1Deparment of Chemistry, Motilal Nehru College, University of Delhi, Delhi, India.
2Deparment of Chemistry, Sri Aurobindo College, University of Delhi, Delhi, India.
Corresponding author email: krishanchem@gmail.com;
Article Publishing History
Received: 15/12/2021
Accepted After Revision: 20/03/2022
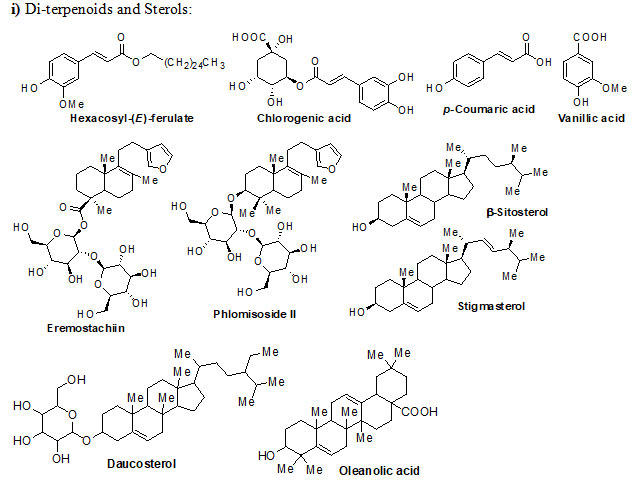
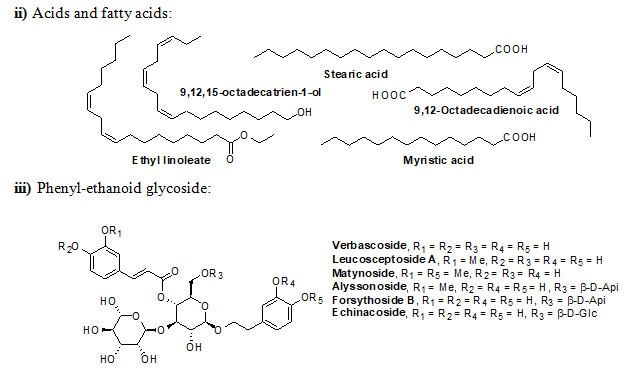
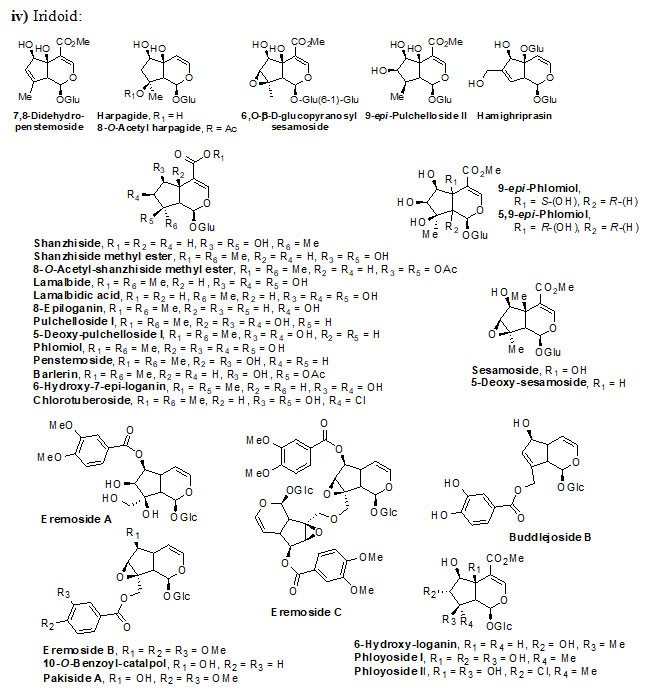
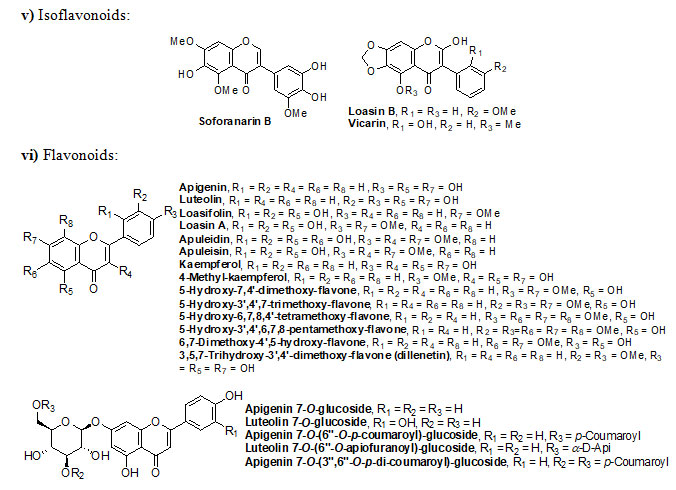
Genus Eremostachys Bunge is a key medicinal plant grown in Eastern Europe, Central and Western Asia and Middle East. The plants of this genus have numerous secondary metabolites, which exhibit both traditional and pharmacological applications. Eremostachys contains several classes of reactive chemical ingredients such as flavonoids (viz. Apigenin, Luteolin, Loasifolin, Loasin A, Apuleisin, Apigenin and Kaempferol etc), isoflavonoids (viz. Soforanarin B, Loasin B and Vicarin), iridoid glucosides (viz. Shanzhiside, Lamalbide, Lamalbidic acid, Epiloganin, Pulchelloside, Harpagide, Pulchelloside, hamighriprasin, Eremoside, Phloyoside and Barlerin etc.), phenylethanoid glycosides (viz. Verbascoside, Leucosceptoside A, and Echinacoside etc,), acids, hydrocarbons, terpenes, diterpenoids and sterols (viz. Eremostachiin, Phlomisoside II, Stigmasterol, β-Sitosterol, Daucosterol and Oleanolic aicd) etc.
These metabolites are well known for their pharmacological applications such as antibacterial, anti-inflammatory, antioxidant, antirheumatic, anti-poisonous, antimalarial, anticancer, antimalarial, antiallergic, antiarthritic and antidepressant etc. Before the identification of chemical constituents, genus Eremostachys was used by few countries since ancient viz. by China, Iran, India, Pakistan, Tajakistan and few middle and south Asian countries etc. This genus has been used by people of these region since ancient as analgesic, anti-inflammatory, wound healing, ant-insecticidal, antiparasitic, antiallergic, liver care, joint pain, arthritis, antioxidant, antibacterial, antidepressant, antimalarial, perfumery, detergent, soap, beauty products. In India, E. superba has been used as a food for cattle to increase milk production. In the present review, the important traditional uses of some important species of the genus Eremostachys have been briefly discussed due to their availability and affordability. The number of medicinal and pharmacological applications of the plant genus Eremostachys are also summarized in the paper.
Anti-Inflammatory; Antioxidant; Diterpenoid; Eremostachys; Secondary Metabolites,.
Khan A. M, Agnihotri N. K, Singhv. K , Joshi M. C, Kumar K. Conventional Medicinal Uses and Chemical Structure of Important Secondary Metabolites in the Genus Eremostachys: A Literature Review . Biosc.Biotech.Res.Comm. 2022;15(1).
Khan A.M, Agnihotri N.K, Singhv.K , Joshi M.C, Kumar K. Conventional Medicinal Uses and Chemical Structure of Important Secondary Metabolites in the Genus Eremostachys: A Literature Review. Biosc.Biotech.Res.Comm. 2022;15(1). Available from: <a href=”https://bit.ly/3LiIOki“>https://bit.ly/3LiIOki</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Genus Eremostachys, known as desert rod, belongs to the family Lamiaceae. Presently, around 80 species of this genus have been documented, which are mainly distributed in Eastern Europe, Central and Western Asia and the Middle East (Harley et al. 2004). However, more than 45 species are distributed only in Azerbaijan, Armenia, Turkey, Iran, and Turkmenistan (Azizian et al. 1982; Hedge et al. 1986). It is an Irano-Turanian genus and majorly distributed in the desert mountains of the Iranica area especially covering Central Asia. However; few species viz. E. laciniata, E. molucelloides and E. vicaryi expanded their distribution towards Turkey, Pakistan and Afghanistan etc.
Overall, genus Eremostachys has been represented by 52 taxa of Flora in the USSR (Former Soviet Union); 41 taxa of Flora found in Iranica; 16 species in Iran; 8 species in Pakistan; 5 species in China; 2 taxa of Flora in Palaestina and 1 taxa in Flora Europeae and one critically endangered taxa of the flora is found in Northern Himalaya of Uttarakhand, Himachal Pradesh and Jammu & Kashmir of India (Knorring et al. 1954; De Filipps et al. 1972; Shishkin et al. 1977; Zohary et al. 1978; Azizian et al. 1982; Rechinger et al. 1982; Chowdhary et al.1984; Jain et al; 1984; Radcliffe-Smith et al. 1986 ; Hedge et al. 1990; Li et al. 1994; Rao et al. 1994; The Hindu 10 Mar, 1997; The Daily Excelsior 17 Oct, 1997; Kalvandi et al. 2007; Hariri et al. 2021).
The morphology of genus Eremostachys has been characterized by a robust or erected pubescent stem, laciniate or crenate leaves, large calyces, large yellow, creamy or white corollas, beared nutlets and tuberous roots (Pignatti 1982). Phytochemical studies of genus Eremostachys have revealed the presence of many potent secondary metabolites viz. alkaloids, phenylethanoids, iridoid glycosides, acids, flavonoids, terpenoids, hydrocarbons and essential oils etc. Due to the variety of secondary metabolites present in the genus Eremostachys, this genus is well known for its medicinal properties viz. as strong antidepressant, free radical scavenging and cytotoxic activity (Delazar et al. 2004a; Delazar et al. 2004b; Delazar et al. 2005; Delazar et al. 2006).
Some species like E. azerbaijanica, E. glabra, E. labiosa, E. laciniata, E. laevigata, E. loasifolia, E. macrophylla and E. vicaryi are excessively explored for their secondary metabolites and their medicinal importance (Delazar et al. 2004; Delazar et al. 2005; Erdemoglu et al. 2006; Navaei et al. 2006; Amiri et al. 2007; Calis et al. 2007; Nori-Shargh et al. 2007; Javidnia et al. 2008; Modaressi et al. 2009; Khan et al. 2010; Rustaiyan et al. 2011; Ali et al. 2012; Al-Jaber et al. 2012; Esmaeili 2012; Mughal et al. 2010 and 2012; Imran et al. 2012; Akhlaghi et al. 2015; Vaez et al. 2015; Asnaashari et al. 2016 a; Asnaashari et al. 2016 b; Faryabi et al. 2021; Hariri et al. 2021).
From India point of view, there is only one species E. superba Royale ex Benth., of genus Eremostachys that was identified as a critically endangered plant species due to lack of proper knowledge, grazing by herbivores, plucking of the flowers by travelers, and overexploitation by local people (Verma et al. 2003). It was described from Mohand and Khree Pass (Siwaliks of Saharanpur) by Royle in 1839, which was a very sophisticated and beautiful plant found in Uttarakhand, Himachal Pradesh, Jammu & Kashmir province of India (Sharma et al. 1981; Jain et al. 1984; Panwar et al. 2015; Hariri et al. 2021).
The genus Eremostachys is one of the important medicinal plants due to the presence of numerous potent secondary metabolites. The number of medicinal and pharmacological applications of the plant genus Eremostachys are also summarized in the paper. The chemical structure of the important reactive chemical ingredients of the secondary metabolites isolated and identified from the genus Eremostachys are given in the present paper. The important secondary metabolites of genus Eremostachys reported in the literature are compiled along with their pharmacological applications. It is well evident from the literature reports that substantive number of species of Genus Eremostachys got extinct or at the verge of extinction. The present review is aimed to recognize medicinal importance, traditional uses among society and also to document status report of ever becoming critically endangered species of medicinal flora (Hariri et al. 2021).
Taxonomic description of Genus Eremostachys (Ved et al. 2003)
Kingdom: Plantae Subkingdom: Tracheobionta
Superdivision: Spermatophyta Division (Phylum): Tracheophyta
Class: Magnoliopsida (Dicotyledons) Subclass: Magnoliidae Novak ex Takht.
Order: Lamiales Family: Lamiaceae
Genus: Eremostachys
Species: E. adenantha, E. azerbaijanica, E. baissunensis, E. glabra, E. labiosa, E. labiosiformis, E. laciniata, E. laevigata, E. lehmanniana, E. loasifolia, E. macrophylla, E. molucelloides, E. pulvinaris, E. speciosa, E. superba, E. thyrsiflora, E. vicaryi etc.
Traditional Uses of Eremostchys: Conventionally, the genus Eremostchys is used by South Asian and West Asian countries for the treatment of various ailments. Eremostachys has been used as an anti-inflammatory and analgesic agent and applied topically for the treatment of bruises and localized pain and swelling (Said et al. 2002; Delzar et al. 2004b; Erdemoglu et al. 2006; Hariri et al. 2021).
Traditionally, E. laciniata is used in various illnesses viz, to treat allergies, headache and various liver diseases, asthma, cough & cold, alleviate inflammation and used as a herbal tea (from root and flower) (Said et al. 2002; Modaressi et al. 2009). The number of plants of this genus is also used for traditional and folk medicine for treating a number of ailments are described briefly in Table 1. In India genus Eremostachys superba Royle ex Benth is used to restore mulching by mixing it with cattle feed and fed to goats, cows, and buffaloes etc., which stop yielding milk (Khan et al. 2020; Hariri et al. 2021).
Table 1. Traditional uses of some species of genus Eremostachys
| Species | Parts Used for Treatment | Traditional Uses |
| E. glabra | Rhizomes | Used as a native analgesic and anti-inflammatory agent in Iran (Delazar et al. 2004a). |
| E. laevigata | Whole plant | Used as therapeutics against many infectious diseases, as food preservatives and have shown insecticidal and antiparasitic properties (Burt et al. 2004). Also used in cosmetic and household products, (www.inchem.org). |
| E. laciniata | Roots, flower and rhizomes | Roots and flower decoction have been used orally for the treatment of allergy, headache and liver disease. It is known by the local name “Chelle-Daghi” in Iran and its rhizomes are used to relieve pain related to rheumatoid arthritis (Said et al. 2002 and Delazar et al. 2013), as an antioxidant (Erdemoglu et al. 2006), antibacterial (Modaressi et al. 2009), antidepressant (Nisar et al. 2011), antiinflammatory (Hariri et al. 2021) & analgesic in various places of middle south East & south Asia (Delazar et al. 2009). |
| E. macrophylla | Aerial and rhizome | Aerial & rhizome, used as a folk medicine in Iran, comprises therapeutic ingredients against joints pain, infectious wound healing, snakebite, rheumatism and antimalarial (Nori-Shargh et al. 2007, Mosaddegh et al. 2012, Asnaashari et al. 2015 and Asnaashari et al. 2016 (a and b)). |
| E. superba | Whole plant | Used as an antidepressant and antioxidant. This species is less reported towards medicinal importance except for the local report according to Gujjars, where they used root tubers as food to buffaloes to increase the milk production. It is used for curing mastitis and restoration of mulching in cattles (Verma et al. 2003 and Sharma et al. 2015) and against fish poisoning (Ajaib et al. 2014). |
| E. vicaryi | Whole plant and seed | Used for poisoning fish in the Eusufzai near Peshawar (Radcliffe-Smith et al. 1986) and seeds are utilized as cooling agents to lower fever in the Balochistan province (Pakistan) (Tareen et al. 2016). |
Pharmacological Importance: Genus Eremostachys is one of the important plants, which are known for their diversified medicinal and pharmacological applications (Table 2). Few plants of this species are widely studied viz. E. laciniata, E. loasifolia, E. macrophylla, E. glabra, E. laevigata, E. azerbaijanica, E. labiosa, E. labiosiformis, E. pulvinaris etc. However; most of the species are still need to be explored with respect to their pharmacological applications and secondary metabolites.
Table 2. Secondary metabolites and Pharmacological Uses of some species of genus Eremostachys
| Species | Secondary metabolites | Pharmacological application |
| E. adenantha Jaub.
Et Spach |
Dodecanal, tetradecanal, undecanal, tetradecanoic acid, hexadecanoic acid, 6,10,14-trimethyl-2-pentadecanone, caryophyllene oxide (from aerial part) (Javidnia et al. 2008). | Antioxidant (from leaves) (Firuzi et al. 2010). |
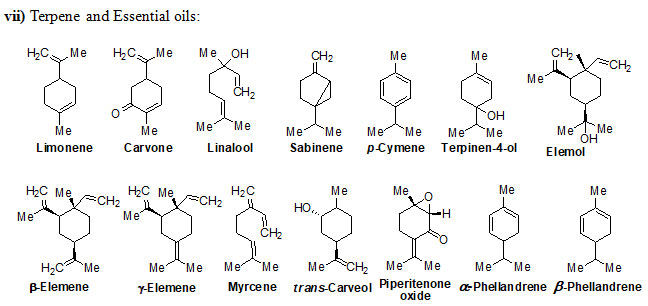
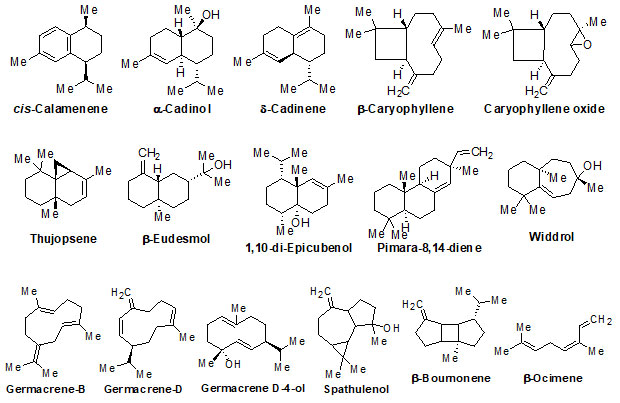
| E. azerbaijanica Rech. f | Tricosane, hexahydrofarnesyl acetone, 2-methyl-6-propyl-dodecane, flavonoid (luteolin-7-O-rutinoside), phenylethanoid (verbascoside) (Asnaashari et al. 2016a), sesquiterpenes, steroids, coumarins (Asnaashari et al. 2016b), Phlomisoside II, eremostachiin, alyssonoside, forsythoside B, lamalbide, pulchelloside I, sesamoside, 6-hydroxyloganin, shanzhiside methyl ester (from roots) (Modarresi et al. 2013, Fouladnia et al. 2012 and Asnaashari et al. 2018), dodecanal, hexadecanoic acid, 6,10,14-trimethyl-2-penta-decanone, tetradecanal, undecanal, tetradecanoic acid, caryophyllene oxide (Javidnia et al. 2008), carvone, β-caryophyllene, limonene, β-bourbonene, germacrene D, transcarveol, cis-calamenene (Manafi et al. 2010), hexahydrofarnesyl acetone, 2-methyl-6-propyl-dodecane (Asnaashari et al. 2016a). | Radical scavenging activity (Asnaashari et al. 2016a), antioxidant, antimicrobial, and cytotoxic activity (Asnaashari et al. 2017), antimalarial activity (aerial part showed IC50 values of 0.949 ± 0.061 mg mL–1 and rhizomes showed 0.382 ± 0.011 mg mL–1) (Asnaashari et al. 2016b), antiproliferative (Delazar et al. 2017). |
| E. glabra Boiss. ex Benth. | furanolabdane diterpene glycoside (Eremostachiin) (Delazar et al. 2006), methyl ester, iridoid glycosides (6,9-epi-8-O-acety- lshanziside 5,9-epi-penstemoside, 5,9-epi-7,8-didehydro-penstemoside (Delazar et al. 2004b), hexacosyl-(E)-ferulate, leucosceptoside A (Delazar et al. 2004a), iridoids (Barlerin, 8-O-acetyl-shanziside, penstemoside, 7,8-didehydro -penstemoside) (Jensen et al. 2007), β-sitosterol, verbascoside, stigmasterol, phlomisoside II, forsythoside B, 9-epi-phlomiol, lamalbide, 5,9-epiphlomiol, penstemoside, 9-epi-pulchelloside ІІ, 6-hydroxy-7-epi-loganin, 6′-O–β-D-glucopyranosyl sesamoside, shanzhiside methyl ester, phloyoside ІІ, hexacosyl-(E)-ferulate (from Rhizomes) (Delazar et al. 2013). | Free-radical scavenging activity, antioxidant (hexacosyl-(E)-ferulate showed RC50 = 0.0976 mg/mL and leucosceptoside-A showed 0.0148 mg mL–1) ((Delazar et al. 2004a)) and antibacterial (Delazar et al. 2004b and 2005, Erdemoglu et al. 2006). |
| E. labiosa Bunge | α-Pinene, 1,8-cineole, 6,10,14- trimethyl 2-pentadecanone, sabinene, hexadecane, α-phellandrene, β-phellandrene, tetradecane, p-cymene (from aerial and stem part) (Rustaiyan et al. 2011). | Anticancer, anti-inflammatory, antileishmanicidal (Rabe et al. 2014). |
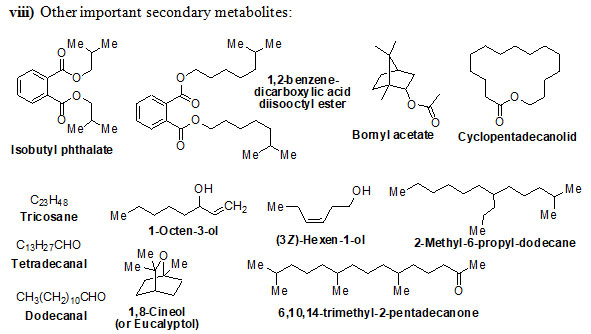
| E. labiosiformis (Popov) | Harpagide (from flowers), 9,12-octa-decadienoic acid, octadecanoic acid, hexadecanoic acid, 1,2-benzene-dicarboxylic acid diisooctyl ester, 9,12,15-octa -decatrien-1-ol (from aerial part) (Kooiman 1972). | Antioxidant, anti-Alzheimer (Samandari-Bahraseman et al. 2018), antibacterial (Vahedi et al. 2013). |
From a medicinal point of view, genus Eremostachys is playing a key role in Ayurvedic and Unani medicine due to the presence of the number of chemical reactive secondary metabolites. The whole plant is important for medicinal purposes as all parts of the plant contain some vital secondary metabolites. Secondary metabolites reported in the literature along with their important pharmacological applications are summarized in Table 2 (i) (ii), (iii) and (iv) (Khan et al. 2020; Hariri et al. 2021).
Table 2(ii).Secondary metabolites and Pharmacological Uses of some species of genus Eremostachys
| Species | Secondary metabolites | Pharmacological application |
| E. laciniata a (L.) Bunge | Acidic iridoid glucoside (Calis et al. 2008), iridoid glucosides (phloyoside I, phlomiol pulchelloside I) (Modaressi et al. 2009), furanolabdane diterpene glycosides, monoterpenes, sesquiterpenes, iridoid glucosides and flavonoids (Navaei et al. 2006; Delazar et al. 2008; Eftekharsadat et al. 2011), luteolin, apigenin, 5,8-dihydroxy-6,7-dimethoxy-flavone, 5,7-dihydroxy-6,8-dimethoxy-flavone, luteolin 7-O–β-glucosides (Nisar et al. 2011), phlomisoside II, verbascoside, leucosceptoside A, martynoside, forsythoside B, apigenin 7-O-glucoside, luteolin 7-O-(6”-O– apiofuranosyl)-glucoside, apigenin 7-O-(6”-O-p-coumaroyl)-glucoside, sesamoside, 5-deoxysesamoside, 6-β-hydroxy-7-epi-loganin, 5-deoxy- pulchelloside-I, Chlorotuberoside, lamalbide, lamalbidic acid, phloyoside I (7-epi-phlomiol), phloyoside II, phlomiol, shanzhiside, shanzhiside methyl ester, 8-Oacetyl-shanzhiside methyl ester, dodecanol, widdrol, germacrene B and D, thujopsene, 3-octanone, (3Z)-hexen-1-ol, n-hexanol, benzacetaldehyde, 1-octen -3-ol, α-pinene, linalool, 6,10,14-trimethyl-2-pentadecanone, limonene, p-cymene, δ-cadinene, (2E)-dodecenal, dehydrolinalool, cyclo-pentadecanolide, (E)-β-ocimene, 1,8-cineole, terpinen-4-ol (Navaei et al. 2006, Al-Jaber et al. 2012 and Delazar et al. 2013) (aerial part). | Anti-inflammatory (Hariri et al. 2021 and Delazar et al. 2013), antibacterial (MIC = 0.05-0.50 mg mL–1) (Modaressi et al. 2009 and Ur Rahman et al. 2015), free radical scavenging, antioxidant properties, anti-inflammatory, dietary supplement (Hariri et al. 2021, Mosaddegh et al. 2012 and Bajalan et al. 2017), effective in the treatment of mild and moderate Carpal Tunnel Syndrome (CTS) in combination with the wrist night splint, especially in alleviating the severity of the syndrome and increasing the palmer prehension power (Eftekharsadat et al. 2011), antipain (Gharabagy et al. 2013) anti-depressants (Nisar et al. 2011 and Hakimi et al. 2020). |
| E. laevigata Bunge | Benzaldehyde, 1,8-cineole, piperitenone oxide, cis– piperitoneoxide, 1-octen-3- ol, dodecanal, germacrene-D, β-caryophyllene, caryophyllene oxide (Amiri et al. 2007 and Esmaeili et al. 2012) (from whole plant).
|
Antibacterial, antioxidant activity (IC50 (μg mL–1): 277.1 (flowers), 495 (stems), 212.6 (root) (Esmaeili et al. 2012), β-caryophyllene possesses anti-inflammatory, anti-carcinogenic activities and plant defense (Cai et al. 2002), germacrene-D is anti-insect (Altug et al. 2004), Dodecanal is non-toxic, food additive (GRAS in USA and inchem in UE) and used in perfumery as in soap, detergent, beauty care and household products (www.inchem.org). |
Table 2(iii). Secondary metabolites and Pharmacological Uses of some species of genus Eremostachys
| Species | Secondary metabolites | Pharmacological application |
| E. laciniata a (L.) Bunge | Acidic iridoid glucoside (Calis et al. 2008), iridoid glucosides (phloyoside I, phlomiol pulchelloside I) (Modaressi et al. 2009), furanolabdane diterpene glycosides, monoterpenes, sesquiterpenes, iridoid glucosides and flavonoids (Navaei et al. 2006; Delazar et al. 2008; Eftekharsadat et al. 2011), luteolin, apigenin, 5,8-dihydroxy-6,7-dimethoxy-flavone, 5,7-dihydroxy-6,8-dimethoxy-flavone, luteolin 7-O–β-glucosides (Nisar et al. 2011), phlomisoside II, verbascoside, leucosceptoside A, martynoside, forsythoside B, apigenin 7-O-glucoside, luteolin 7-O-(6”-O– apiofuranosyl)-glucoside, apigenin 7-O-(6”-O-p-coumaroyl)-glucoside, sesamoside, 5-deoxysesamoside, 6-β-hydroxy-7-epi-loganin, 5-deoxy- pulchelloside-I, Chlorotuberoside, lamalbide, lamalbidic acid, phloyoside I (7-epi-phlomiol), phloyoside II, phlomiol, shanzhiside, shanzhiside methyl ester, 8-Oacetyl-shanzhiside methyl ester, dodecanol, widdrol, germacrene B and D, thujopsene, 3-octanone, (3Z)-hexen-1-ol, n-hexanol, benzacetaldehyde, 1-octen -3-ol, α-pinene, linalool, 6,10,14-trimethyl-2-pentadecanone, limonene, p-cymene, δ-cadinene, (2E)-dodecenal, dehydrolinalool, cyclo-pentadecanolide, (E)-β-ocimene, 1,8-cineole, terpinen-4-ol (Navaei et al. 2006, Al-Jaber et al. 2012 and Delazar et al. 2013) (aerial part). | Anti-inflammatory (Hariri et al. 2021 and Delazar et al. 2013), antibacterial (MIC = 0.05-0.50 mg mL–1) (Modaressi et al. 2009 and Ur Rahman et al. 2015), free radical scavenging, antioxidant properties, anti-inflammatory, dietary supplement (Hariri et al. 2021, Mosaddegh et al. 2012 and Bajalan et al. 2017), effective in the treatment of mild and moderate Carpal Tunnel Syndrome (CTS) in combination with the wrist night splint, especially in alleviating the severity of the syndrome and increasing the palmer prehension power (Eftekharsadat et al. 2011), antipain (Gharabagy et al. 2013) anti-depressants (Nisar et al. 2011 and Hakimi et al. 2020). |
| E. laevigata Bunge | Benzaldehyde, 1,8-cineole, piperitenone oxide, cis– piperitoneoxide, 1-octen-3- ol, dodecanal, germacrene-D, β-caryophyllene, caryophyllene oxide (Amiri et al. 2007 and Esmaeili et al. 2012) (from whole plant).
|
Antibacterial, antioxidant activity (IC50 (μg mL–1): 277.1 (flowers), 495 (stems), 212.6 (root) (Esmaeili et al. 2012), β-caryophyllene possesses anti-inflammatory, anti-carcinogenic activities and plant defense (Cai et al. 2002), germacrene-D is anti-insect (Altug et al. 2004), Dodecanal is non-toxic, food additive (GRAS in USA and inchem in UE) and used in perfumery as in soap, detergent, beauty care and household products (www.inchem.org). |
Chemical structure of Secondary metabolites: Numerous secondary metabolites were identified from the genus Eremostachys. Sterols, essential oils, linear hydrocarbons, iridoid glucosides, flavonoids, isoflavonoids, terpenoids, and their derivatives, acid derivatives and phenylethanoid glycosides etc. are found in a majority. Most of them are represented and specifies by their core structures as follows:
Table 2(iii):Secondary metabolites and Pharmacological Uses of some species of genus Eremostachys
| Species | Secondary metabolites | Pharmacological application |
| E. loasifolia Benth. | Iridoid glucosides (eremosides A-C) (Mughal et al. 2012), 6,7-dihydroxy-coumarin, luteolin 4′-O-β-d-gluco pyranoside (Delazar et al. 2006), loasins A & B, apuleisin, apuleidin (Mughal et al. 2010), loasifolin, eremoside A and B, kaempferol (3,4′,5,7-tetrahydroxy flavone), 5-hydroxy-6,7,8,4′-tetra-methoxy flavone, 5-hydroxy-3′,4′,6,7,8- pentamethoxy-flavone, apigenin (4′,5,7-trihydroxy-flavone), luteolin (3′,4′,5,7-tetrahydroxy-flavone), apigenin 7-O–β-D-glucopyranose, 4-methyl kaempferol (3,5,6-trihydroxy-4-methoxy flavone), 5-hydroxy-7,4-dimethoxy flavones, 6,7-dimethoxy-4,5-hydroxyflavone, 3,5,7-trihydroxy-3,4-dimethoxy-flavone (Altug et al. 2004). (whole plant). | Antioxidant activity (Altug et al. 2004). |
| E. macrophylla Montbret & Aucher ex Benth | α-Cadinol, germacrene B and D, isobutyl phthalate, hexadecanoic acid, ethyl linoleate, 6-methyl-α-ionone, myrcene, non-terpenoids, oxygenated nonterpenes, nonterpene hydrocarbons, β-elemene, γ-elemene, β-phellandrene (Navaei et al. 2006 and Asnaashari et al. 2016a), spathulenol, hexadecanoic acid, caryophyllene oxide (oils), β-caryophyllene, pimara-8,14-diene, ethyl linoleate, β-eudesmol, 6,10,14-trimethyl-2- pentadecanone (Rustaiyan et al. 2011), verbascoside, lamalbide, sesamoside, phlomiol, luteolin-7-O-Glucoside, apigenin- 7-O-rutinoside, kaempferol-3-O-glucosides with iridoid, phenylethanoid, flavonoid structures (Imran et al., 2018; Asgharian et al., 2020) (from the whole plant), 1,8-cineole, germacrene D-4-ol, α-pinene, 1,10-diepicubenol, elemol, bornyl acetate, 1,10-diepicubenol (Navaei et al., 2006). | Antimalarial activities (IC50 = 0.797 ± 0.016 mg mL–1 in aerial parts and 0.324 ± 0.039 mg mL–1 in rhizomes compared to positive control (Chloroquine, IC50 = 0.014 ± 0.003 mg mL–1, IC90 = 0.163 ± 0.004 mg mL–1) (Delazar et al. 2009), used to treat wounds, snake bites, rheumatism and joint pains (Navaei et al. 2006), antioxidant, general toxicity, anti- proliferative and anti- microbial (Delazar et al. 2018) anti-oxidant, anti-proliferative (Nori-Shargh et al. 2007 and Asgharian et al. 2017a and 2017b). |
| E. molucelloides Bunge | Phloyoside II, p-coumaric acid, sesamoside, 5-deoxysesamoside, 6-hydroxy-7-epiloganin, 2β,14-dihydroxy-11-formyl-12-carboxy-13-des-isopropyl-13- hydroxymethyl-abieta-8,11,13-triene-16(17)-lactone, 12,18-dicarboxy-14-hydroxy-13-des-isopropyl-13-hydroxymethyl-abieta-8,11,13-triene-16(17)-lactone, echinacoside, lamalbide, vanillic acid, verbascoside, 5-hydroxy-3′,4′,7-trimethoxyflavone, 5-hydroxy-7,4′-dimethoxy-flavone, apigenin-7-O-glucoside, luteolin-7 –O-glucoside, apigenin-7-O-(6”-O– pcoumaroyl)-glucoside, apigenin-7-O– (3”,6”-β–O–p-dicoumaroyl)-glucoside, luteolin-7-O– (6”-O-apiofuranosyl)- glucoside, 5-deoxy- pulchelloside I, shanzhiside methylester, chlorogenic acid, lamalbidic acid (Qui et al. 2019). | It might be expected to have an excellent pharmacological insight due to the presence of many potent chemical constituents. |
Table 2(iv):Secondary metabolites and Pharmacological Uses of some species of genus Eremostachys
| Species | Secondary metabolites | Pharmacological application | |
| E. pulvinaris Jaub. & Spach | Phenylethanoid glycosides (forsythoside B, leucosceptoside A, verbascoside) (Delazar et al. 2004) (from rhizomes). | Free radical scavenging activity and toxicity, antioxidant (RC50 = 0.0064, 0.0148 & 0.0079 mg mL–1 for forsythoside B, leuco-sceptoside A & verbascoside, respectively) (Delazar et al. 2004). | |
| E. speciosa Rupr. | luteolin 7-O–β-D-glucoside Gella et al. 1972. | Antioxidant and anti-inflammatory (from epigeal parts) (Gella et al. 1972). | |
| E. superba Bunge | less studied due to critically endangered species in India (Shrivastava et al. 2017 and Srivastava et al. 2018). | A very handsome plant used as an ornament (Duthie, 1903-29), tuberous roots are used for increasing lactation in cattle (Koul et al. 1997, Vaez et al. 2015 and Pant et al. 2011), treatment of liver, stomach and gout related diseases (Srivastava et al. 2018).
|
|
| E. thyrsiflora Benth. | Alkaloids, steroids, flavonoids, phenols, tannins, saponins, terpenoids, fats, glycosides, coumarins, xanthoproteins, carbohydrates, carboxylic acids and volatile oils (Behlil et al. 2019). | Antioxidant activity (from the whole plant) (Behlil et al. 2019). | |
| E. vicaryi Benth. ex Hook. f. | Vicarin, soforanarin B, luteolin 7-O–β-D-glucopyranoside, hamighriprasin (Calis et al. 2007). | Seeds are utilized as cooling agent to lower fever in the Balochistan of Pakistan (Ajaib et al. 2014). | |
| E. baissunensis Popov
|
Barlerin, lamalbide, 5-deoxysesamoside (from aerial part) (Bobaev et al. 2015). | Not studied much. | |
| E. lehmanniana | Fatty acids from seeds (Bagci et al. 2007) | E. lehmanniana Bunge is not studied much. | |
CONCLUSION
The findings of the present study has shown that the genus Eremostachys is very important with proven medicinal impacts due to the presence of numerous secondary metabolites and their known biological applications viz. antibacterial, anti-inflammatory, antioxidant, painkilling, antirheumatic, anti-poisonous. Further, it can be a potential agent towards antimalarial, anti-Parkinson’s and anticancer etc. as few reports are based on such studies. Therefore, in this review, the important secondary metabolites extracted from the genus Eremostachys viz., flavonoids, isoflavonoids, iridoid glucosides (chemotaxonomic markers), phenyl-ethanoid glycoside, acids, hydrocarbons, essential oils, terpenes, diterpenoids and sterols etc. are summarized along with chemical structure.
The traditional uses and pharmacological applications of this genus Eremostachys reported in the literature are compiled in tabular form. Unfortunately, only a few species (viz. E. laciniata, E. azerbaijanica, E. glabra, and E. macrophylla) have been majorly studied so far, however; most of the species of this genus are still need to be explored. The genus Eremostachys superba Royle ex Benth is an only endangered species in India, having an ornamental value as very few studies on their medicinal properties are reported in literature.
ACKNOWLEDGEMENTS
The literature survey facilities were provided by the Motilal Nehru College, Delhi University, India and Delhi University, India.
Conflicts of Interests: Authors declare no conflicts of interests to disclose.
Data Availability Statement: The database generated and /or analysed during the current study are not publicly available due to privacy, but are available from the corresponding author on reasonable request.
REFERENCES
Ajaib M, Khan Z and Zikrea A, (2014). Ethnobotanical survey of some important herbaceous plants of District Kotli, Azad Jammu & Kashmir. Biologia (Pakistan), Vol. 60(1), 11-22.
Akhlaghi H, (2015). Chemical composition of the essential oil from aerial parts of Eremostachys macrophylla Montbr. & Auch from northeast of Iran, Nat Prod Chem Res, Vol. 3(1), 1000159, https://doi.org/10.4172/2329-6836.1000159
Ali B, Mehmood R, Mughal UR, et al., (2012). Eremosides A–C New Iridoid Glucosides from Eremostachys loasifolia, Helv Chim Acta, Vol. 95(4), 586-593.
Al-Jaber HI, Al-Qudah MA, Barhoumi LM, et al., (2012). Variation in the essential oil composition of Eremostachys laciniata from Jordan at different flowering stages, J Essent Oil Res, Vol. 24(3), 289-297, https://doi.org/10.1080/10412905.2012.676799
Altug IG, Bouwmeester HJ and Konig WA, (2004). Isolation and functional expression of cDNAs encoding sesquiterpene synthases, including the enantiomeric (+)- and (-)-Germacrene D Synthases from Solidago canadensis L. Proceedings (poster or lecture-abstract) of the annual fall meeting, German Society for Biochemistry and Molecular Biology (GBM), Munster (Westfalen), Germany, September, 19-22, (www.gbm-online.de).
Amiri H, Al Sadat MHM and Yazdi HL, (2007). Chemical composition of the essential oil of Eremostachys laevigata bung, DARU J Pharm Sci, Vol. 15(1), 34-40.
Asnaashari S, Afshar FH, Ebrahimi A, et al., (2015). In vitro antimalarial activity of different extracts of Eremostachys macrophylla, Montbr. & Auch. Bioimpacts, Vol. 5(3), 135-140.
Asnaashari S, Afshar FH, Ebrahimi A, et al., (2016a). Chemical composition and radical scavenging activity of essential oil and methanolic extract of Eremostachys azerbaijanica Rec. F. from Iran, Res Pharm Sci, Vol. 11(2), 113-119.
Asnaashari S, Afshar FH, Moghadam SB et al., (2016b). Evaluation of in vitro antimalarial activity of different extracts of Eremostachys azerbaijanica Rech.F. Iran J Pharm Res, Vol. 15(3), 523-529.
Asnaashari S, Delazar A, Asgharian P, et al., (2017). In-vitro bioactivity and phytochemical screening of extracts from rhizomes of Eremostachys azerbaijanica rech. F. growing in Iran, Iran J Pharma Res, Vol. 16(1), 306-314.
Asnaashari S, Delazar A and Moghaddam SB, (2018). Iridoid and furanolabdanetype diterpene glycosides from rhizomes of Eremostachys azerbaijanica Rech. f. Res J Pharmacog, Vol. 5(3), 7-13.
Asgharian P, Delazar A, Vatankhah AM, et al., (2017a). In vitro bioactivity and phytochemical evaluation of extracts from aerial parts of Eremostachys macrophylla Montbr. & Auch. growing in Iran. Res J Pharmacog, Vol. 4(2), 65-73.
Asgharian P, Delazar A, Lotfipour F et al., (2017). Bioactive properties of Eremostachys macrophylla Montbr. & Auch. rhizomes growing in Iran. Pharm Sci, Vol. 23(3), 238-243.
Asgharian P, Delazar A and Asnaashari S, (2020). Chemical constituents of Eremostachys macrophylla Montbr. & Auch. aerial parts, Pharma Sci, Vol. 26(2), 203-208.
Azizian D and Moore DM, (1982). Morphological and palynological studies in Phlomis L., Eremostachys bunge and Paraphlomis prain (Labiatae). Bot J Linnean Soc, Vol. 85(4), 225-248.
Bagci E, (2007). Fatty acids and tocochromanol patterns of some Turkish Apiaceae (Umbelliferae) plants; a chemotaxonomic approach. Acta Bot Gallica, Vol. 154(2), 143-151.
Bajalan I, Zand M, Goodarzi M et al., (2017). Antioxidant activity and total phenolic and flavonoid content of the extract and chemical composition of the essential oil of Eremostachys laciniata collected from Zagros. Asian Pac, J Trop Biomed, Vol. 7(2), 144-146.
Behlil F, Khan NS, Akbar A, et al., (2019). Phytochemical screening and antioxidant activity determination of some medicinally important plants of Balochistan, Pak J Bot, Vol. 51(2), 601-608.
Bobaev ID, Bobakulov KM, Ramazanov NS et al., (2015). Iridoid glycosides from Eremostachys baissunensis, Chem Nat Compd, Vol. 51(5), 991-992.
Burt S, (2004). Essential oils: their antibacterial properties and potential applications in foods – a review, Int J Food Microbiol, Vol. 94(3), 223-253.
Cai Y, Jia JW, Crock J, et al., (2002). cDNA clone for β-caryophyllene synthase from Artemisia annua. Phytochem, Vol. 61(5), 523-529.
Calis I, Guvenc A, Armagan M, et al., CH, (2007). Jensen SR. Iridoid Glucosides from Eremostachys moluccelloides Bunge. Helv Chim Acta, Vol. 90(8), 1461-1466.
Calis I, Guevenc A, Armagan M, et al., (2008). Secondary metabolites from Eremostachys laciniata, Nat Prod Commun, Vol. 3(2), 117-124.
Chowdhary HJ and Wadhawa BM, (1984). Flora of Himachal Pradesh, Botanical Survey of India, Howrah, 2.
Delazar A, Sarker SD, Kumarasamy Y, et al., (2004). Three antioxidant phenylethanoid glycosides from the rhizomes of Eremostachys pulvinaris (family: Labiatae), Iranian J Pharma Res, Vol. 3(2), 23-24.
Delazar A, Shoeb M, Kumarasamy Y, et al., (2004a). Two bioactive ferulic acid derivatives from Eremostachys glabra. DARU, Vol. 12(2), 49-53.
Delazar A, Byres M, Gibbons S, et al., (2004b). Iridoid glycosides from Eremostachys glabra. J Nat Prod, Vol. 67(9), 1584-1587.
Delazar A, Gibbons S, Kumarasamy Y, et al., (2005). Antioxidant phenylethanoid glycosides from the rhizomes of Eremostachys glabra (Lamiaceae), Biochem Sys Ecol, Vol. 33(1), 87-90.
Delazar A, Modarresi M, Shoeb M, et al., (2006). Eremostachiin: a new furanolabdane diterpene glycoside from Eremostachys glabra. Nat Prod Res, Vol. 20(2), 167-172.
Delazar A, Modarresi M, Nazemiyeh H, et al., (2008). Furanolabdane diterpene glycosides from Eremostachys laciniata. Nat Prod Commun, Vol. 3(6), 873-876.
Delazar A, Asl H, Mohammadi O, et al., (2009). Evaluation of analgesic activity of Eremostachys laciniata in mice. J Nat Remedies, Vol. 9(1), 1-7.
Delazar A, Sarker SD, Nahar LJ et al., (2013). Rhizomes of Eremostachys laciniata: isolation and structure elucidation of chemical constituents and a clinical trial on inflammatory diseases, Adv Pharma Bulletin, Vol. 3(2), 385-393.
Delazar A, Asharian P and Asnaashari S, (2017). Biological and phytochemical screening of Eremostachys azerbaijanica Rech. F. aerial parts. Jundishapur, J Nat Pharm Prod, Vol. 12(3 (Supp), e64312.
Delazar A and Asnaashari S, (2018). Two iridoid structures from Eremostachys macrophylla Montbr. & Auch. Rhizomes, J Rep Pharma Sci, Vol. 7(2), 221-226.
De Filipps RA and Richardson IBK, (1972). Eremostachys binge. In: Heywood VH, Tutin GT, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA. (eds): Cambridge Univ. Press, Cambridge. Flora Europaea, Vol. 3, 205.
Duthie JF, (1915). Flora of the upper gangetic plain and of the adjacent Siwalik and sub-Himalayan tracks. Reprinted (ed.) Singh B, and Singh MP. Dehradun, India, 1903-29.
Eftekharsadat B, Shakouri SK, Shimia M, et al., (2011). Effect of E. laciniata (L) ointment on mild and moderate carpal tunnel syndrome: a double-blind, randomized clinical trial. Phytother Res, Vol. 25(2), 290-295.
Erdemoglu N, Turan NN, Cakici I, et al., (2006). Antioxidant activities of some Lamiaceae plant extracts. Phytother Res, Vol. 20(1), 9-13.
Esmaeili A, (2012). Biological activities of Eremostachys laevigata Bunge. grown in Iran. Pak J Pharm Sci, Vol. 25(4), 803-808.
Faryabi E, Noori M, Mousavi A, et al. (2021). Chemotaxonomy of Wild Lamiaceae Taxa Based on Their Flavonoids Profiles. J Rangeland Sci, Vol. 11(3), 269-282.
Firuzi O, Javidnia K, Gholami M, et al., (2010). Antioxidant activity and total phenolic content of 24 lamiaceae species growing in Iran, Nat Prod Commun, Vol. 5(2), 261-264.
Fouladnia FM and Modarresi M, (2012). Phenylethanoid glycosides from Eremostachys azerbaijanica Rech. f., Res Pharm Sci, Vol. 7(5), 760-761.
Gella EV, Vavilov VI and Ermolov NG, (1972). Luteolin 7-glucoside from Eremostachys speciosa. Chem Nat Compd, Vol. 8(5), 660.
Gharabagy PM, Zamany P, Delazar A, et al., (2013). Efficacy of Eremostachys laciniata herbal extract on mitigation of pain after hysterectomy surgery. Pakistan J Biol Sci, Vol. 16(17), 891-894.
Hakimi S, Mohammadpour S, Delazar A, et al., (2020). Eremostachys laciniata as effective as rectal diclofenac suppository in cesarean section pain relief: A triple-blind controlled clinical trial. J Endometriosis & Pelvic Pain Disorders, Vol. 12(1), 26-34.
Hariri AS, Shayesteh S, Asgharian P, et al., (2021). Eremostachys Binalodensis, A Potential Therapeutic Choice for Gingival Inflammatory Wounds. J Contemporary Medical Sci, Vol. 7(3), 140-144.
Harley RM, Atkins S, Budantsev AL, et al. (2004). Labiatae. In: Kubitzki K and Kadereit JW, (2004), (eds), The families and genera of vascular plants, Berlin & Heidelberg: Springer.Vol. 7, 167.
Hedge IC, (1986). Labiatae of south-west asia: Diversity, distribution and endemism. Proc R Soc Edinb. Sec B. Biol Sci, Vol. 89, 23-35.
Hedge IC and Ali SI, (1990). Labiatea. In: (eds.) Flora of Pakistan, University of Karachi, Katrachi, Pakistan, Vol. 192, 193.
Imran M, Mehmood R, Mughal UR, et al., (2012). Vicarin, a new isoflavone from Eremostachys vicaryi, J Asian Nat Prod Res, Vol. 14(3), 293-296.
Imran M, Mughal UR, Iqbal M, et al., (2018). Studies of chemical constituents from Eremostachys loasifolia. Pak J Sci Ind Res Ser A Phys Sci, Vol. 61A(3), 126-131.
Jain SK and Shastry ARK (1984). The Indian Plant Red Data Book, I, Botanical Survey of India, p. 90.
Javidnia K, Miri R, Soltani M et al., (2008). Essential oil composition of two species of Eremostachys from Iran (E. adenantha Jaub. et Spach and E. macrophylla Montbr et. Auch), J Essen Oil Res, Vol. 20(3), 226-228.
Jensen SR, Calıs I, Gotfredsen CH et al., (2007). Structural revision of some recently published iridoid glucosides. J Nat Prod, Vol. 70(1), 29-32.
Kalvandi R, Safikhani K, Najafi Gh et al., (2007). Identification of medicinal plants of Hamedan province. Iranian J Med Arom Plants, Vol. 23(3), 350-374.
Khan S, Nisar SM, Simjee SU, et al., (2010). Evaluation of micronutrients level and antinociceptive property of Eremostachys laciniata (L) Bunge. Afr J Biotechnol, Vol. 9(5), 775-777.
Knorring OE (1954). Eremostahcys Bunge and Phlomis L. In: Schischkin BK, (ed), Flora of the USSR (English version), Jerusalem: Keter Publishing House, 21: 3.
Kooiman P, (1972). The occurrence of iridoid glycosides in the Labiatae, Acta Bot Need, Vol. 21(4), 417-427.
Koul AK, Mangotra R and Verma S. (1997). Is Eremostachys superba Royle ex. Benth. Really at the verge of extinction? Curr Sci, Vol. 73, 313-314.
Li HW, Hedge C, Wu ZY, et al. (1994). Flora of China, Beijing and St. Louis: Science Press, 17.
Manafi H and Shafaghat A, (2010). Chemical composition of essential oil of Eremostachys azerbaijanica Rech.f. from Iran, J Essent Oil-Bear Plants, Vol. 13(4), 412-415.
Mughal UR, Fatima I, Malik A et al., (2010). Loasifolin, a new flavonoid from Eremostachys loasifolia. J Asian Nat Prod Res, Vol. 12(4), 328-330.
Mughal UR, Fareed G, Zubair A, et al., (2012). Loasins-A and B, new flavonoids from Eremostachys loasifolia, Nat Prod Res, Vol. 27(20), 1906-1910.
Modaressi M, Delazar A, Nazemiyeh H, et al., (2009). Antibacterial iridoid glucosides from Eremostachys laciniata, Phytother Res, Vol. 23(1), 99-103.
Modarresi M, Foladnia M, Rafiee Z, et al., (2013). Iridoid glycosides from Eremostachys azerbaijanica Rech. F. root, J Med Plants, Vol. 12(46), 66-77.
Mosaddegh M, Naghibi F, Moazzeni H, et al., (2012). Ethnobotanical survey of herbal remedies traditionally used in Kohghiluyeh va Boyer Ahmad province of Iran, J Ethnopharmacol, 141(1), 80-95.
Navaei MN and Mirza M, (2006). Chemical composition of the oil of Eremostachys laciniata (L.) Bunge from Iran, Flavour Fragr J, Vol. 21(4), 645-646.
Nori-Shargh D, Kiaei SM and Deyhimi F, (2007). The volatile constituent’s analysis of Eremostachys macrophylla Montbr. & Auch. From Iran, Nat Prod Res,Vol. 21(8): 733-735.
Nisar M, Khan S, Dar A, et al., (2011). Antidepressant screening and flavonoids isolation from Eremostachys laciniata (L) Bunge, Afr J Biotechnol, Vol. 10(9), 1696-1699.
Pant S and Pant VS, (2011). Status and conservation management strategies for threatened plants of Jammu and Kashmir, J Phytology, Vol. 3(7): 50-56.
Panwar GS, Srivastava SK and Uniyal PL, (2015). In vitro propagation of Eremostachys superba Royle ex Benth. – an endangered, medicinal and ornamental herb of North-West Himalaya, Med Plants – Int J Phytomed Related Industries, Vol. 7(4), 264-271.
Pignatti, S, (1982). Flora d’Italia; Edagricole: Bologna.
Qiu F, Khutsishvili M, Fayvush G, et al., (2019). Phytochemical investigation of Eremostachys moluccelloides Bunge (Lamiaceae), Biochem Syst Ecol, Vol. 84, 17-20.
Rabe SZT, Mahmoudi M, Ahmadsimab H, et al., (2014). Investigation of the biological activity of methanol extract from Eremostachys labiosa Bunge, Food Agr Immunol, Vol. 25(4), 578-585.
Radcliffe-Smith A, (1986). Flora of Pakistan, edited by Nasir E, Ali I, Shamim Printing Press, Karachi, Vol. 192: pp. 1, 224.
Rao RR and Garg A, (1994). Can Eremostachys superba be saved from extinction, Curr Sci, Vol. 67(2): 80-81.
Rechinger KH, (1982). Eremostachys, Phlomis. In: Rechinger KH. (ed.), Flora Iranica, no. Graz: Akademische Druck-u. Verlagsanstalt, Vol. 150, 256.
Rustaiyan A, Masudi S, Ezzatzadeh E, et al., (2011). Composition of the aerial part, flower, leaf and stem oils of Eremostachys macrophylla Montbr. & Auch. and Eremostachys labiosa Bunge From Iran. J Essent Oil Bear Plant, Vol. 14(1), 84-88.
Said O, Khalil K, Fulder S et al. (2002). Ethnopharmacological survey of medicinal herbs in Israel, the golden heights and the West bank region, J Ethnopharmacol, Vol. 83(3), 251-265.
Samandari-Bahraseman MR, Jahanshahi M, Barbariha SA et al., (2018). Altered micro-RNA regulation and neuroprotection activity of Eremostachys labiosiformis in Alzheimer’s disease model, Ann Neurosci, Vol. 25(3), 161-165.
Sharma BM and Kachroo P, (1981). Flora of Jammu and Plants of Neighbourhood, Singh BP, and Singh MP, Dehradun, Vol. 1, 264.
Sharma N, Sharma A and Singh D, (2015). Ethno-medical uses of floristic diversity of sub-tropical forests of Jammu, Jammu and Kashmir, India. Int J Develop Res, 5(3) 3945-3954.
Shishkin BK and Bobrow EG, (1977). Flora of USSR, Izdatel’stvo Akademii Nauk SSSR, Moscow and Leningrad, Vol. 21, 3-41.
Shrivastava A, Sharma YP, Vidyarthi OPS et al., (2017). Conservation status assessment and new population record of the threatened golden himalayan spike Phlomoids superba (Royle ex Benth.) Kamelin & Makhm. From Jammu & Kashmir, India., J Threat Taxa, 9(4), 10089-10095.
Srivastava A, Dey S, Shrivastava SK et al., (2018). Lectotypification of Eremostachys superba (Lamiaceae, Phlomideae), J Jpn Bot, Vol. 93(6), 401-403.
Tareen NM, Rehman SU, Ahmad M, et al., (2016). Ethnomedicinal utilization of wild edible vegetables in district Harnai of Balochistan province – Pakistan, Pak J Bot, Vol. 48(3), 1159-1171.
Ur Rehman H, Saeed R, Iqbal T, et al., (2015). Antibacterial and phytochemical evaluation of the crude extract and fractions of Eremostachys laciniata, Int J Basic Medical Sci Pharma, Vol. 5(1): 20-23.
Vaez H, Arab M, Delazar A et al., (2015). Study of the antioxidant effects of Eremostachys laciniata rhizome extracts in isolated rat hepatocytes, Trends in Pharma Sci, Vol. 1(3), 139-148.
Vahedi H, Lari J, Halimi M et al., (2013). Chemical compositions of Eremostachys labiosiformis growing wild in Iran and antimicrobial activities against phytopathogenic bacteria. Chem Nat Compd, Vol. 49, 958-960.
Ved DK, Kinhal GA, Ravikumar K, et al., (2003). Conservation assessment and management prioritisation for the medicinal plants of Jammu and Kashmir, Himachal Pradesh and Uttaranchal. FRLHT, Bangalore. pp; 53-54.
Verma S, Magotra R and Koul AK, (2003). Restoration of Eremostachys superba Royle ex Benth. – a critically endangered species. Curr Sci, Vol. 84(10), 1307-1308.
Zohary M and Feinbrun-Dothan N, (1978). Flora Palaestina. Jerusalem Acad. Press, 3, 117-118, 190-191.


