Department of Biological Science and Engineering, Maulana Azad National Institute of Technology, Bhopal (India)
Corresponding author email: rashiraizada@gmail.com
Article Publishing History
Received: 10/07/2020
Accepted After Revision: 23/09/2020
The Chikungunya virus (CHIKV) cases were ubiquitously reported in several countries of the North American region, but with time this virus has been spread throughout the world. The Indian subcontinent is not an exception. Till date, the absence of any appropriate drugs or vaccines against the CHIKV makes the research scenario more challenging towards the identification and development of novel lead compounds essential for the same. The Cysteine protease (nsp2) has been identified as a key drug target molecule for combating infections induced by alpha-viruses like the CHIKV. CHIKV nsp2 has an extremely compact structure with RNA-binding surface domains, which make nsp2 more efficient for genome replication during pathogenesis. The present study aims to investigate the novel inhibitors for the nsp2 protein domain using in-silico approach. The Tertiary structure of target protein and various antimicrobial drugs were retrieved from protein data bank and drug bank database respectively. The docking studies are performed and it is observed that Telaprevir is having the highest binding affinity followed by Doxycycline, Sennoside A, Acarbose, and Trobicin. Telaprevir is a widely used antiviral drug for the treatment of chronic Hepatitis c virus. Therefore these drugs can be reprofiled as a potential inhibitor of nsp2.
Antiviral Drugs, Chikungunya Virus, Molecular Docking, Nsp2, Drug Reprofiling.
Raizada R, Pandey K. M. Chikungunya Virus: New Drug Prospects Emerging From Molecular Docking Studies for Medicinal Biotechnology. Biosc.Biotech.Res.Comm. 2020;13(3).
Raizada R, Pandey K. M. Chikungunya Virus: New Drug Prospects Emerging From Molecular Docking Studies for Medicinal Biotechnology. Biosc.Biotech.Res.Comm. 2020;13(3). Available from: https://bit.ly/322xp4B
Copyright © Raizada and Pandey This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Chikungunya (CHIKV) is an epidemic arbovirus that is often used to describe both the virus and the disease. The virus is transmitted mainly to humans through the bite of an infected mosquito of the genus Aedes ( Pialoux et al., 1953). The disease generally consists of such a severe infection that cause fever, rashes, and musculoskeletal pain (to walk bent over) is the hallmark of chikungunya that characterizes this dengue-like illness (Staples, Breiman and Powers, 2009; M Dubrulle et al – 2009; Caglioti et al., 2013; Lo Presti et al., 2014).
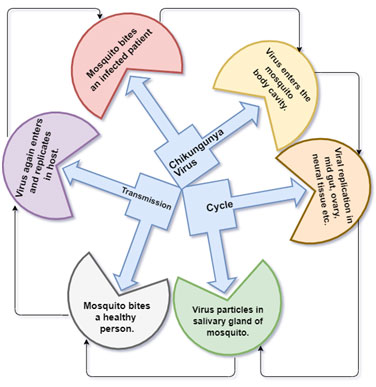
There have been several CHIKV outbreaks that have been contributed to describing chikungunya fever in detail and identified maculopapular rash predominantly on the thorax, facial edema a bullous rash with pronounced sloughing, and localized petechial rash. It intensely, affects main extremities, large and small joints eg: ankles, wrists, phalanges (Lo Presti et al., 2014). CHIKV is been carried by an infected female mosquito to the host the mosquito inoculates virus-containing saliva into the bloodstream of a new victim (Lo Presti et al., 2014) (Fig. 1).
Figure 1: Transmission cycle of CHIKV
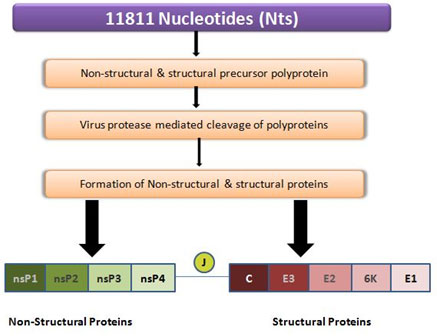
CHIKV is an enveloped, spherical body of about 70nm in diameter. The virion genome consists of a Monopartite, linear single-stranded (ss), positive-sense RNA molecule of approximately 11.8 kb long, where the 5’ end is capped with a 7-methylguanosine while the 3’ end is poly-adenylate. The viral genome contains 2 polyproteins represent four non-structural proteins and five structural proteins (Fig. 2). The replication and propagation of the virus is regulated by nsp2 protein, therefore, it is hypothesized that a compound that inhibits the nsp2 will be a promising and potential drug molecule. In the Era of drug reprofiling efforts can be made to identify a promising inhibitory molecule from the existing antiviral drugs for the treatment of CHIKV the identified potential inhibitors for CHIKV may serve as an inhibitory molecule for other viruses also.which may provide a clear potential path towards the identification of broad-spectrum drugs. (Singh et al., 2011) (Fig. 2).
Figure 2: Schematic description of both structural and nonstructural proteins within the polyprotein CHIKV. CHIKV RNA 11811 bases (top bar, purple color), translates into non-structural and structural precursor polyproteins of 2474 and 1244 residues, respectively, after maturation by protease cleavage, it gives 4 non-structural proteins (left bar, green color) and 5 structural proteins (right bar, red color).
MATERIAL AND METHODS
Retrieval of Target and Lead molecule: The nsp2 crystal structure was retrieved from the protein data bank (PDB) (www.rcsb.org). The retrieval of protein was followed by energy minimization using PYMOL (a user-sponsored molecular visualization system, version 2). The minimization process includes the removal of water molecules, sodium ion, l-peptide linking, and the gaps between amino acids. The lead compound for nsp2 protease was retrieved from PubChem and Drug bank database (Table1). The small molecules were optimized with AVOGADRO: open-source molecular builder and visualization tool( version 1). The optimization process was done with the false parameters that is the force field is off, steps per update is 4, the algorithm is the steepest descent.
Table 1. List of ligands involved in protein‐ligand interaction.
| S.No. | Drugs | REFERENCE |
| 1. | (R)‐Chloroquine | Andersag H et al., 1941 |
| 2. | Acarbose | S. P. Clissold et al 1988 |
| 3. | Acetaminophen | Kis B et al., 2005 |
| 4. | Amikacin Sulfate | Overington JP et al., 2006. |
| 5. | Aspirin | Sneader W ., 2000 |
| 6. | Arbidol | Hui Peng et al., 2020 |
| 7. | Baicalein (Natural Compound) | Oliveira et al., 2017 |
| 8. | Bisdesethylchloroquine | Ajayi FO et al., 1989 |
| 9. | Boceprevir | Jennifer J Kiser et al, 2013 |
| 10. | Boswellic acid | Arne Henkel et al, 2012 |
| 11. | Cefadroxil (Sumacef) | Leonardo Marsili., 1978 |
| 12. | Celecoxib | Yi Yu Ke et al., 2020 |
| 13. | Chloroquine | Vincent MJ et al., 2005 |
| 14. | Cletoquine | Dongre VG et al., 2009 |
| 15. | Curcumin | Fatemeh Zahedipour et al, 2020 |
| 16. | Desethylchloroquine | Frisk-Holmberg M et al., 1984 |
| 17. | Didesethylchloroquine Hydroxyacetamide | Abraham MJ et al., 2015 |
| 18. | Dihydrostreptomycin Sulfate | CURCI G ., 1951 |
| 19. | Diminazene Aceturate | R. Ghildiyal et al., 2019 |
| 20. | Docosanol | Hardman et al 2001 |
| 21. | Doxycycline | Dahl EL et al 2006 |
| 22. | E-64 (Zinc13493525) | Zheming Wang et al. 2008 |
| 23. | Etidronate (Etidronic Acid) | Rogovin et al 1968 |
| 24. | Fisetin (Natural Compound) | Liu L et al 2019. |
| 25. | Glucosamine Sulphate | Arvind Chopra et al, 2013 |
| 26. | Hesperetin | Samie A et al., 2018 |
| 27. | Hydroxychloroquine | Lim HS et al. 2009 |
| 28. | Ibandronate Sodium | Epstein S et al. 2005 |
| 29. | Ibuprofen | Casper D et al., 2000 |
| 30. | Imatinib | Deininger MW et al2003 |
| 31. | Iron Sucrose | Hörl WH 2007. |
| 32. | Kanamycin Sulfate | Vetting MW et al. 2002. |
| 33. | Ketotifen | Roy W. Bryant et al. 2011 |
| 34. | Leupeptin Hemisulfate | Pérez-Pérez et al 2019 |
| 35. | Mitoxantrone Hydrochloride (Novantrone) | Fox EJ 2006. |
| 36. | N‐Acetyl (Mono) Desthylchloroquine | E. E. Essien et al1989 |
| 37. | Naproxen | Wongrakpanich S et al., 2018 |
| 38. | Nelfinavir | Kaldor SW et al 1997 |
| 39. | Niacin | Briggs gg, et al.,1998 |
| 40. | Officinalis acid | Mohammed Bourhia et al., 2019 |
| 41. | Pemetrexed Disodium Hemipentahydrate | Prateek Kumar et al. |
| 42. | Pirodavir | Jef Peeters et al. 2007 |
| 43. | Pleconaril | Florea NR et al 2003 |
| 44. | Prednisolone | Maryam Daneshpazhooh ., 2020 |
| 45. | Quercetagetin (Natural Compound) | Weiyou Wang et al 2016, |
| 46. | Ribavirin | Sidwell RW et al. 2005 |
| 47. | Ribostamycin Sulfate | Zhou et al.1992 |
| 48. | Sennoside A | Esposito F et al 2016 |
| 49. | Sofosbuvir | Asselah T 2013 |
| 50. | Spectinomycin Hydrochloride Hydrate (Trobicin) | David R. White 1966 |
| 51. | Telaprevir | Kim JJ et al. 2012 |
| 52. | Zinc Acetate | Berni Canani R et al 2011 |
Molecular docking studies: Before performing molecular docking studies, we need to identify the binding pockets of the protein molecule. The Automated active site docking and scoring (AADS) is used in this analysis to identify binding pockets. The AADS (http://www.scfbio-iitd.res.in/dock/ActiveSite_new.jsp) utilizes the 3D structure of target molecules and identify top 10 possible binding sites with 100% precision in identifying the real (active) binding sites (Table 4). Once the protein binding pockets are identified, the Small Molecules Library (Table 1) is screened against these sites to identify the hit molecules using the software. For this study, PyRx and AutoDockVina software were used to analyze the ligand-protein binding properties to the protein.
The blind dockings were performed in which the grid boxes’ size was adjusted to cover the binding site. Once the docking is complete the resulting PDBQT output file was opened in the PyMOL software for converting all protein conformations into one file analysis on further studies. Afterward, each conformation was examined using Discovery Studio 2.5 software, using information like binding affinities, interaction energies, van der Waals energies, electrostatic energies, hydrogen bonding,pi-pi interactions, pi-cation interactions and close contacting residues were obtained and recorded. The compounds were screened against nsp2 using the PyRx tool to identify the ligands with the best conformers to the target protein.
Table 2. Parameters used for molecular docking of top ten ligands with the protein of interest. All grid boxes with a spacing size of 1.000 A˚ have sufficient sizes to cover the entire protein structures during molecular docking.
| S.No. | Ligands with Protein | Center-X | Center-Y | Center-Z | Size-X | Size-Y | Size-Z |
| 1. | 3trk_Acarbose | 12.8155666274 | 26.2634859746 | 21.5992382951 | 82.0109838983 | 84.344657921 | 61.1584777994 |
| 2. | 3trk_Baicalin | 11.697598268 | 23.47479489 | 28.5074738773 | 67.5700208963 | 85.8025203587 | 98.2122134629 |
| 3. | 3trk_Doxycycline | 11.37419874 | 23.0596327058 | 21.6856001381 | 71.6738245391 | 86.7873081266 | 53.1784838552 |
| 4. | 3trk_Fisetin | 28.9252280574 | 24.6848042594 | 19.2251198554 | 115.892612756 | 88.4610580017 | 86.8902924293 |
| 5. | 3trk_Ibandronate sodium | 28.9252280574 | 24.6848042594 | 19.2251198554 | 115.892612756 | 88.4610580017 | 86.8902924293 |
| 6. | 3trk_Mitoxantrone hydrochloride | 28.6614606151 | 20.2939940445 | 19.342323373 | 104.001803984 | 95.7958489908 | 74.5569102783 |
| 7. | 3trk_Quercetagetin | 12.2522153681 | 25.5314252099 | 22.5563495924 | 70.093582201 | 93.8091943285 | 81.4775121373 |
| 8. | 3trk_Sennoside A | 12.2522153681 | 25.5314252099 | 22.5563495924 | 70.093582201 | 93.8091943285 | 81.4775121373 |
| 9. | 3trk_spectinomycinhydrochloride | 16.1736849469 | 22.7882300823 | 17.3275975767 | 88.0971554836 | 88.3232612193 | 73.2683286745 |
| 10. | 3trk_Telaprevir | 12.2522153681 | 25.5314252099 | 22.5563495924 | 70.093582201 | 93.8091943285 | 81.4775121373 |
RESULTS AND DISCUSSION
During Retrieval of the target molecule, the nsP2 with the PDB ID – 3TRK was retrieved and 52 lead compounds were listed (Table1) these lead compounds were screened for potential inhibitory activities against the top 10 binding sites (Table 4) CHIKV’s non-structural protein nsp2. The docking studies for the top 10 binding sites of nsp2 (Table3) the docking studies of all 52 lead compounds with 10 binding sites. The study suggests out of 52 lead compounds the four compound Telaprevir, Doxycycline, Acarbose, Sennoside A showed significant binding affinity whereas spectinomycin hydrochloride (trobicin), Baicalin, Ibandronate sodium, Quercetagetin, Mitoxantrone hydrochloride, and Fisetin showed promising binding affinity ( Table.4 and5.). Telaprevir showed the strongest binding affinity (-12.3kcal/mol), is a member of protease blockers (a group of antiviral medicine). These affinities and energies are due to interaction and bond formation between lead molecules and binding site amino acid of nsp2.
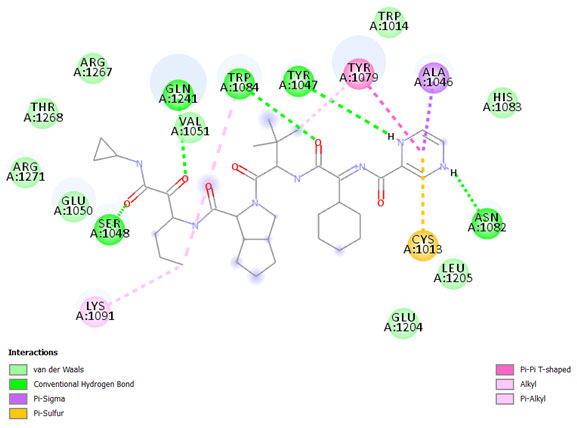
The result shows the amino acid residue found in the binding pocket between Telaprevir and nsP2, are SER1048, GLN1241, TRP1084, TYR1047, ASN1082, TYR1079, ALA1046, CYS1013, LYS1091, GLU1048, VAL1051, ARG1271, THR1268, ARG1267, TRP1014, HIS1083, LEU1205, and GLU1204 a Fig. 3. The hydrogen bonds between Telaprevir, Doxycycline, Acarbose, Sennoside A, spectinomycin hydrochloride (trobicin), Baicalin, Ibandronate sodium, Quercetagetin, Mitoxantrone hydrochloride, Fisetin, and 3TRK are also as shown in (Table 4). between Telaprevir and nsP2, are SER1048, GLN1241, TRP1084, TYR1047, ASN1082, TYR1079, ALA1046, CYS1013, LYS1091, GLU1048, VAL1051, ARG1271, THR1268, ARG1267, TRP1014, HIS1083, LEU1205, and GLU1204 a Fig. 3. The hydrogen bonds between Telaprevir, Doxycycline, Acarbose, Sennoside A, spectinomycin hydrochloride (trobicin), Baicalin, Ibandronate sodium, Quercetagetin, Mitoxantrone hydrochloride, Fisetin, and 3TRK are also as shown in (Table 4).
Table 3. Cavity details of Nsp2 Protein
| S.No. | Cavity Points | V | A | D | R | h | |||
| 1. | 124.023 | 55.309 | 63.320 | 0.94 | 0.39 | 0.42 | 1.00 | 0.68 | 0.6857 |
| 2. | 100.718 | 64.861 | 70.832 | 0.94 | 0.56 | 0.50 | 0.75 | 0.63 | 0.6755 |
| 3. | 109.046 | 35.144 | 85.035 | 1.00 | 0.17 | 0.77 | 0.50 | 0.80 | 0.6465 |
| 4. | 86.588 | 66.635 | 79.871 | 0.71 | 0.39 | 0.46 | 0.62 | 1.00 | 0.6371 |
| 5. | 92.209 | 49.198 | 90.901 | 0.78 | 1.00 | 0.62 | 0.25 | 0.41 | 0.6107 |
| 6. | -16.257 | -22.551 | -4.530 | 0.98 | 1.00 | 1.00 | 0.83 | 0.35 | 0.8326 |
| 7. | -23.802 | -30.007 | 2.100 | 1.00 | 0.22 | 0.57 | 1.00 | 0.61 | 0.6804 |
| 8. | -7.096 | -43.641 | -3.465 | 0.98 | 0.72 | 0.53 | 0.67 | 0.40 | 0.6595 |
| 9. | -5.740 | -45.699 | -23.319 | 0.62 | 0.72 | 0.77 | 0.50 | 0.48 | 0.6173 |
| 10. | -24.954 | -46.789 | 4.600 | 0.30 | 0.67 | 0.47 | 1.00 | 0.53 | 0.5934 |
Table 4. Hydrogen Bonding Between the top hit compounds from the blind docking and CHIKV Nsp2. This table documents the Residues involved in the Discovery Studio 2.5. The binding affinities as ranked by the PyRx 8.0 and Auto Dockvinal 1.5.6 are recorded in the final column of the table.
| Ligands with Protein | Hydrogen bonds | AngleDHA(○) | Distance(A˚) | Binding affinity (kcal/mol) |
| 3trk andTelaprevir | :UNL1:HN – A:ASN1082:O
:UNL1:HN – :UNL1:O :UNL1: HN – A: TYR1047:O |
119.232
133.404 151.652 |
2.43416
2.79452 2.0751 |
-12.3 |
| 3trk and doxycycline | A: TYR1047: HN – :UNK0: O
A: TRP1084: HE1 – :UNK0: O |
146.817
155.005 |
2.86321
1.69013 |
-11.8 |
| 3trk andAcarbose | A: TYR1047: HN – :UNK0: O
A: SER1048: HG – :UNK0: O A: TRP1084: HE1 – :UNK0: O |
148.924
107.507 147.087 |
2.3149
2.42619 2.46832 |
-10.9 |
| 3trk andSennoside A | A: TYR1079: HH – :UNK0: O
A: TRP1084:HE1 – :UNK0: O A: GLN1241: HE22 – :UNK0: O |
152.837
135.362 108.266 |
1.82834
2.37549 2.53045 |
-10.9 |
| 3trk and spectinomycin hydrochloride(trobicin) | A: TRP1084: HE1 – :UNK0: O
A: TRP1084: HE1 – :UNK0: O :UNK0: H – A:TYR1079: OH |
160.856
142.698 94.399 |
2.14139
2.27101 2.72027 |
-8.9 |
| 3trk and baicalin | A: TRP1084: HE1 – :UNK0: O
A: GLN1241: HE22 – :UNK0: O :UNK0: H – A:TYR1079: OH :UNK0: H – A:ASN1082: OD1 |
150.648
99.059 138.871 150.896 |
2.03652
2.87101 2.70173 2.76228 |
-8.1 |
| 3trk and Ibandronate sodium | A: TYR1047: HN – :UNK0: O
A: TRP1084: HE1 – :UNK0: O :UNK0: H – A:TYR1079: OH : UNK0: H – A: TYR1047: O : UNK0: H – A: TYR1047: O |
162.318
132.257 102.006 137.74 147.153 |
2.22615
2.66686 2.77491 2.21746 2.12432 |
-8 |
| 3trk andQuercetagetin | A: TYR1047: HN – :UNK0: O
A: TYR1047: HN – :UNK0: O A: TRP1084: HE1 – :UNK0: O |
149.998
165.015 135.434 |
2.84355
2.2247 2.27803 |
-7.9 |
| 3trk and Mitoxantrone hydrochloride | A: TYR1047: HN – :UNK0: O
A: TRP1084: HE1 – :UNK0: O |
157.204
173.755 |
2.30123
1.82032 |
-7.8 |
| 3trk andFisetin | A: SER1048: HG – :UNK0: O
: UNK0: H – A: TYR1047: O :UNK0: H – A:ASP1246: OD2 |
154.341
140.152 150.743 |
2.30796
2.06479 2.84791 |
-7.7 |
Figure 3: 2D diagram of the interaction between telaprevir and nsP2. The diagram shows the ligand-receptor interactions and close amino acid residues found in the binding pocket.
The result of computational studies recommends that Telaprevir, Doxycycline, Acarbose, Sennoside A can be used as nsp2 inhibitors for chikungunya. These lead compounds already exist and were listed in antiviral medicines especially protease blocker so no harm in exploring these drugs for CHIKV inhibition. This significant outcome is for country path in drug reprofiling studies and here we are proposing molecular docking as a tool for exploring new drug prospects from old drugs.
Table 5. Analysis of ligand-receptor interactions
| S.No. | Ligand | Binding Affinity | Rmsd/Ub | Rmsd/Lb |
| 1. | 3trk_Telaprevir | -12.3 | 2.456 | 1.087 |
| 2. | 3trk_Doxycycline | -11.8 | 5.49 | 1.561 |
| 3. | 3trk_Acarbose | -10.9 | 5.22 | 2.49 |
| 4. | 3trk_Sennoside A | -10.9 | 8.368 | 0.016 |
| 5. | 3trk_Spectinomycin hydrochloride (Trobicin) | -8.9 | 4.485 | 1.768 |
| 6. | 3trk_Baicalin | -8.1 | 8.143 | 5.091 |
| 7. | 3trk_Ibandronate sodium | -8 | 10.415 | 9.23 |
| 8. | 3trk_Quercetagetin | -7.9 | 31.104 | 30.446 |
| 9. | 3trk_Mitoxantrone hydrochloride | -7.8 | 5.817 | 0.058 |
| 10. | 3trk_Fisetin | -7.7 | 6.404 | 2.937 |
| 11. | 3trk_Imatinib | -7.7 | 21.294 | 19.022 |
| 12. | 3trk_Proteinase inhibitor E64 | -7.7 | 12.243 | 10.903 |
| 13. | 3trk_N acetyl Desethylchloroquine | -7.6 | 13.291 | 11.618 |
| 14. | 3trk_Nelfinavir | -7.5 | 28.101 | 24.91 |
| 15. | 3trk_Beta-Boswellic acid | -7.5 | 26.375 | 23.199 |
| 16. | 3trk_Etidronic acid | -7.4 | 2.29 | 0.784 |
| 17. | 3trk_Celecoxib | -7.4 | 5.297 | 3.179 |
| 18. | 3trk_Officinalic acid | -7.4 | 13.228 | 9.67 |
| 19. | 3trk_Pleconaril | -7.2 | 19.127 | 14.524 |
| 20. | 3trk_Hesperetin | -7.1 | 8.237 | 2.364 |
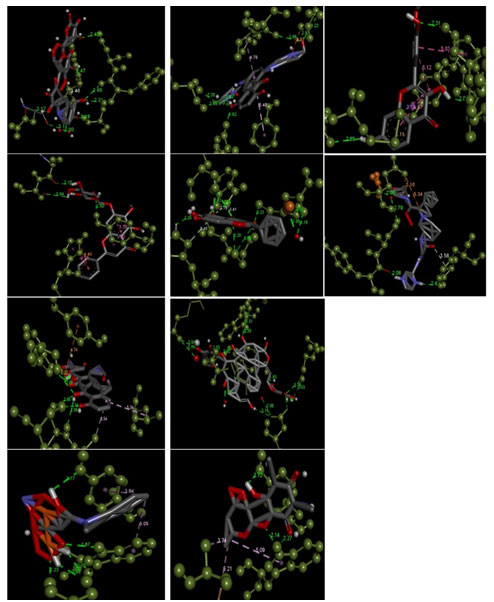
Figure 4: The receptor-ligand interactions, and bonds between them with the highest binding affinities of Acarbose, Baicalin. Doxycycline, Fisetin, Ibandronate sodium, Mitoxantrone hydrochloride, Quercetagetin, Sennoside A, spectinomycin hydrochloride, and Telaprevir (Grey, Red, and Blue stick structure) when docked against Nsp2 protein (dark green colored ball and stick structure).
- 3trk_Telaprevir with the binding affinity of -12.3 kcal/mol
- 3trk_Doxycycline with the binding affinity of -11.8 kcal/mol
- 3trk_Acarbose with the binding affinity of -10.9 kcal/mol
- 3trk_Sennoside A with the binding affinity of -10.9 kcal/mol
- 3trk_Spectinomycin hydrochloride with the binding affinity of -8.9 kcal/mol
- 3trk_Baicalin with the binding affinity of -8.1 kcal/mol
- 3trk_Ibandronate sodium with the binding affinity of -8 kcal/mol
- 3trk_Quercetagetin with the binding affinity of -7.9 kcal/mol
- 3trk_Mitoxantrone hydrochloride with the binding affinity of -7.8 kcal/mol
- 3trk_Fisetin with the binding affinity of -7.7 kcal/mol.
CONCLUSION
In our current study, we conclude briefly that Telaprevir, Doxycycline, Acarbose, Sennoside A possesses interactions with CHIKV non-structural protein to (NSP2) which plays a role in the virus replication cycle. These findings enhance our understandings of the possibility of an existing antimicrobial drug molecule to be used for treatment against chikungunya fever. The repurposing of these old drugs to treat chikungunya will become an attractive proposition because it involves the use of no risk compounds with considerably lower development cost and minimal discovery timeline hence further studies on this target protein and ligands will enhance the development of a novel anti-CHIKV drug.
ACKNOWLEDGEMENTS
The authors are thankful to the Department of Biological Science and Engineering, Maulana Azad National Institute of Technology, Bhopal (India).
Conflict of Interests: We, the authors of the submitted manuscript declare that the work and data present in the manuscript entitled – Chikungunya virus: new drug prospects emerging from molecular docking studies for medicinal biotechnology is genuine research carried out by us. The work finally belongs to the institute. We have not misused the data previously published and have not manipulated the original work.
REFERENCES
Agarwal, T., Asthana, S., and Bissoyi, A. (2015). Molecular Modeling and Docking Study to Elucidate Novel Chikungunya Virus nsP2 Protease Inhibitors. Indian journal of pharmaceutical sciences, 77(4), 453–460. https://doi.org/10.4103/0250-474x.164769
Bora L (2012) Homology Modeling and Docking to Potential Novel Inhibitor for Chikungunya (37997) Protein nsP2 Protease. J Proteomics Bioinform 5: 054-059. doi:10.4172/jpb.1000213
Choi, H. K., Tong, L., Minor, W., Dumas, P., Boege, U., Rossmann, M. G., and Wengler, G. (1991). Structure of Sindbis virus core protein reveals a chymotrypsin-like serine proteinase and the organization of the virion. Nature, 354(6348), 37–43. https://doi.org/10.1038/354037a0
Clissold, S. P., and Edwards, C. (1988). Acarbose. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs, 35(3), 214–243. https://doi.org/10.2165/00003495-198835030-00003
CURCI G. (1951). La idrossistreptomicina [Dihydrostreptomycin]. Archivio di tisiologia e delle malattie dell’apparato respiratorio, 6(3), 104-6.
Delogu, I., Pastorino, B., Baronti, C., Nougairède, A., Bonnet, E., and de Lamballerie, X. (2011). In vitro antiviral activity of arbidol against Chikungunya virus and characteristics of a selected resistant mutant. Antiviral Research, 90(3), 99–107. https://doi.org/10.1016/j.antiviral.2011.03.182
Dongre, V. G., Ghugare, P. D., Karmuse, P., Singh, D., Jadhav, A., and Kumar, A. (2009). Identification and characterization of process-related impurities in chloroquine and hydroxychloroquine by LC/IT/MS, LC/TOF/MS, and NMR. Journal of pharmaceutical and biomedical analysis, 49(4), 873–879. https://doi.org/10.1016/j.jpba.2009.01.013
Dubrulle, M., Mousson, L., Moutailler, S., Vazeille, M., and Failloux, A. B. (2009). Chikungunya virus and Aedes mosquitoes: saliva is infectious as soon as two days after oral infection. PloS one, 4(6), e5895. https://doi.org/10.1371/journal.pone.0005895.
Esposito, F., Carli, I., Del Vecchio, C., Xu, L., Corona, A., Grandi, N., Piano, D., Maccioni, E., Distinto, S., Parolin, C., and Tramontano, E. (2016). Sennoside A, derived from the traditional Chinese medicine plant Rheum L., is a new dual HIV-1 inhibitor effective on HIV-1 replication. Phytomedicine: international journal of phytotherapy and phytopharmacology, 23(12), 1383–1391. https://doi.org/10.1016/j.phymed.2016.08.001
Felix, R. A., 2nd, Kadner, A., and Berrebi, A. S. (2012). Effects of ketamine on response properties of neurons in the superior para olivary nucleus of the mouse. Neuroscience, 201, 307–319. https://doi.org/10.1016/j.neuroscience.2011.11.027
Hahn, C. S., and Strauss, J. H. (1990). Site-directed mutagenesis of the proposed catalytic amino acids of the Sindbis virus capsid protein auto protease. Journal of virology, 64(6), 3069–3073.
Hörl W. H. (2007). Clinical aspects of iron used in the anemia of kidney disease. Journal of the American Society of Nephrology: JASN, 18(2), 382–393. https://doi.org/10.1681/ASN.2006080856
Kaur, P., Thiruchelvan, M., Lee, R. C., Chen, H., Chen, K. C., Ng, M. L., and Chu, J. J. (2013). Inhibition of chikungunya virus replication by harringtonine, a novel antiviral that suppresses viral protein expression. Antimicrobial agents and chemotherapy, 57(1), 155–167. https://doi.org/10.1128/AAC.01467-12
Kawatkar, S., Wang, H., Czerminski, R., and Joseph-McCarthy, D. (2009). Virtual fragment screening: an exploration of various docking and scoring protocols for fragments using Glide. Journal of computer-aided molecular design, 23(8), 527–539. https://doi.org/10.1007/s10822-009-9281-4
Lani, R., Hassandarvish, P., Chiam, C. W., Moghaddam, E., Chu, J. J., Rausalu, K., Merits, A., Higgs, S., Vanlandingham, D., Abu Bakar, S., and Zandi, K. (2015). Antiviral activity of silymarin against the chikungunya virus. Scientific reports, 5, 11421. https://doi.org/10.1038/srep11421
Lee, N., Wong, C. K., Lam, W. Y., Wong, A., Lim, W., Lam, C. W., Cockram, C. S., Sung, J. J., Chan, P. K., and Tang, J. W. (2006). Chikungunya fever, Hong Kong. Emerging infectious diseases, 12(11), 1790–1792. https://doi.org/10.3201/eid1211.060574
Lopresti, A. L., Maes, M., Maker, G. L., Hood, S. D., and Drummond, P. D. (2014). Curcumin for the treatment of major depression: a randomized, double-blind, placebo-controlled study. Journal of affective disorders, 167, 368–375. https://doi.org/10.1016/j.jad.2014.06.001
National Center for Biotechnology Information (2020). PubChem Database. Etidronic acid, CID=3305, https://pubchem.ncbi.nlm.nih.gov/compound/Etidronic-acid (accessed on January 9, 2020)
Nguyen, P. T., Yu, H., and Keller, P. A. (2014). Discovery of in silico hits targeting the nsP3 macro domain of the chikungunya virus. Journal of molecular modeling, 20(5), 2216. https://doi.org/10.1007/s00894-014-2216-6
Overington, J. P., Al-Lazikani, B., and Hopkins, A. L. (2006). How many drug targets are there?. Nature reviews. Drug discovery, 5(12), 993–996. https://doi.org/10.1038/nrd2199
Perera, R., Owen, K. E., Tellinghuisen, T. L., Gorbalenya, A. E., and Kuhn, R. J. (2001). Alphavirus nucleocapsid protein contains a putative coiled-coil alpha-helix important for core assembly. Journal of Virology, 75(1), 1–10. https://doi.org/10.1128/JVI.75.1.1-10.2001
Pialoux, G., Gaüzère, B. A., Jauréguiberry, S., and Strobel, M. (2007). Chikungunya, an epidemic arbovirosis. The Lancet. Infectious diseases, 7(5), 319–327. https://doi.org/10.1016/S1473-3099(07)70107-X
Schuster, R. K., Wibbelt, G., and Kinne, J. (2014). On the life cycle and morphology of development stages of Paraspiralatus sakeri Gibbons et al., 2004 (Nematoda: Spiroidea, Spirocercidae), a heterogenic stomach parasite of falcons. Parasitology Research, 113(6), 2047–2051. https://doi.org/10.1007/s00436-014-3852-6
Singh KhD, Kirubakaran P, and Nagarajan S (2012) Homology modeling, molecular dynamics, e-pharmacophore mapping, and docking study of Chikungunya virus nsP2 protease. J Mol Model. 2012; 18(1):39-51. DOI: 10.1007/s00894-011-1018-3
Soni, A., Pandey, K. M., Ray, P., and Jayaram, B. (2013). Genomes to hits in silico – a country path today, a highway tomorrow: a case study of chikungunya. Current pharmaceutical design, 19(26), 4687–4700. https://doi.org/10.2174/13816128113199990379
Staples, J. E., Breiman, R. F., and Powers, A. M. (2009). Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America, 49(6), 942–948. https://doi.org/10.1086/605496
Sun, L., Wu, J., Du, F., Chen, X., and Chen, Z. J. (2013). Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science (New York, N.Y.), 339(6121), 786–791. https://doi.org/10.1126/science.1232458
Taubitz, W., Cramer, J. P., Kapaun, A., Pfeffer, M., Drosten, C., Dobler, G., Burchard, G. D., and Löscher, T. (2007). Chikungunya fever in travelers: clinical presentation and course. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America, 45(1), e1–e4. https://doi.org/10.1086/518701
Vetting, M. W., Hegde, S. S., Javid-Majd, F., Blanchard, J. S., and Roderick, S. L. (2002). Aminoglycoside 2′-n-acetyltransferase from mycobacterium tuberculosis in complex with coenzyme a and aminoglycoside substrates. Nature structural biology, 9(9), 653-658. https://doi.org/10.1038/nsb830
Vincent, M. J., Bergeron, E., Benjannet, S., Erickson, B. R., Rollin, P. E., Ksiazek, T. G., Seidah, N. G., and Nichol, S. T. (2005). Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virology journal, 2, 69. https://doi.org/10.1186/1743-422X-2-69
Wang, Z., Gu, C., and Colby, T. (2008). β-Lactone probes identify a papain-like peptide ligase in Arabidopsis thaliana. Nat Chem Biol 4, 557–563 https://doi.org/10.1038/nchembio.104
Wu, D., Wu, J., Zhang, Q., Zhong, H., Ke, C., Deng, X., Guan, D., Li, H., Zhang, Y., Zhou, H., He, J., Li, L., and Yang, X. (2012). Chikungunya outbreak in Guangdong Province, China, 2010. Emerging infectious diseases, 18(3), 493–495. https://doi.org/10.3201/eid1803.110034
Zhang, W., Fisher, B. R., Olson, N. H., Strauss, J. H., Kuhn, R. J., and Baker, T. S. (2002). Aura virus structure suggests that the T=4 organization is a fundamental property of viral structural proteins. Journal of virology, 76(14), 7239–7246. https://doi.org/10.1128/jvi.76.14.7239-7246.2002
Zhou, Q. S., Zhao, Y. M., Bai, X., Li, P. X., and Ruan, C. G. (1992). Effect of new-breviscapine on fibrinolysis and anticoagulation of human vascular endothelial cells. Acta pharmacologica Sinica, 13(3), 239–242.