular levels of ROS followed by activating the caspases 3, 8, and 9 to induce apoptosis. This compound was found as well to repress the expression of RANTES and MCP-1 which are important determinants in the recruitment of macrophages at ovarian tumor sites; and to decrease the levels of factors that promote cancer like VEGF.
Similar findings were reported on studies done on the glioblastoma cell line U87; Ailanthus altissima was found to induce oxidative stress and endoplasmic reticulum stress sequentially which lead to the activation of the caspases, eventually leading to apoptosis of the malignant cells. Moreover, a chloroform fraction of Ailanthus altissima induced apoptosis on the human lung cancer cell line A549, as well as it was responsible for the upregulation of Bax, the pro-apoptotic factor, and the downregulation of the gene BCL-2 which encodes a protein that regulates the programmed cell death (Wang et al., 2011).
These anti-proliferative and cytotoxic effects are not restricted to the two plants Albizia procera and Ailanthus altissima, but they are seen among other plants as well. Urtica pilulifera and Lagenariasiceraria was found to inhibit the proliferation and induce apoptosis on the three cell lines HEP-3b (hepatocellular carcinoma), HeLa (cervical cancer) and PC-3 (prostate cancer). Meanwhile, the plant Ficus carica was ascertained to have cytostatic and anti-proliferative effects on the same 3 cell lines; it reduces the amount of metabolically active cells, leading to a decrease in the number of dividing cells. Another study done by (Réthy et al., 2007)) concluded that the plants Elodea canadensis, Erigeron annuus, Ambrosia artemisiifolia, Helianthus annuus and Xanthium italicum exhibited potent cytotoxic effects against the cell lines MCF-7, HeLa, and A431 (epidermoid carcinoma) with IC50 values that range between 1.84 and 19.91 µg/ml. Four other Malaysian plants were investigated in research in research done by Chu et al., (2013).
The first one, Acalypha wilkesiana, had a GI50 of 15.9 μg/ml on breast cancer cells, as well as extracts of this plant, had the ability to impair the colony forming and cell survival mechanisms of the cancerous cells. The GI50 value demonstrates the concentration of the drug needed to reduce the proliferation of malignant cells by 50%. The second investigated plant was Archidendron ellipticum, whose leaf and bark extracts showed respective GI50 values of 9.3 and 1.7 μg/ml on the breast cancer cell line.
These extracts lead to the senescence of the cells primarily then induced apoptosis. The third plant was Duabanga grandiflora. Leaf and bark extracts of this plant showed a growth inhibition activity against the colorectal cancerous cell line HCT-116 by activating the protein caspase 3 to induce apoptosis. The last tested plant was Pseuduvaria macrophylla which inhibited the growth of the HCT-116 cells as well, with a GI50 1.6 μg/ml. The mechanism of action of this plant was found to activate the protein caspase 3 and induce apoptosis, similarly to Duabanga grandiflora (Chu et al., 2013).
In this study, 4 extracts from Albizia procera and 3 extracts from Ailanthus altissima were tested for their cytotoxicity effect on 3 human tumor cell lines colorectal cancer (HCT-116), breast cancer (MCF-7), and hepatocellular carcinoma (HEPG-2) in a monolayer cell culture; as well as they were tested on the colorectal carcinoma cell line in a 3D model where the cells were cultured in spheroids. The investigation was done using 2 colorimetric-based assays: MTT assay for the monolayer cell culture, and acid phosphatase assay for the 3D model system.
The results showed differential cytotoxicity effects on the 3 cell lines as well as there were significant differences between the monolayer and the spheroids cell cultures of the same cell line HCT-116. However, extract 4, leaves and flowers of Albizia procera) seems to have high cytotoxicity effects on all the tumor cell lines; while extracts 4, 5, 6, and 7 appear to have significant cytotoxic effects on the breast cancer cell line MCF-7. According to the National Cancer Institute Guidelines, plant extracts having an IC50 less than 20 µg/ml are considered as active extracts having a significant cytotoxic or anti-proliferative effect on the cancerous cell lines. In fact, IC50 is a pharmacokinetic measure that reveals the potency of the drug and lower IC50 value is the higher activity of the drug.
In the monolayer cell culture, extract 4 exhibited the highest efficacy (100% mean percentage inhibition) and potency (IC50 0.23 µg/ml) on the HCT-116 cell lines, which means that only 0.23 µg/ml of the extract is needed to inhibit 50% of the colorectal cancer cells. It was the only active cytotoxic extract on this cell line with IC50 less than 20 µg/ml. The same extract had the highest efficacy and potency on the breast cancer cell line (MCF-7) as well, with a mean percentage inhibition reaching 99.8% and IC50 2.53 µg/ml. Moreover, extracts 5, 6, and 7 demonstrated active cytotoxic effects on this cell line having IC50 less than 20 µg/ml. Meanwhile, their potency against this cell line is differential, with extract 4 having the highest potency, followed by extract 5, and extract 7 having the lowest potency. Nevertheless, extract 7 has the second highest efficacy after extract 4, with a mean percentage inhibition reaching 98.9%.
As HCC forms a therapeutic challenge and doesn’t show a typical cytotoxic pattern, almost all of the extracts didn’t show a significant cytotoxic effect against the HEPG-2 cell line, having no efficacy in the first place. Nevertheless, although extract 4 and 7 showed high efficacy using high concentrations, extract 7 is considered inactive as its IC50 value is more than 20; and the potency of the fourth extract couldn’t be determined with the current results. However, extract 4 could conceal promising cytotoxic effects as it shows high mean percentage inhibition in 3 out of the 4 concentrations used, suggesting that its IC50 value could present an active, highly potent compound.
The experiment done on the HCT-116 spheroids showed a significant decrease in the mean percentage inhibition using a concentration of 100 µg/ml with regard to the monolayer cell culture. However, extract 4, which was actively cytotoxic in the 2D culture model, inhibited almost three-quarters of the malignant cells in the 3D model, confirming its cytotoxicity against this cell line. The decrease in the cytotoxic effect of the extracts in the 3D system and the altered drug response in these two culture models is due to several facts; firstly, the unnatural and altered microenvironment of the cells in the monolayer cell culture leads to an altered cellular response to the drug, secondary, the varied gene expression in both models alters the cell’s sensitivity to the drugs (Edmondson et al., 2014; Gurski et al., 2010).
Moreover, cells cultured in 3D models seem to be more resistant to drugs as cellular interactions and signals from the ECM and neighboring cells enter in the process, as well as the drug suffers from a restricted diffusion into the spheroid, accompanied by hypoxia which activates genes involved in the drug sensitivity and cell survival (Walker et al., 2003, Trédan et al., 2007). These resistance mechanisms which are present in vivo and observed in the 3D spheroids are not developed in the monolayer cell cultures, making the 3D spheroids a more reliable source to better understand the cellular behavior in response to a particular drug (Edmondson et al., 2014).
This study showed 4 potential drugs against the 3 tested cell lines, one was potent against the all three tested cell line while the other 3 extracts showed efficiency against the breast cancer cell line only. They could be used as direct drugs against these cancer types, or they could be used for the production of different compounds by semi-synthesis. However, the research regarding these 4 potential extracts should be extended to confirm their potency in vivo. Some of the limitations of the present investigation include the lack of secondary screening of extract 4 on the HEPG-2 cell line to be able to calculate its respective IC50 and evaluate its potency. Additionally, the safety of the 3 extracts 5, 6, and 7, showing potency against the breast cancer cell line, was not tested; their safety on normal cells was supposed to be evaluated on normal, non-cancerous, cells to confirm their possible use as a safe drug.
Hence, this research could be extended by undertaking the secondary screening of extract 4 on the HEPG-2 cell line and by testing the potential extracts on 3D cell cultures of the 2 cell lines MCF-7 and HEPG-2 to have a closer look at their cytotoxic effect and their actual efficacy and potency on the respective cell lines in vivo, as the 3D cell cultures highly mimic the in vivo cells. Furthermore, the phytochemical compounds present in the potential extracts could be identified, isolated, and lastly produced to be used as direct drugs. Moreover, the molecular mechanism of the 4 cytotoxic extracts could be evaluated to determine the activated apoptotic pathways or the disrupted oncogenic pathways. Hence, in case a phytochemical compound caused the disruption of a particular oncogenic pathway, this compound could be used against other types of cancer where this pathway is activated.
Moreover, since extract 7 showed the second highest efficacy against the MCF-7 cell line, the toxicity of this extract could be tested to determine the highest dose that remains safe and kills the malignant cells altogether. Lastly, the normal extension of this research is to test these 4 potential extracts in vivo, on mice or rats to confirm their cytotoxicity along with the physiological functions and responses.
CONCLUSION
After the screening of 7 plant extracts from Albizia procera and Ailanthus altissima, on the 3 cell lines HCT-116, MCF-7, and HEPG-2 in monolayer cell cultures and on HCT-116 on spheroids as well, 1 plant extract from A. procera exhibited privilege for its therapeutic effects against the 3 cancer types: colorectal cancer, breast cancer, and hepatocellular carcinoma; and 3 other plant extracts from A. altissima manifested potent cytotoxic effects against breast cancer only. These extracts could be investigated further to harness their therapeutic potential in medical achievements and benefits by being promising to be utilized as potential drugs against their respective cancer types.
REFERENCES
Adelstein BA, Macaskill P, Chan SF, et al. (2011) Most bowel cancer symptoms do not indicate colorectal cancer and polyps: a systematic review. BMC Gastroenterol; 11:65.
Albouchi F., Hassen I., Casabianca H., Hosni K. (2013) Phytochemicals, antioxidant, antimicrobial and phytotoxic activities of Ailanthus altissima (Mill.) Swingle leaves. South African Journal of Botany, 87: 164–174.
Albright T. P., Chen H., Chen L., Guo Q (2010). The ecological niche and reciprocal prediction of the disjunct distribution of an invasive species: the example of Ailanthus altissima. Biological Invasions, 12(8): 2413–2427.
Al-Sayed E and Esmat A . (2016). Hepatoprotective and antioxidant effect of ellagitannins and galloyl esters isolated from Mela leuca styphelioides on carbon tetrachloride-induced hepatotoxicity in HepG2 cells. J Pharmaceutical Biology, Vol 54( 9): P 1727-1735.
Awad MM, Oxnard GR, Jackman DM, Savukoski DO, Hall D, Shivdasani P, Heng JC, Dahlberg SE, Jänne PA, Verma S, Christensen J, Hammerman PS, ShollL M. (2016) MET Exon 14 Mutations in Non-Small- Cell Lung Cancer Are Associated with Advanced Age and Stage-Dependent MET Genomic Amplification and c-Met Overexpression. Clin Oncol., 34(7):721-30.
Bertram JS (2000) The molecular biology of cancer. Mol Aspects Med. 21: 167-223.
Bishayee A. (2012) Editorial: Current advances in cancer prevention and treatment by natural products. Curr Pharm Biotechnol., 13: 115–116.
Boulos L. (2005). Flora of Egypt: Monocotyledons, Alismataceae-Orchidaceae, Vol. 4, Al Hadara Pub, Egypt.
Boulos L (2002) Flora of Egypt: Verbenaceae-Compositae. Vol. 3. Al Hadara Pub, Egypt.
Cawkill D and Eaglestone S S.Evolution of Cell-Based Reagent Provision.Drug Discov Today. 2007 Oct;12(19-20):820-5.
Chu M, Yuxiang S, Jinliang P, Xiangyun D, Haikuo L, Qingsheng W, and Donglu S (2013). “Near-infrared laser light mediated cancer therapy by photothermal effect of Fe3O4 magnetic nanoparticles. Biomaterials, 34(16): 4078-4088.
da Rocha AB, Lopes RM, Schwartsmann G (2001) Natural products in anticancer therapy. Curr Opin Pharmacol., 1:364–369.
Des Guetz G, Uzzan B, Morere JF, et al. (2010) Duration of adjuvant chemotherapy for patients with non-metastatic colorectal cancer. Cochrane Database Syst Rev., 20:CD007046.
Edmondson R, Broglie J J., Adcock A F., and Yang L. (2014) Three-dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors.Assay Drug Dev Technol.,12(4):207-18.
Edmondson R, Broglie JJ, Adcock AF (2014) Three-dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev Technol. May;12(4):207-18.
Efferth T, Li PC, Konkimalla VS, Kaina B. (2007) From traditional Chinese medicine to rational cancer therapy. Trends Mol Med, 13: 353–361.
El-Mostafa K, El Kharrassi Y, Badreddine A, Andreoletti P, Vamecq J, El Kebbaj M S, Latruffe N, zard G, Nasser B, Cherkaoui-Malki M. (2014) Nopal Cactus (Opuntia Ficus-indica) as a Source of Bioactive Compounds for Nutrition, Health and Disease. Molecules. 19(9):14879-901.
Fayad W, El-Hallouty SM, El-Manawaty MA, Mounier MM, Soliman AF, Mahmoud K, Yousry AA, Farghaly AA, Fahmy MA, Hasasn ZM, Linder S (2017) A systematic multicellular spheroids screening approach lead to the identification of antineoplastic activity in three different plant extracts from the Egyptian flora. J. of App. Pharmac Sci, Vol 7(6): P 13-22.
Fayad Z, Mani V, Woodward M, Kallend D, Abt M, Burgess T, Fuster V, Ballantyne C M, Stein E A, Tardif J, Rudd J H F, Farkouh M E, Tawakol A. (2011). Safety and Efficacy of Dalcet rapib on Atherosclerotic Disease Using Novel Non-Invasive Multimodality Imaging (dal-PLAQUE): A Randomised Clinical Trial. Lancet., 29;378(9802):1547-59.
Friedrich TL,Vessey WB, Schuelke MJ, Ruark GA and Mumford MD. (2009) A framework for understanding collective leadership: The selective utilization of leader and team expertise within networks. The Leadership Quarterly Yearly Review of Leadership, Vol 20( 6) 33-39.
Ho JW, Leung YK, Chan CP. (2002) Herbal medicine in the treatment of cancer. Curr Med Chem Anticancer Agents, 2: 209–214.
Ibrahim AS, Khaled HM, Mikhail NN, et al. (2014) Cancer incidence in Egypt: results of the national population-based cancer registry program. J Cancer Epidemiol; 437971 10.
Jungwoo Lee, Meghan J Cuddihy, Nicholas A Kotov. (2008). Three-dimensional Cell Culture Matrices: State of the Art. Mar;14(1):61-86.
Khan A. S. (2017) Trees with Antimicrobial Activities, Medicinally Important Trees. Springer International Publishing.
Khoobchandani M, Ojeswi BK, Sharma B, Srivastava MM (2009) Chenopodium album prevents progression of cell growth and enhances cell toxicity in human breast cancer cell lines. Oxid Med Cell Longev., 2:160–165.
Maluccio M and Covey A (2012). Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. Cancer J Clin.; 62:394-9.
Melek FR, Miyase T, Ghaly NS, Nabil M (2007) Triterpenoid saponins with N-acetyl sugar from the bark of Albizia procera. Phytochemistry. 68: 1261-1266.
Mosmann, T. (1983) Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. Journal of Immunological Methods, 65, 55-63.
Nguyen NH , Ta Q , Pham Q, Luong T , Phung V , Duong T and Vo V G (2020). Anticancer Activity of Novel Plant Extracts and Compounds from Adenosma bracteosum (Bonati) in Human Lung and Liver Cancer Cells. Molecules 25, 2912.
Newman DJ, Gordon M and Cragg GM (2007) Natural Products as Sources of New Drugs Over the Last 25 Years.J Nat Prod., Mar;70(3):461-77.
Omata M., Cheng AL., Kokudo N, Kudo M, Lee J.M., Jia J., et al. (2017) Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma. Hepatol. Int., 11 P. 317-370.
Réthy B, Zupkó I, Minorics R, Hohmann J, Ocsovszki I, Falkay G (2007). Investigation of Cytotoxic Activity on Human Cancer Cell Lines of Arborinine and Furanoacridones Isolated from Ruta graveolens. Planta Med., 73(1):41-8.
Roy PS and Saikia BJ (2016) Cancer and cure: A critical analysis. Indian J cancer 53: 441-442.
Rukunga GM., Waterman P G (1996). New macrocyclic spermine (budmun chiamine) alkaloids from Albizia gummifera: with some observations on the structure activity relationships of the budmun chiamines. J. Nat. Prod., 59: 850-853.
Sivakrishnan S and KottaiMuthu A (2014) Phytochemical Evaluation of Ethanolic Extract of Aerial Parts of Albizia procera. Br Biomed Bull. 2: 235-241.
Trédan O, Geay J-F, Touzet S, Delva R, Weber B, Cretin J, Provencal J, Martin J, Stefani L, E Pujade- Lauraine, G Freyer. (2007) Carboplatin/cyclophosphamide or carboplatin/paclitaxel in Elderly Patients With Advanced Ovarian Cancer? Analysis of Two Consecutive Trials from the Groupe. Ann Oncol., 18(2):256-62.
Walker M, Boey G, McDonald LA (2003) The pathology of oral cancer. Pathology, Vol. 35(5) P 376-383.
Wang R, Zhang J, Chen S, LU M, Luo X, Yao S, Liu S, Qin Y, Chen, H. (2011) Tumor-associated macrophages provide a suitable microenvironment for non-small lung cancer invasion and progression. Lung Cancer,Vol 74( 2) P 188-196.
Wang R., Lu Y., Li H., et al. (2018) Antitumor activity of the Ailanthus altissima bark phytochemical ailanthone against breast cancer MCF7 cells. Oncology Letters, 15(4): 6022–6028.
Wang Y, Wang WJ, Su C, Zhang DM, Xu LP, He RR, Wang L, Zhang J, Zhang XQ, Ye W (2013) Cytotoxic quassinoids from Ailanthus altissima. Bioorg Med Chem Lett., 23: 654–657.
Wong M.C., Jiang J.Y., Goggins W.B., Liang M., Fang Y., Fung F.D et al. (2017) International incidence and mortality trends of liver cancer: a global profile. Sci. Rep., 7, P. 45846.


