Department of Microbiology and Biotechnology, University School of
Sciences, Gujarat University, Ahmadabad, Gujarat, India.
Corresponding author email: charanniky@gmail.com
Article Publishing History
Received: 15/07/2023
Accepted After Revision: 25/09/2023
Cumin is the current leading currency earning crop in India, as per Agriwatch production estimate, Jeera production for 2021-22 marketing period is estimated at 391,291 MT, generating 8,600 crore rupees. Despite its importance, cumin is threatened by a serious, destructive disease called Cumin Wilt, which is caused by Fusarium oxysporum. The disease’s impact has reduced income at both the household and national levels. The current study was carried out to look into chemical control strategies for the disease. The research was carried out from November to March (Rabi season) in Ravipura Kampa, Gujarat, India. Field observation of infected cumin wilt was followed by laboratory isolation, characterization of the causative agent, and assessment of the effects of various fungicides. In chemical management different fungicides were used. In laboratory tests, F. oxysporum, which is characterized by whitish mycelia growth and chlamydospores, was found to be the causative agent of the cumin wilting symptoms. There are two types of conidia: macro conidia and micro conidia.
After 120 days of application, the tested chemical fungicides showed significant effects on the suppression of Fusarium wilt disease, thus enhancing cumin recovery. All the fungicides showed varying effects against F. oxysporum. This resulted in a complete stoppage of mycelial growth, followed by 5% EC formulation of Hexaconazole 2800 ppm, 50% WP CAPTAN3500ppm, Azoxystrobin 1600ppm, Azoxystrobin 11% + Tebuconazole 18.3% w/w SC 1000 ppm, Epoxyconazole + Flyxapyroxald 3500 ppm, Isopropanol azole 4000 ppm, Mancozeb 5000 ppm, Pyarclostrobin 13.3%+ Epoxyconazole 5% EC 4000 ppm, and Tebuconazole 25.9% EC 2000 ppm reduced severity and accelerated recovery. These findings support the administration of these chemicals in the appropriate concentrations for timely disease management in the affected areas. Studies are being conducted to determine the effects of the chemicals used in order to improve and continue applications to combat the disease.
Chemical Fungicides, Cumin, Fusarium Wilt, in Vitro.
Charan N. D, Rajput K. N, Panchal R. R. Characterization and Chemical Management of Cumin Wilt Disease Caused by Fusarium oxysporum. Biosc.Biotech.Res.Comm. 2023;16(3).
Charan N. D, Rajput K. N, Panchal R. R. Characterization and Chemical Management of Cumin Wilt Disease Caused by Fusarium oxysporum. Biosc.Biotech.Res.Comm. 2023;16(3). Available from: <a href=”http://surl.li/lsjot“>http://surl.li/lsjot</a>
INTRODUCTION
India has been a land of spices and the largest producer, consumer, and exporter of spices. Rajasthan and Gujarat are the important states to produce cumin. The crop suffers from several diseases. Among the major threats to the cumin is wilt. When stem was cut longitudinally, brownish discoloration is observed. Mathur and Mathura (1956) reported wilt of cumin from Rajasthan and identified the casual organism to be Fusarium oxysporum. Based on host specificity, Patel and Prasad finally named it as Fusarium oxysporum f. sp. cumini. Fusarium is largest genus of filamentous fungi widely distributed in agricultural soils. It contains large number of destructive plant pathogens such as F. avenaceum, F. eumartii, F.oxysporum and particularly F. solani, which is potential cause of vascular wilt, root rot and fruit rot as well as influence seed germination in different host plants. The fungus is soil borne in nature and commonly found in all crop growing areas in world. The fungus belongs to ascomycetes and cause wilt disease of several important crops. Fungus produces micro conidia, macro conidia and chlamydospores, which persist in soil for several years. The pathogen is seed borne in nature too and transmits from one place to another through seed material (Watt, 2006, Akter et al 2021).
Crop plants are highly susceptible to attack of Fusarium spp. during the pre- and post-emergence stages (Nawar, 2007). Pathogens can quickly develop in light sandy soils, triggering root rot disease in a variety of crops in different parts of the world (Celar, 2000). The fungus produces toxic substances that are responsible for inducing wilt symptoms in susceptible cultivars. Brown blotches form on the wilted area. The disease causes withering signs in the seedling stage. Plants are damaged during every phase of its development. The infected plants turn yellowish and show characteristic dropping of leaves leading to mortality of plant. Brown discolorations can also be seen on the vascular bundle, and the plants are easily pulled from the soil. Although there are a lot of approaches and procedures for controlling plant diseases, only a handful have been proven effective. The pathogen is managed using a combination of biological, cultural, and chemical methods. Varieties of systemic and non-systemic fungicides have been tested against the pathogen, having varied results. Several scientists have concluded that systemic fungicides are superior for disease management (Rafique et al., 2016).
MATERIAL AND METHODS
Isolation of pathogen: The Pathogen of cumin wilt was isolated from wilted part of cumin plant (Ravipura kampa-Gujarat India). Small (5 by 5 mm) sections were excised from margins of browning stem bases and surface-sterilized in 75% ethanol for 10 s, then sterilized in 5 % sodium hypochlorite for 1.5 min, followed by three rinses with sterile water. The fragments were then placed on to potato dextrose agar (PDA) and incubated at 27°C. Fungal growth was examined daily for up to7 days. Isolates were transferred to fresh PDA and purified by single-spore culturing. All fungal isolates were placed on fresh PDA slants and stored at 4°C Akter et al,.(2021).
Effect of temperature, carbon sources, different nutrient media, pH and salt on linear growth of pathogen: There were four temperatures selected for study 4°C, 28°C, 35°C and 40°C. Twelve sterile Petri-dishes with PDA medium were used (three plates for each treatment). With the help of 2 mm-diameter sterile cup borer a disc of inoculum of a 7- day old cultures, and then transferred to the center of each plate in close contact with the medium. The plates were incubated for 7 days at the following temperature range: 4°C, 28°C, 35°C and 40°C. The fungal growth was estimated daily by measuring the colony size and results were recorded for each degree of temperature, (Khilare and Ahmed, 2012).
Effect of carbon sources on linear growth of pathogen was investigated by using four different sources. Those carbon sources were dextrose, sucrose, maltose and starch. The amount of 20 gm of each sugar was added to the potato agar medium. Four new media PDA, PSA, PMA and PSTA were taken for study. The Petri-dishes were inoculated with 2 mm disc taken from 7 days old culture of the isolate and incubated in complete darkness at 28°C for 7 days. Readings were taken daily by measuring the growth diameters and the average was recorded.
Effect of different nutrient medium on linear growth of pathogen was assessed by taken five types of media those were potato dextrose agar (PDA), malt extract agar (MEA) and Czepek’s Dox agar, Rose bangle agar, white bran medium. Four plates (9 cm diameter) were prepared from each medium and then the plates were inoculated with 2 mm disc from 7 days old culture of isolate. The plates were incubated in complete darkness for 7 days at 28°C and then the rate of fungal growth was estimated daily by measuring the colony size ( Chittem and Srikant Kulkarni, 2008)
Effect of pH and salinity on linear growth of pathogen:5.0, 6.0, 7.0, 8.0 and 9.0 pH were taken. The pH was adjusted in PDAmediumbyadding1NHCland1NNaOH. Salinity was adjusted with NaCl in medium. Four concentrations of NaCl were taken for study 0.10,0.25,0.50and0.75percent were maintained in growth PDA media in Petri plates. All Petri plates were incubated at 28°C for a week. Radial growth observations were taken at 24 hour intervals up to one week, radial growth was measured by scale Gordon et al, (2019).
Effect of different fungicides on pathogen at laboratory level: 5% EC formulation of Hexaconazolek, 50% WP CAPTAN, Azoxystrobin, Azoxystrobin 11% + Tebuconazole 18.3% w/w SC, Epoxyconazole + Flyxapyroxald, Isopropanol azole, Mancozeb, Pyarclostrobin 13.3%+ Epoxyconazole 5% EC, and Tebuconazole 25.9% EC Fungicide tested to analyze their effect on growth of pathogen . Each fungicides was dissolved in water to obtain a final concentration 100 ppm, 500 ppm, 1000 ppm, 1500 ppm, 2000 ppm, 2500 ppm, 3000 ppm, 3500 ppm, 4000 ppm, then inoculated into sterile PDA with three replicate .
These five dilutions for each chemical were made based on their recommended dose. Three plates poured with PDA medium served as control. Then 2 mm disc was cut from the edge of 7 days old culture of the fungus and disc was placed in the center of Petri dish and kept in BOD incubator for 8 days for the full growth of fungus was observed, in case of control where no chemicals were applied in medium. The inhibition in the radial growth of pathogen mycelium was recorded and percent inhibition in the mycelium of pathogen was recorded by comparing the mycelial growth of fungus in the treated plates with control. The effect of each chemical was evaluated as percentage of reduction (Sultana & Ghaffar 2010).
Where: R = dc – dt x 100/dc 25 Whereas: R = Percentage of reduction
dc = diameter of control colony dt = diameter of treatment colony
Effect of different fungicides on pathogen at field level: An experiment was conducted at Ravipurakampa, Aravalli, Gujarat, for one year during rabi season 2019-20. There were three replications in RBD with plot size 2×3 sq m. Sowing was done in the month of November. Seeds were treated with different systemic and contact fungicides. Two concentrations of each Fungicide were tested in farm.
RESULTS AND DISCUSSION:
In the present study, the experiments were carried out under laboratory conditions including all the available management practices and further their efficacy was tested in farm conditions. Standard tissue isolation technique was followed to get culture of causal organism from the wilted plants (figure 1), showing the symptoms. Fusarium was isolated consistently from wilted cumin sample. Repeated sub-culturing was done to obtain pure culture on basal medium PDA (figure 2).
Figure 1: Wilted cumin sample

Figure 2: Growth of pathogen on PDA

Figure 3: Microscopic observation of macro and micro conidia
Hyphal tip method was used to obtain pure culture as detailed in material and methods. The pathogen was identified as based on their morphological and cultural characters as F.oxysporum by referring to Booth (1971)
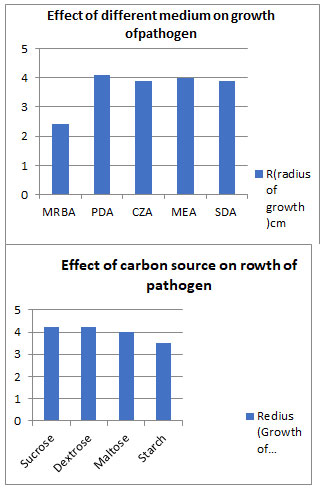
Cultural studies: Cultural characters were studied on five different solid media and four carbon sources were studied to analyze effect of carbon source on linear growth of pathogen, maximum growth was observe in Potato dextrose agar medium, dextrose and sucrose carbon source.
Figure 4
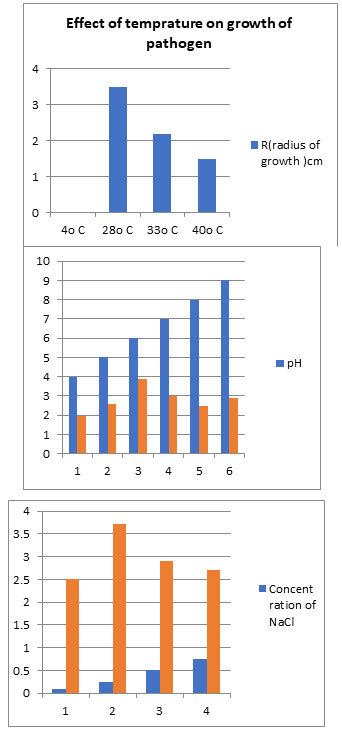
For the majority of the isolates, the 27°C temperature, pH of 6, and salt content of 0.25 percent resulted in the fastest in vitro radial growth.
Figure 5
The symptoms of Fusarium wilt caused by Fusarium oxysporum appeared as leaf chlorosis, yellowing of foliage followed by wilting and dropping of leaves. The xylem became brown in colour of the stem and finally death of the above ground parts. Similar symptoms were also reported by Altinok (2005) and Mac Hardy and Beckman (1981). Isolated fungal pathogens were identified based on cultural and microscopic characteristic and using available literatures.
Figure 6
Altinok (2005) and Mwaniki et al. (2011) isolated F. oxysporum from the eggplant vascular tissues in Tanzania. Sahar et al. (2013) also isolated Fusarium oxysporum from infected roots of egg plant in Pakistan. F. oxysporum was isolated from Fusarium wilt of tomato plant by Amini and Sidovich (2010). F.oxysporum was also isolated from roots and stem of wilt infected tomato plant (Nirmaladevi and Srinivas, 2012 and Joshi et al., 2013).
Results indicate that temperature 28°C, basic pH (8.0) and 0.25 5% NaCl salinity is optimum for the of growth Fusarium oxysporum sp. cumini. While dextrose and sucrose carbon sources, as well as Potato Dextrose Agar media, are ideal for pathogen growth. It is confirmed that environment plays a significant role in disease development. The prevalence of Fusarium wilt disease appeared in November month, suitable temperature ranges is11°C to 29°Candrelative humidity78% to 82% for disease development. This finding corroborates with that of Bruchl (1987).
Table 1. Effect of fungicides on the mycelial growth of pathogen
| Name of active compound | Effective inhibition dose ppm | Radial growth (mm) |
| 5% EC formulation of Hexaconazole | 2800 | 0.00 |
| 50% WP CAPTAN | 3500 | 0.00 |
| Azoxystrobin | 1600 | 0.00 |
| Azoxystrobin 11% + Tebuconazole 18.3% w/w SC | 1000 | 0.00 |
| Epoxyconazole + Flyxapyroxald | 3500 | 0.00 |
| Isopropanol azole | 4000 | 0.00 |
| Mancozeb | 5000 | 0.00 |
| Pyarclostrobin 13.3%+ Epoxyconazole 5% EC | 4000 | 0.00 |
| Tebuconazole 25.9% EC | 2000 | 0.00 |
Chemical agents used were found to be effective for the inhibition of mycelial growth of Fusarium oxysporum under in vitro conditions (Table 1). The data clearly indicated that there was significant inhibition of mycelial growth of F.oxysporumf. sp. Melongenae due to the application of fungicides. Among the fungicides tested in this study, carbendazim was found to be significantly superior (96.7% inhibition), followed by Mancozeb (83.46% inhibition), Copper oxychloride (81.83% inhibition), and Captan (77.76% inhibition).Ridomil was noticed to have the least effective inhibition at 41.43%. Fusarium oxysporum at 0.2% concentration gave maximum inhibition of mycelial growth. The next best were Mancozeb (89%), Copper oxychloride (83%), and Captan (80.6%) percent inhibition at the same concentration.
The least inhibition of mycelial growth was observed in Ridomil (44.8%) at a 0.2% concentration level (25). The findings of the present study are in agreement with those of Pandey and Pandey (2001). They reported that Fusarium is the best controlled by the use of Bavistin and Mancozeb. Song et al. (2004) also reported that carbendazim was found to be the most effective fungicide in inhibiting the mycelial growth of F. oxysporum sp. lycopersici. Carbendazim at its highest concentration (1000 ppm) caused the highest reduction of mycelial growth (9.40 mm) in solidified poisoned PDA observed by Nisa et al. (2011).
Khan et al. (1997) and Sharma (2006) found that Bavistin completely inhibited the mycelial growth of F. oxysporum. Hossain and Bashar (2011) also reported that complete inhibition of mycelial growth was observed in Fusarium oxysporum and F. pallidoroseum with Dithane M-45 and Cupravit at 50 and 800 ppm concentrations, respectively. The effectiveness of these fungicides against other fungi has been reported (Daradhiyar, 1980; Sommer, 1982; Kalra and Sokhi, 1985; Singh et al., 1997; Patel et al., 2005; Banyalet al., 2008).
Allen et al. (2004) revealed that Benomyl at 10 g/ml completely inhibited fungal growth of F. oxysporum, F. solani, and F. proliferatum. Wilt incidence was lower at 30 days after sowing compared to 60 DAS. Carbendazim and combi products (carbendazim 25% + mancozeb 50%) were very effective in managing the disease completely up to 60 DAS, which was statistically on par and significantly superior to all other treatments. Significantly, the highest seed yield and wilt reduction were recorded in Bavistin (Carbendazim) treated plots and Sunvit treated plots, and the lowest yield was obtained from control plots, which was followed by Rovral. Hoque and colleagues (2014) and Siddique et al. (2014) noticed that Aimcozim 50 WP (Carbendazim) inhibited fungal growth in vitro at the lowest concentration, and that Trimethylthiuramdisulphide (0.1 g g-1 seeds) was the best fungicide for wilt control, on par with Propiconazole, Carbendazim, and Copper oxychloride (0.1 g g-1 seeds).Klix et al. (2007) found that prothioconazole, the active ingredient, inhibits a key Fusarium enzyme (14-demethylase), which is required for ergo sterol.
CONCLUSION
The observed wilt syndromes on cumin fields are the fungal disease symptoms caused by a soil borne pathogen known as Fusarium oxysporum. Salinity, pH, carbon source and temperature have a significant effect on fungal growth. These characteristics can aid in disease monitoring and raising farmer awareness.
ACKNOWLEDGEMENTS.
All authors have contributed to the manuscript preparation, read, and approved the final manuscript.
Funding This study was financially supported by the department of biochemistry at the university.
Availability of data and materials : All data and material used can be availed from corresponding author upon request.
Ethics Approval and consent to participate: Not applicable.
Competing Interests: Authors declare that they have no competing interest.
REFERENCES
Akter, N., Islam, M. R., Hossain, M. B., Islam, M. N., Chowdhury, S. R., Hoque, S.&Tasnin, R. (2021). Management of Wilt Complex of Eggplant (Solanum melongena L.) Caused by Fusarium oxysporum, Ralstonia solanacearum and Meloidogyne spp. American Journal of Plant Sciences, 12(7), 1155-1171.
Allen, T. W., Enebak, S. A., & Carey, W. A. (2004). Evaluation of fungicides for control of species of Fusarium on longleaf pine seed. Crop Protection, 23(10), 979-982.
Altınok, H. H. (2005). First report of Fusarium wilt of eggplant caused by Fusarium oxysporum sp. melongenae in Turkey. Plant Pathology, 54(4), 577-577.
Amini, J., &Sidovich, D. (2010). The effects of fungicides on Fusarium oxysporum sp. lycopersici associated with Fusarium wilt of tomato. Journal of plant protection research.
Celar, F. (2003). Competition for ammonium and nitrate forms of nitrogen between some phytopathogenic and antagonistic soil fungi. Biological Control, 28(1), 19-24
Chittem, K., & Kulkarni, S. (2010). Effect of Media on the Growth of Fusarium oxysporum sp. gerberae and Fusarium oxysporum f. spdianthi. Karnataka Journal of Agricultural Sciences, 21(2)
Gordon, T. R., Stueven, M., Pastrana, A. M., Henry, P. M., Dennehy, C. M., Kirkpatrick, S. C., &Daugovish, O. (2019). The effect of pH on spore germination, growth, and infection of strawberry roots by Fusarium oxysporum sp. fragariae, cause of Fusarium wilt of strawberry. Plant disease, 103(4), 697-704.
Hossain, K. S., & Bashar, M. A. (2011). In vitro effect of plant extracts, fungicides and antibiotics on the fungal isolates associated with damping-off disease of crucifers. Identities, 10, 100.
Joshi, M., Srivastava, R., Sharma, A. K., & Prakash, A. (2013). Isolation and characterization of Fusarium oxysporum, a wilt causing fungus, for its pathogenic and non-pathogenic nature in tomato (Solanum lycopersicum). Journal of Applied and Natural Science, 5(1), 108-117.
Khan, M. A., Ahmad, M. and Saeed, M. A. 1995. Evaluation of fungicides on the growth of Alternaria alternata in-vitro and the control of the post harvest tomato fruit rot Pakistan Journal of Phytopathology, 7(2): 166-168.
Khilare, V. C., & Ahmed, R. (2012). Effect of different media, pH and temperature on the growth of Fusarium oxysporum sp. ciceri causing chickpea wilt. International Journal of Advanced Biological Research, 2(1), 99-102.
Klix MB Beyer and Verreet JA (2008) Effects of cultivar, agronomic practices, geographic location, and meteorological conditions on the composition of selected Fusarium species on wheat heads. Canadian Journal of Plant Pathology,30: 46-
MacHardy, W. E., Beckman, C. H. (1981). “Vascular wilt Fusaria: infection and pathogenesis,” in fusarium: diseases, biology, and taxonomy. Eds. Nelson, P. E., Toussoun, T. A., Cook, R. J. (University Park, Pennsylvania: The Pennsylvania State University Press), 365–391.
Mathur B L & Mathur R L 1965 Metabolites of Fusarium oxysporum in relation to cumin wilt. Indian Phytopathol. 18: 335–339.
Mwaniki, P. K., Abang, M. M., Wagara, I. N., Wolukau, J. N., &Schroers, H. J. (2011). Morphology, pathogenicity and molecular identification of Fusarium from wilting eggplants in Tanzania. In African Crop Science Conference Proceedings (Vol. 10, pp. 217-221).
Nawar,L.S.20 Pathological and rhizospherical studies on root-rot disease of squash in Saudi Arabia and its control African Journal of Biotechnology,6:219-226.
Nirmaladevi, D., & Srinivas, C. (2012). Cultural, morphological, and pathogenicity variation in Fusarium oxysporum sp. lycopersici causing wilt of tomato. Batman Üniversitesi Yaşam Bilimleri Dergisi, 2(1), 1-16
Nisa, T. U., Wani, A. H., Bhat, M. Y., Pala, S. A., & Mir, R. A. (2011). In vitro inhibitory effect of fungicides and botanicals on mycelial growth and spore germination of Fusarium oxysporum. Journal of Biopesticides, 4(1), 53.
Patel P N & Prasad N 1963 Fusarium wilt of cumin (Cuminum cyminum) in Gujarat State, India. Plant Dis. Report. 74: 528–531
Rauf, C. A., Rafique, K., Naz, F., & Kang, S. (2016). First report of lentil wilt caused by Fusarium nygamai in Pakistan. Journal of Plant Pathology, 98(3).
Sahar, P., Sahi, S. T., Jabbar, A., Rehman, A., Riaz, K., &Hannan, A. (2013). Chemical and biological management of Fusarium oxysporum spmelongenae. Pakistan journal of Phytopathology, 25 (2), 155-159.
Sharma, P., Sharma, K. D., Sharma, R., & Plaha, P. (2006). Genetic variability in pea wilt pathogen Fusarium oxysporum sp. pisi in north-western Himalayas.
Siddique, S. S., Bhuiyan, M. K. A., Momotaz, R., Bari, G. M. M., & Rahman, M. H. (2014). Cultural characteristics, virulence and In-vitro chemical control of Fusarium oxysporum sp. phaseoli of bush bean (Phaseolus vulgaris L.). The Agriculturists, 12(1), 103-110.
Song, W., Zhou, L., Yang, C., Cao, X., Zhang, L., & Liu, X. (2004). Tomato Fusarium wilt and its chemical control strategies in a hydroponic system. Crop protection, 23(3), 243-247.
Sultana, N., & Ghaffar, A. (2010). Effect of fungicides, microbial antagonists and oilcakes in the control of Fusarium solani, the cause of seed rot, seedling and root infection of bottle gourd, bitter gourd and cucumber. Pak. J. Bot, 42(4), 2921-2934.
Vincent, J.M. (1947) Distortion of Fungal Hyphae in Presence of Certain Inhibition. Nature,159,850. https://doi.org/10.1038/159850b0
Watt, B. A. (2006). Fusarium rot of cucurbits. Fact sheet of insect and plant disease diagnostic lab., pest management office, University of Marine Cooperative Extension. UMCE Home page.


