1Department of studies in Biotechnology, University of Mysore, Manasagongothri, Mysore, Karnataka, India.
2Department of Biotechnology and Bioinformatics, School of Life Sciences, JSS Academy of Higher Education and Research, Sri Shivarathreeshwara Nagara, Mysuru, Karnataka, India.
3Department of Biotechnology, Sri Dharmasthala Manjunatheshwara College (Autonomous), Ujire, Karnataka, India.
Corresponding author email: shrishanaik@gmail.com
Article Publishing History
Received: 29/01/2022
Accepted After Revision: 29/03/2022
Memecylon umbellatum, M. edule, M. talbotianum, M. malabaricum, and M. wightii species belong to the family Melastomataceae. The genus is well-known traditional medicinal herb used to treat skin diseases. The goal is to compare the metabolite makeup of the five Memecylon spp. and to evaluate the extract’s anti-inflammatory and antiprotease activities. UPLC-ESI-QTOF-MS was used to profile untargeted metabolites in a water/methanol extract, followed by statistical analysis with PCA and HCA. Anti-inflammatory and antiprotease activity were determined by inhibiting soybean 15-lipoxygenase and Protease. Phenols and flavonoids are the most abundant secondary metabolites in Memecylon spp. Principal component analysis (PCA) was used to identify marker chemicals from five species, tocopherol, isorhamnetin 3-glucoside, isothalic acid, stearoylglycerol, and pyrrolidine.
The Memecylon spp. maybe clustered into two groups based on principal component analysis, with M. malabaricum & M. wightii clustered together and M. umbellatum, M. edule, & M. talbotianum forming another clustered. The anti-inflammatory (Soybean 15-lipoxygenase inhibition) and antiprotease activities (Trypsin and thrombin Inhibition) of crude extracts suggested that M. malabaricum and M. talbotianum extracts exhibited higher inhibition compared to the other three species. These data suggest that differences in metabolite profiles might be connected to differences in the bioactivity of the five plant extracts examined. The untargeted UPLC-ESI-QTOF-MS technique is efficient for identifying bioactive components of Memecylon spp.
Memecylon spp, Metabolites PCA, Untargeted.
Bharathi, T.R, Ramu R, Bajpe S.N, Prakash H.S. Comparing Anti-Inflammatory, Antiprotease Activities and Untargeted Metabolite Profiling Based on Ultra-Performance Liquid Chromatography-Mass Spectroscopy of Five Memecylon species from Western Ghats Karnataka, India. Biosc.Biotech.Res.Comm. 2022;15(1).
Bharathi, T.R, Ramu R, Bajpe S.N, Prakash H.S. Comparing Anti-Inflammatory, Antiprotease Activities and Untargeted Metabolite Profiling Based on Ultra-Performance Liquid Chromatography-Mass Spectroscopy of Five Memecylon species from Western Ghats Karnataka, India. Biosc.Biotech.Res.Comm. 2022;15(1). Available from: <a href=”https://bit.ly/35uCJlu“>https://bit.ly/35uCJlu</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Memecylon spp. of the Melastomataceae family are small trees found in Western and Eastern Ghats and are extensively renowned for treating herpes and skin allergies, as well as exhibiting a wide variety of biological activity (Bharathi et al. 2015; Bharathi et al. 2016a; Bharathi et al. 2016b). Phytoconstituents found in Memecylon spp. includes umbelactone, oleanolic acid, sitosterol, α-tocopherol, epigallocatechin gallate, myricetin, and quercetin-7-O-rhamnoside.
The extract of Memecylon spp leaves has antioxidant, anti-diabetic, antibacterial, and anti-inflammatory activities. The biochemical analysis and inductively coupled plasma-mass spectrometry were performed on the fruits of M. grande, M. randerianum, and M. umbellatum, it was discovered that they were rich in phenolic, alkaloids, flavonoids, and terpenoids, as well as various trace metals (Sree et al. 2021). GC-MS study of M. Umbellatum contained α-Tocopherol, Campesterol, Stigmasterol, β-Sitosterol, β-Amyrin which a potent inhibitor of enzymes related to diabetes, steroid metabolism, and cancer (Perumal et al. 2021).
Ursolic acid extracted from M. edule was tested for anti-proliferative action against human leukemic monocyte lymphoma (U-937) and human acute promyelocytic leukaemia (HT-60) cell lines, which showed significant growth suppression and Topoisomerase II inhibition (Srinivasan et al. 2021). New species of such as M. viswanathanii have been identified in Kalakkad- Mundanthurai Tiger Reserve, and M. pachaimalayanum Pachaimalayanum, in the Eastern Ghats of India (Rajesh et al. 2021a; Rajesh et al. 2021b).
Plant products from the same species may have mass spectral properties that are comparable. Metabolite study and multivariate statistical profiling of undiscovered Mass Spectroscopic (MS) features provides fine resolution for differentiating closely similar plant species (Patil et al. 2021). Metabolite findings might be used as crucial supplemental evidence for taxonomic classification of plants with high medicinal potential and close genetic similarities (Xin et al. 2014; Li et al. 2016, Ramasetty et al. 2016). Plant bioactive research is essential in the identification of new medications. Highly advanced chromatographic methods Liquid Chromatography-MS(LC-MS) and Gas Chromatography-MS(GC-MS) are required for metabolite profiling from plant extracts (Antunes et al. 2020; Kiran et al. 2020; Pushpa et al. 2021).
According to the literature review and prior research, Memecylon spp exhibits a wide range of biological activities. Screening of metabolites based on activity from biochemical assays and standard isolation methods results in the identification of just a few biomolecules. As a result, in the current work, a high number of potentially bioactive compounds were identified utilizing MS, untargeted metabolite composition profiling, compound identification, and multivariate statistical analysis of the five Memecylon spp. (M. wightii, M. talbotianum M. umbellatum, M. edule, and M. malabaricum) was performed. Anti-inflammatory using Soybean 15-lipoxygenase (LOX-15) and antiprotease using trypsin and thrombin inhibition was conducted to investigate the potential anti-viral bioactivity of Memecylon spp.
MATERIAL AND METHODS
Five of Memecylon spp (M. umbellatum, M. edule, M. talbotianum, M. malabaricum, and M. wightii)
were gathered from the Western Ghats of India in April-May 2014 and authenticated (voucher specimens #IOELP0001a, #IOELP0002, #IOELP0001h, # IOELP0003, # IOELP0004). Fresh leaves were collected, washed with distilled water, and then immersed in liquid nitrogen. For the extract’s preparation, Memecylon samples that had been frozen and crushed were lyophilized (Telstar, Thermo scientific).
16 mL of Hydro-methanol [80:20 (v/v)] was added to 0.5gm of lyophilized Memecylon samples and kept in a sonicator for 30 minutes at room temperature. In a round bottom flask, the supernatant was collected after centrifugation for 15 minutes at 4000 rpm. A rotary evaporator was used to evaporate the solvent under vacuum at 40°C. 500 ul of Hydro-methanol was added to the dry residue and vortexed to completely dissolve the extract. The extracts were filtered and kept at 20oC using a 0.2 m syringe filter.
For the MS analysis, the LC system was connected to a Waters Acquity Series UPLC/SYNAPT G2 HDMS (Milford, MA, USA) with electrospray ionization for qualitative analysis of the metabolites. At a drying temperature of 350oC, the nebulizer pressure was 60 psi, and the nitrogen flow rate was 10 L/min. A 5ul aliquot of the hydro-methanol extract of leaves was evaluated following the protocol described in previous studies (Stark et al. 2015). The data was analyzed, and the correct mass was determined using MassLynx 4.1 SCN 9.16 (Waters, Manchester, UK).
The pentapeptide leucine enkephaline (m/z-554.2615) was used to lock mass in a solution (1 ng/L) of MeCN/0.1 percent HCO2H (1:1, v/v). Progenesis QI was used to handle the raw data of all samples and replicates acquired from MS analysis. The following peak picking settings were used: all runs, limits automated, sensitivity 3, and retention time limits 0.59 minutes. An ANOVA with a p-value of 0.05 and a fold change of 2 was used to filter data. The matrix was analyzed using PCA and Pareto scaling after the data was transferred to EZinfo. Hierarchical clustering analysis was carried out using neighbor-joining and Pearson’s coefficient matrix (HCA) (Deng et al.
2014; Martucci et al. 2014; Ramu et al. 2016). Soybean 15-lipoxygenase (LOX-15) was used in the anti-inflammatory experiment. Inhibition experiments employing 0.2 M linoleic acid as the substrate and product in a solubilized form in 0.2 M borate buffer (pH 9.0) were used to evaluate the loss of soybean -LOX- 15activity (5g). A UV-Vis spectrophotometer (Beckman Coulter, DU 730 Life Sciences) was used to record inhibition tests in the presence of various doses of extracts (20 to 40 g/ml) and quercetin is used as a reference.
The IC50, or the concentration required to block LOX-15 activity by 50%, was also calculated. (Rackova et al. 2007).For protease inhibition assay, the substrate N-benzoyl-DL-arginine-paranitroanilide hydrochloride (BApNA) was dissolved in DMSO (20 mg/mL). Enzyme Stock solution was prepared by dissolving 2 mg each of trypsin and thrombin was in 10 mL of 1.0 mM HCl. The enzymes (0.3 mL) and 100 µ L of plant extracts in the concentration range of 10 to 50 g/mL were incubated at 37oC for 15 minutes subsequently, 0.6 mM substrate was added and volume was adjusted to 2.5 mL with Tris buffer (100 mM, pH 7.5).
The reaction mixture was incubated at 37°C for 30 minutes. The enzyme reaction was stopped by adding 100 uL of 30% acetic acid. Phenyl methane sulfonyl fluoride (PMSF) was employed as a positive inhibitor. A UV/Vis spectrophotometer was used to detect absorbance at 410 nm (Jedinak et al. 2010; Ramu et al. 2015).
RESULTS AND DISCUSSION
Putative identification of peaks by UPLC-ESI-QTOF-MS: The primary components of Memecylon spp. analyzed in the current study were phenolic acid derivatives, flavonoid derivatives, and hydroxyl derivatives (Table1). The compounds Enoxolone, Stearamide, and Dibutyl phthalate were identified in M. talbotianum, M. umbellatum, and M. edule. Methyl 9,10-Dihydroxystearate, Stearoyl glycerol, isorhamnetin 3-glucoside, Deiten, Myricetin were identified in M. malabaricum and M. wightii.
Table 1. Putative metabolites identified
| Sl.No | Metabolite | MU | ME | MT | MM | MW |
| 1 | (-)-Cholesteryl acetate | 0 | 0 | 1 | 1 | 0 |
| 2 | (-)-Vindoline | 0 | 0 | 0 | 0 | 1 |
| 3 | (−)-Epigallocatechingallate | 1 | 1 | 1 | 1 | 0 |
| 4 | (E)-Diethylstilbestrol | 1 | 0 | 0 | 0 | 0 |
| 5 | 2,3,23-Trihydroxyurs-12-en-28-oic acid | 1 | 1 | 1 | 1 | 0 |
| 6 | 3,6-diacetyl-9-isopropylcarbazole | 1 | 0 | 0 | 0 | 0 |
| 7 | Alpha-tocopherol | 1 | 1 | 1 | 1 | 1 |
| 8 | Baicalin | 1 | 0 | 1 | 1 | 0 |
| 9 | Daphnoretin | 1 | 0 | 0 | 0 | 0 |
| 10 | Deiten | 0 | 0 | 0 | 0 | 1 |
| 11 | D-Glucosaminide | 0 | 0 | 0 | 0 | 1 |
| 12 | Enoxolone | 1 | 1 | 1 | 1 | 0 |
| 13 | Fucoxanthinol | 0 | 0 | 1 | 1 | 0 |
| 14 | Fustin | 0 | 0 | 1 | 1 | 0 |
| 15 | Iso-Quercitrin 6″-acetate | 0 | 0 | 1 | 1 | 0 |
| 16 | Isorhamnetin 3-glucoside | 1 | 1 | 1 | 1 | 1 |
| 17 | Iso-Scopoletin | 0 | 1 | 0 | 0 | 0 |
| 18 | Kaempferol-3-Glucoside-3”-Rhamnoside | 0 | 1 | 0 | 0 | 0 |
| 19 | Kaempferol-3-O-beta-D-galactoside-7-O-alpha-L-rhamnoside | 0 | 1 | 0 | 0 | 0 |
| 20 | Kojic acid | 0 | 0 | 1 | 1 | 0 |
| 21 | Methyl 9,10-Dihydroxystearate | 1 | 1 | 1 | 1 | 1 |
| 22 | Myricetin | 0 | 0 | 0 | 0 | 1 |
| 23 | Myrtillin | 0 | 1 | 0 | 0 | 0 |
| 24 | N-(b-Pyrrolidinoethyl) phenothiazine | 1 | 0 | 0 | 0 | 0 |
| 25 | Nicotianamine | 1 | 0 | 0 | 0 | 0 |
| 26 | Peltatoside | 0 | 1 | 0 | 0 | 0 |
| 27 | P-hydroxyphenylacetamide | 0 | 0 | 1 | 1 | 0 |
| 28 | Procyanidin B1 | 0 | 0 | 1 | 1 | 0 |
| 29 | Pyrrolidine, 1-oleoyl- | 1 | 0 | 0 | 0 | 0 |
| 30 | Pyrrolidine, 1-stearoyl- | 1 | 1 | 1 | 1 | 1 |
| 31 | Quercetin-7-O-rhamnoside | 0 | 1 | 0 | 0 | 0 |
| 32 | R-(-)-Phenylephrine | 0 | 1 | 0 | 0 | 0 |
| 33 | Razoxane | 0 | 0 | 0 | 0 | 1 |
| 34 | Stearamide | 1 | 1 | 1 | 1 | 0 |
| 35 | Stearoylglycerol | 1 | 1 | 1 | 1 | 1 |
| 36 | Syringetin-3-glucoside | 0 | 0 | 1 | 1 | 0 |
| 37 | Trimethylsilyl (9Z)-9-hexadecenoate | 0 | 0 | 1 | 1 | 0 |
umbellatum = MU, M. edule= ME, M. talbotianum=MT, M. malabaricum= MM and M. wightii=MW. 0=Absent,1=Present.
All five Memecylon spp. included marker chemicals such as α-tocopherol, Pyrrolidine, 1-stearoyl-, Stearoylglycerol, and isorhamnetin 3-glucoside (Table 1). The variations in chemicals found from one species to the next may, however, be reflected in the PCA analysis, indicating that all five Memecylon spp. are differentiated from one another. Some of the compounds identified from Memecylon spp. such as Kaempferol-3-Glucoside-3-Rhamnoside, isorhamnetin 3-glucoside, Quercetin-7-O-rhamnoside, tiliroside, Myricetin are also reported by several other workers in several medicinal plants such as Bidens pilosa, Cucumber, Citrullus lanatus (Chiang et al. 2004; Abu-Reidah et al. 2012; Abu-Reidah et al. 2013).
α-tocopherol identified from Memecylon spp. is also identified in other plants such as Cyamopsis tetragonoloba, Moringa oleifera, Stevia rebaudiana, Millingtonia hortensis, and Jasminum sambac (Tomar and Rijhwani 2015). The identified compounds Kaempferol-3-Glucoside-3-Rhamnoside has Anti-inflammatory activity; isorhamnetin 3-glucoside and Quercetin-7-O-rhamnoside has Anti-inflammatory, hepatoprotective, antiviral against influenza virus, Myricetin has Anti-microbial, Anti-diabetes, Cardio-cerebrovascular protection, Anti-inflammatory, Anti-tumor activities (Lee et al. 2019; Nile et al. 2020; Ahn et al. 2020; Hu et al. 2021; Kumar et al. 2021; Song et al. 2021). α-tocopherol, Kojic acid (present in M. talbotianum, M. malabaricum) are well known anti-aging metabolite which further validated Memecylon spp use in folk medicine for skin diseases (Świątek, et al. 2021).
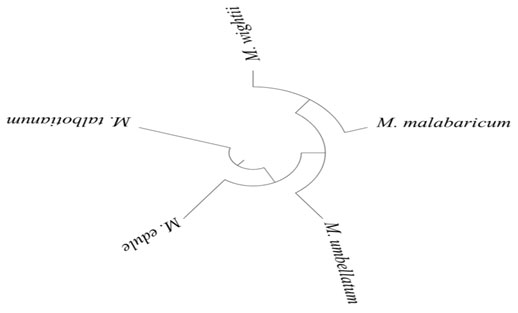
Multivariate PCA analysis: By using an ANOVA with a p-value of 0.05 and a fold change of 2, the number of compounds utilized for PCA was reduced, resulting in the PCA shown in Fig 2. The repeatability of the UPLC-ESI-QTOF-MS profiling approach was confirmed after analysis of each species in three replicates revealed a satisfactory clustering of each duplicate in the vicinity. The PCA result revealed that the greatest differences were found between all five Memecylon spp. along PC1, but that all five Memecylon spp. could be clearly distinguished along PC2. In addition, hierarchical cluster analysis (HCA) (Figure 1) demonstrates that M. umbellatum, M. edule, and M. talbotianum clustered together (Świątek, et al. 2021).
The chemical constituents present in different Memecylon spp. differ significantly when metabolite profiling and multivariate analysis results are compared but grouping in PCA and HCA analysis showed that plants like M. umbellatum, M. talbotianum, and M. edule are grouped together, as are M. malabaricum and M. wightii. The grouping is in accordance with to morphotypes as described by Saldhana (1996) and Gamble (1967) in their floras.
This confirms that variations also occur at metabolic level (Martucci et al. 2014). Similar type of conclusions were drawn while studying, characterizing and comparing the metabolite profiles of the medicinal plants such as Panax ginseng, Lonicera spp., Fritillaria bulbs, genus Vernoni, Garcinia buchananii, Garcinia oblongifolia and genus Panax (Kim et al. 2011; Gao et al. 2012; Xin et al. 2014; Stark et al. 2015; Li et al. 2016; Nguyen et al. 2016; Świątek, et al. 2021).
Figure 1: Memecylon spp. Hierarchical cluster analysis (HCA)
Anti-inflammatory and antiprotease potential of Memecylon spp. extracts: Lipoxygenase inhibitory activity was tested at different doses of extracts ranging from 20 to 40 g/mL. At 40 g/mL, the LOX inhibitory activity of hydro-methanol extracts ranged from 66 to 95%. M. talbotianum (95%) and M. malabaricum (86%) hydro-methanol extracts had the strongest LOX inhibitory efficacy (Table 2).
Quercetin was utilized as a control, with a 95.7 % inhibition in a 40 g/reaction concentration and an IC50 of 20 1.2 g for LOX. The reference standard PMSF had an inhibitory activity of 84.8 % at 50 g/reaction concentration and an IC50 of 97% 1.2g/ml for trypsin and thrombin. Data were expressed as mean ± SD (n=3). Antiprotease activity was found in hydro-methanol extracts of five Memecylon spp. at 50 g/mL, hydro-methanol extract inhibited trypsin and thrombin in the range of 55 and 83% for trypsin and 53 and 80% for thrombin, respectively.
The hydro-methanol extracts of M. malabaricum and M. talbotianum had the greatest antiprotease activity, with trypsin activity of 68% and 83% and thrombin activity of 66% and 80%, respectively (Table 2). M. malabaricum and M. talbotianum hydro-methanol extracts had the strongest anti-inflammatory and antiprotease efficacy compared to other Memecylon spp. extracts. Crude methanol leaf extracts of Memecylon spp. substantially suppressed the LOX and COX in vitro (Bharathi et al. 2014; Aliter and Al-Horani 2021).
Herpes simplex viruses stimulate thrombin synthesis to make cells more susceptible to infection via a process requiring PAR1-mediated cell regulation (Sutherland et al. 2007). Thrombin was also discovered to exacerbate the inflammation caused by the influenza virus. In reality, influenza viruses can cause thrombin production, which can lead to platelet activation-mediated lung inflammation (Keller et al. 2006). Human metapneumovirus and respiratory syncytial virus, two enveloped, negative-sense, single-stranded RNA viruses that cause respiratory infections, have also been connected to thrombin. Thrombin was shown to promote the replication of these viruses and to aggravate the accompanying inflammation in these experiments (Aliter and Al-Horani 2021).
Table 2. Hydro-methanol extracts Inhibition of LOX, trypsin, and thrombin enzyme by five Memecylon spp extracts.
| Plant | M U | ME | MT | MM | MW |
| Lipoxygenase enzyme% | 66 ± 0.9 | 78 ± 08 | 95 ± 0.3 | 86 ± 0.5 | 72 ± 0.6 |
| Trypsin % | 55 ± 0.3 | 63 ± 0.8 | 83 ± 0.4 | 68 ± 0.6 | 62 ± 0.2 |
| Thrombin enzyme% | 53 ± 2.1 | 64 ± 2.2 | 80 ± 2.1 | 66 ± 1.2 | 58 ± 2.1 |
| IC50 values in μg | 30, 2,63, 60.7 | 20.8,71.8, 75.5 | 20,95,
91.5 |
20,77.8,
73.2 |
20, 71,
66.4 |
CONCLUSION
The findings of the present study have shown that the UPLC with ESI-QTOF-MS approach is a viable chromatographic method for denoising and identifying phytochemicals from the genus Memecylon. The use of Waters Progenesis QI software to analyze the data resulted in the discovery of more phytoconstituents in the plant extracts than could have been discovered using the method used. The results obtained using ESI-QTOF-MS, PCA, and HCA show that the discovered molecules may be linked to variations in bioactivity of the plant species. This study validates the ethno-medicinal use of Memecylon spp in the treatment of skin diseases mainly viral infection.
ACKNOWLEDGEMENTS
The mass spectroscopic facility and services were provided by the Institution of excellence, Vijnana Bhanvan, University of Mysore.
Conflict of Interests: Authors declare no conflicts of interests to disclose.
Data Availability Statement: The database generated and /or analysed during the current study are not publicly available due to privacy, but are available from the corresponding author on reasonable request.
REFERENCE
Abu-Reidah, I. M., Arráez-Román, D., and Quirantes-Piné, R., (2012). HPLC–ESI-Q-TOF-MS for a comprehensive characterization of bioactive phenolic compounds in cucumber whole fruit extract. Food Research International, 46, 108-117.
Abu-Reidah, I. M., Arráez-Román, D., and Segura-Carretero, A. (2013). Profiling of phenolic and other polar constituents from hydro-methanolic extract of watermelon (Citrullus lanatus) by means of accurate-mass spectrometry (HPLC–ESI–QTOF–MS). Food Research International, 51, 354-362.
Ahn H. J., You, H. J., Park, M. S., et al. (2020). Microbial biocatalysis of quercetin-3-glucoside and isorhamnetin-3-glucoside in Salicornia herbacea and their contribution to improved anti-inflammatory activity. RSC Advances.;10(9):5339-50.
Aliter K, F and Al-Horani R. A. (2021). Thrombin inhibition by argatroban: potential therapeutic benefits in COVID-19. Cardiovascular Drugs and Therapy. Apr;35(2):195-203.
Antunes, E. R., Duarte, R., S., and Moritz, T., (2020). Differentiation of two Maytenus species and their hybrid via untargeted metabolomics. Industrial Crops and Products. Dec 15;158:113014
Bharathi, T., Madhusudhan, M., and Kumar, P. P. (2015). Antimicrobial potential of Memecylon L. species from Western Ghats against clinical isolates of pathogenic bacteria. Research Journal of Pharmaceutical Biological Chemical Sciences, 6, 1280-1287.
Bharathi, T., Nadafi, R. and Prakash, H. (2014). In vitro antioxidant and anti-inflammatory properties of different solvent extracts of Memecylon talbotianum Brandis. Int J Phytopharm, 4, 148-52.
Bharathi, T., Kumara, K.S. and Prakash, H. (2016a). Memecylon species: A review of traditional information and taxonomic description. Int J Phar Pharm Sci, 8, 1-9.
Bharathi, T. R., Shailasree, S., Sampath Kumara, K., et al. (2016b). Metabolite profiling by UPLC-PDA-ESI/HDMS and antibacterial activity of Memecylon talbotianum Brandis. Pharmacogn Commun b, 6, 1-10.
Chiang, Y. M., Chuang, D. Y., Wang, S. Y., et al. (2004). Metabolite profiling and chemopreventive bioactivity of plant extracts from Bidens pilosa. Journal of Ethnopharmacology, 95, 409-419.
Deng, W., Wang, Y., and Liu, Z., (2014). HemI: a toolkit for illustrating heatmaps. PloS one, 9, e111988.
Gamble, J. (1967). Flora of the Presidency of Madras. Botanical Survey of India. Calcutta, India.
Gao, W., Yang, H., Qi, L. W., et al. (2012). Unbiased metabolite profiling by liquid chromatography–quadrupole time-of-flight mass spectrometry and multivariate data analysis for herbal authentication: Classification of seven Lonicera species flower buds. Journal of Chromatography A, 1245, 109-116.
Hu W. H., Dai, D. K., Zheng, B. Z., et al. (2021). The binding of kaempferol-3-O-rutinoside to vascular endothelial growth factor potentiates anti-inflammatory efficiencies in lipopolysaccharide-treated mouse macrophage RAW264. 7 cells. Phytomedicine. Jan 1;80:153400.
Jedinak, A., Valachova, M., and Maliar, T. (2010). Antiprotease activity of selected Slovak medicinal plants. Die Pharmazie-An International Journal of Pharmaceutical Sciences, 65, 137-140.
Keller, T. T., van der Sluijs K. F., de Kruif M. D., et al. (2006). Effects on coagulation and fibrinolysis induced by influenza in mice with a reduced capacity to generate activated protein C and a deficiency in plasminogen activator inhibitor type 1. Circulation research. Nov 24;99(11):1261-9.
Kim, N., Kim, K., Choi, B. Y., et al. (2011). Metabolomic approach for age discrimination of Panax ginseng using UPLC-Q-Tof MS. Journal of agricultural food chemistry, 59, 10435-10441.
Kiran, K. R, Swathy, P. S, Paul, B, et al. (2021). Untargeted metabolomics and DNA barcoding for discrimination of Phyllanthus species. Journal of Ethnopharmacology. Jun 12;273:113928
Kumar, K. A, Ramu, R, Chandan, S, et al. (2020). Kaempferol–A dietary flavonoid isolated from Blepharis integrifolia. Pharmacognosy Research.; 12 (3).
Li, P., Kumar, H.A.S., Wu, S. B., et al. (2016). Comparative UPLC-QTOF-MS-based metabolomics and bioactivities analyses of Garcinia oblongifolia. Journal of Chromatography B, 1011, 179-195.
Lee, S., Lee, J., Lee, H., et al. (2019). Relative protective activities of quercetin, quercetin‐3‐glucoside, and rutin in alcohol‐induced liver injury. Journal of food biochemistry. Nov; 43 (11): e13002.
Martucci, M. E. P., De Vos, R. C., Carollo, C. A., et al. (2014). Metabolomics as a potential chemotaxonomical tool: application in the genus Vernonia Schreb. PLoS One, 9, e93149.
Nile S. H., Kim D. H., Nile, A., et al. (2020). Probing the effect of quercetin 3-glucoside from Dianthus superbus L against influenza virus infection-In vitro and in silico biochemical and toxicological screening. Food and Chemical Toxicology. Jan 1; 135:110985.
Nguyen, H. T., Lee, D. K., Lee, W. J., et al. (2016). UPLC-QTOFMS based metabolomics followed by stepwise partial least square-discriminant analysis (PLS-DA) explore the possible relation between the variations in secondary metabolites and the phylogenetic divergences of the genus Panax. Journal of Chromatography B, 1012, 61-68.
Patil, S. M, Ramu, R., Shirahatti, P. S., et al.. (2021). A systematic review on ethnopharmacology, phytochemistry and pharmacological aspects of Thymus vulgaris Linn. Heliyon. 2021 May 1;7(5):e07054.
Perumal, G. M., Prabhu, K., Rao, M.R., et al. (2021). The GC-MS Analysis of Ethyl Acetate Extract of One Herbal Plant, Memecylon umbellatum. Natural volatiles & essential oils journal|. Nov 22:6355-63.
Pushpa, V.H., Jayanthi, M.K., Patil, S.M., et al. (2021). Pharmacological profile of Shiva Gutika: an uncharted and versatile polyherbal drug. All Life. Jan 1;14(1):215-219.
Rackova, L., Oblozinsky, M., and Kostalova, D. (2007). Free radical scavenging activity and lipoxygenase inhibition of Mahonia aquifolium extract and isoquinoline alkaloids. Journal of inflammation, 4, 1-7.
Ramasetty, B. T., Bajpe, S. N., Kadappa, S. K. K., et al. (2016). Identification and genetic diversity analysis of Memecylon species using ISSR, RAPD and Gene-Based DNA barcoding tools. Electronic Journal of Biotechnology, 24, 1-8.
Ramu, R., S. Shirahatti P., Zameer, F., et al. (2016). Assessment of in vivo antidiabetic properties of umbelliferone and lupeol constituents of banana (Musa sp. var. Nanjangud Rasa Bale) flower in hyperglycaemic rodent model. PLoS One. Mar 22;11(3):e0151135.
Ramu, R., Shirahatti, P.S., Zameer, F., et al. (2015). Investigation of antihyperglycaemic activity of banana (Musa sp. var. Nanjangud rasa bale) pseudostem in normal and diabetic rats. Journal of the Science of Food and Agriculture. Jan;95(1):165-173.
Rajesh, R., Viswanathan, M., and Silambarasan, R. (2021). Memecylon pachaimalayanum (Melastomataceae)—a new species from the Eastern Ghats of Tamil Nadu in India. Phytotaxa. Apr 9;496(1):69-76.
Rajesh, R., Sakthidhasan P., and Chinnaiyan, R. (2021). Memecylon viswanathanii, a new species of Melastomataceae from Kalakkad-Mundanthurai Tiger Reserve (KMTR), India. Webbia. Apr 7;76(1):71-6.
Saldhana, C. (1996). Flora of Karnataka Vol-I-IV. Oxford and IBH Publishing Co. Pvt. Ltd, New Delhi.
Song, X., Tan, L., Wang, M., et al. (2021). Myricetin: A review of the most recent research. Biomedicine & Pharmacotherapy. Feb 1;134:111017.
Sree, P. R., and Thoppil, J. E. (2021). Comparative Seed Morphology, Pharmacognostic, Phytochemical, and Antioxidant Potential of Memecylon L. Fruits. Turkish Journal of Pharmaceutical Sciences. Apr;18(2):213.
Srinivasan, R., Aruna, A., Lee, J. S., et al. (2020). Antioxidant and antiproliferative potential of bioactive molecules ursolic acid and thujone isolated from Memecylon Edule and Elaeagnus Indica and their inhibitory effect on topoisomerase II by molecular docking approach. BioMed research international. Feb 14;2020.
Sutherland, M. R., Friedman, H. M., and Pryzdial, E. L., (2007). Thrombin enhances herpes simplex virus infection of cells involving protease‐activated receptor 1. Journal of Thrombosis and Haemostasis. May;5(5):1055-61.
Świątek, Ł., Sieniawska, E., Sinan, K. I., et al. (2021). LC-ESI-QTOF-MS/MS Analysis, Cytotoxic, Antiviral, Antioxidant, and Enzyme Inhibitory Properties of Four Extracts of Geranium pyrenaicum Burm. f.: A Good Gift from the Natural Treasure. International journal of molecular sciences. Jan;22 (14):7621.
Stark, T. D., Lösch, S., Wakamatsu, J., et al. (2015). UPLC-ESI-TOF MS-based metabolite profiling of the antioxidative food supplement Garcinia buchananii. Journal of agricultural food chemistry, 63, 7169-7179.
Tomar, K. and Rijhwani, S. (2015). Evaluation of antibacterial activity of phytoconstituents isolated from Jasminum sambac L. and their identification through GC-MS. Int J Eng Technol Manag Appl Sci, 3, 451-9.
Xin, G. Z., Hu, B., Shi, Z. Q., et al. (2014). Rapid identification of plant materials by wooden-tip electrospray ionization mass spectrometry and a strategy to differentiate the bulbs of Fritillaria. Analytica chimica acta, 820, 84-91.


