Department of Biological Sciences, College of Science, King Abdulaziz University, Jeddah, Saudi Arabia.
Corresponding author email :aahmadi1000@hotmail.com
Article Publishing History
Received: 25/04/2021
Accepted After Revision: 24/06/2021
Diabetes mellitus is a lifelong metabolic condition resulting from chronic hyperglycemia. Non-alcoholic fatty liver and cirrhosis are among the most important complications of diabetes, which are associated with increased mortality.This study aimed to re-emphasize the protective effects of Ajwa seeds (Phoenix dactylifera seeds) against hepatic damage caused by diabetes, and to investigate the mechanisms underlying it, specifically the antioxidant, anti-inflammatory, and anti-apoptotic ones.Five groups (n = 5) of adults male Wistar rats were created. Group 1 was the control, group 2 was the control treated with Ajwa seeds (1g/kg), group 3 was the diabetes (STZ, 35 mg/kg), group 4 was the diabetes treated with Metformin (150 mg/kg), and group 5 was the diabetes treated with Ajwa seeds (1 g/kg). Metformin and Ajwa seeds suspension were administered orally using oral gavage six days a week, for four weeks.
Ajwa seeds suspension significantly lowered STZ-induced hyperglycemia and increasing insulin secretion. It reduced the elevated liver enzymes (ALT, AST, ALP, and LDH), and improved liver tissue pathological features.Ajwa seeds increasedliver concentrations of enzymatic (SOD and CAT) and non-enzymatic (GSH) antioxidants and reduced levels of oxidative stress products (MDA and AGE).Ajwa seeds lowered the levels of inflammatory mediators (TNF-α and NF-κB) and NF-κBprotein expression. Moreover Ajwa seeds lowered the hepatic protein expression of pro-apoptotic marker, caspase 3.The antioxidant, anti-inflammatory, and antiapoptotic properties of Ajwa seeds can explain their hepatoprotective effects in this diabetes model.
Ajwa Seeds;Diabetes; Antioxidants; Anti-Inflammatory; Antiapoptotic
Alahmadi A. A, Banayah H. M. Certain Hepatoprotective Effects of the Ajwa Date (Phoenix dactylifera) Seeds on Streptozotocin-Induced Diabetic Rats:. Biosc.Biotech.Res.Comm. 2021;14(2).
Alahmadi A. A, Banayah H. M. Certain Hepatoprotective Effects of the Ajwa Date (Phoenix dactylifera) Seeds on Streptozotocin-Induced Diabetic Rats:. Biosc.Biotech.Res.Comm. 2021;14(2). Available from: <ahref=”https://bit.ly/3iC8Nbu“>https://bit.ly/3iC8Nbu</a>
Copyright © This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY). https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provided the original author and sources are credited.
INTRODUCTION
Diabetes mellitus is a lifelong metabolic condition resulting from chronic hyperglycemia(Heindel et al., 2017). Diabetes mellitus affects roughly 425 million individuals globally, and it is expected to affect approximately 642 million people worldwide by 2040, (Meo et al., 2017, 2019). Many liver disorders, including non-alcoholic fatty liver and hepatobiliary ailments, are related to diabetes(Afrin et al., 2015). Similarly, unregulated glycogen accumulation in the liver can exacerbate insulin resistance, which, when combined with hyperglycemia, can damage the liver and contribute to higher morbidity and mortality among diabetes patients. .Diabetes mellitus is most often linked with non-alcoholic steatohepatitis, alcoholic cirrhosis, chronic hepatitis C, and hemochromatosis (Dewidar et al., 2020; Garcia-Compean et al., 2009).The mortality rate from diabetes-induced cirrhosis is significantly higher than that of cardiovascular diseases( Harrison, 2006, Mohamed et al., 2016;Thobaiti and Abu Zeid, 2019 Naseri et al., 2020); .
The common anti-diabetic medications are ineffective in preventing the progression of liver-related illness. Consequently, there is a real need for complementary and adjunctive medications to manage diabetes mellitus-related severe complications ( Chaudhury et al., 2017, Al-Thobaiti and Abu Zeid, 2019) Herbal remedies and natural treatments are safer, more effective, more affordable, and less expensive alternatives to oral diabetes medications ( Kooti et al 2016 Al-Attar and Alsalmi, 2019).The date is one of many traditional supplements that is utilized in several developing nations’ healthcare systems.Folk medicine providers widely use dates in country areas of many countries. The date palm tree is a member of the Arecaceae family (Angiosperms, monocotyledon), which includes over 2,500 species and 200 genera.
Phoenix, which includes Phoenix dactylifera L., is one of the genera with nearly 14 species(Eoin, 2016). Phoenix dactylifera L. (var. Ajwa) is one of Southwest Asia’s and North Africa’s oldest and most important staple and historic plants (Al-Harrasi et al., 2014).Ajwa date is one of the most famous date varieties which recognized by Muslim communities for its therapeutic and religious importance (Nematallah et al., 2018).Ajwa fruits are rich in antioxidants, antibacterial, antifungal, and anti-proliferative effects, and have a greater nutritional and medicinal importance (Al-Alawi et al., 2017; Temitope Idowu et al., 2020). Ajwaseeds are traditionally dumped products of date fruit. It accounts for about 10% of the fruit’s total weight. Dietary fiber, protein, carbohydrates, phenols, and minerals make up the bulk of the date seeds. Antioxidant and antimicrobial actions are parts of the numerous biological effects of these compounds(Mrabet et al., 2020).
A previous study has documented the antidiabetic potential of Ajwa date seeds extract in STZ-induced hyperglycemic model in rats; besides, it normalized the elevated liver transaminases in diabetic rats (Hasan and Mohieldein, 2016).Date seeds in an aqueous suspension may help to mitigate the early complications of diabetes, especially hepatic and renal problems(Abdelaziz et al., 2015).The aim of this study was to re-emphasize the protective properties of Ajwa seeds(Phoenixdactylifera seeds) against hepatic damage caused by diabetes, and to investigate the mechanisms underlying it, specifically the antioxidant, anti-inflammatory, and anti-apoptotic mechanism.
MATERIAL AND METHODS
Chemicals: Streptozotocin (STZ) S0130-1G was purchased from Sigma- Aldrich, (St. Louis, MO, USA); Metformin (Glucophage, 500 mg tablet, Merck Santé, France) was obtained from Alnahdi pharmacy, Jeddah, Saudi Arabia. Ajwa seeds used in this study were collected from Ajwa dates purchased from the Oasis Lina, Al-Madinah Al-Munawara, Saudi Arabia.
Preparation of Ajwa seeds suspension: The seeds were removed from the fruits, then washed to remove residues. Seeds were dried for 2-3 weeks at room temperature in a well-ventilated room. The dried Ajwa seeds were hammer-milled into a fine powder. A fresh aqueous suspension of Ajwa seeds was made by mixing 1 gram of powdered seeds with 10 mL of Tween-80 vehicle.
Animals:This study utilized 25 adults male Wistar rats, their mean body weight ranged from 150 ± 250 g. Rats were bought from King Fahad Research Centre, King Abdulaziz University, Jeddah, Saudi Arabia. After the one-week adaptation period, the experiment was performed under the standard laboratory conditions of temperature, moisture, and 12:12 h light/dark period. No limitations were applied to the rats on water and food. The Biomedical Ethics Research Council, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia, approved the study’s procedure (346-19).
Induction of diabetes: The rats were injected once with intraperitoneal dose of STZ (35 mg/kg)(Meng et al., 2017). After 7 days fasting blood sugar concentrations were determined using ACCU-CHEK. Diabetic model rats were described as those with blood glucose levels greater than 200 mg/dL(Zhang et al., 2008).
Study design: Rats were equally classified in 5 groups (n = 5). The 1st group was the control group, the 2nd group was the control group treated with Ajwa seeds (1g/kg) (Khan et al., 2017), the 3rd group was the diabetes group, the 4th group was the diabetes group treated with Metformin (150 mg/kg) (El-Sayed et al., 2020), and the 5th group was the diabetes group treated with Ajwa seeds (1 g/kg). Metformin and Ajwa seeds were administered orally using oral gavage six days a week, for four weeks.
Sample Collection: At the end of the experiment, rats were fasted before blood collection and dissection. All animals were euthanized using diethyl ether, then blood was collected from the orbital plexus sinus. The blood was centrifuged for 15 min at 3500 rpm at -4°C to separate the serum which was kept in the freezer at -80°C. Using rodent guillotine rats ware decapitated and dissected. Liver’s ware taken out and washed with normal saline then cut. For each organ, some of pieces were preserved in 10% formalin and some kept frozen at -80°C.
Measurement of serum indicators of hyperglycemia: Fasting serum glucose was measured by using the colorimetric kit of Reactivos GPL, Barcelona, Spain.Fasting serum glucose was measured by using the rat ELISA assay kit, Immunospec, CA.
Measurement of serum indicators of liver dysfunction: Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), Alkaline phosphatase (ALP), lactate dehydrogenase (LDH), and total protein (TP) were measured using the kits of Human, Germany for all the enzymes and Crescent Diagnostics kit, Saudi Arabia for the TP.
Measurement of serum indicators of liver oxidative stress: Hepaticconcentration of reduced glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), and malondialdehyde (MDA), were measured using the kits of Biodiagnostic, Egypt. and advanced glycation product (AGE)were measured using the kits of My Bio Source, USA.
Measurement of serum indicators of liver inflammation: Hepaticconcentration of tumor necrosis factor alpha (TNF-α) and nuclear factor kappa beta (NF-κB)were measured using the rat ELIZA kits of Abcam, USA.
Histopathological investigation of the liver tissue (hematoxylin and eosin (H & E) staining):Formalin-fixed liver specimens were packed with paraffin and cut into 4 μm segments. The segments were dyed with H&E and analyzed and photted utilizing light microscopy.
Immunohisto chemistry investigation of apoptotic and inflammatory markers: An immunoperoxidase (peroxidase/antiperoxidase, PAP) protocol was utilized to stain the liver segments for NF-κB and caspase-3proteins. The antibodies (bought from Lab Vision, Fremont, CA)were diluted in 1:200 dilution. The segments were analyzed and photted, utilizing light microscopy.
Statistical analysis: The mean and SE were used to express the data. The data were analyzed statistically using ANOVA and Tukey’s post-hoc test. A p 0.05 was chosen as the significant level. SPSS for Windows, version 22, Armonk, NY, was used to conduct the statistical analysis.
RESULTS AND DISCUSSION
Impact of Ajwa seeds on serum indicators of hyperglycemia: There was no significant difference in glucose levels between the Ajwa seeds rats and the control rats.The diabetes rats showed a significantly increased serum glucose contrasted to the control rats. In comparison to the diabetes rats, the diabetic rats who were given Metformin and Ajwa seeds had significantly lower serum glucose levels(Table 1).The insulin level between Ajwa seeds rats and the control rats showed no significant difference. The diabetes rats showed a significantly decreased serum insulin contrasted to the control rats. In comparison to the diabetes rats, treatment with Metformin and Ajwa seeds substantially increased serum insulin levels; however, Ajwa seeds was found to significantly increased serum insulin contrasted to the Metforminrats(Table 1).
Table 1. Impact of Ajwa seeds on serum indicators of hyperglycemia
| Test Rats | Glucose (mg/dL) | Insulin (µIU/mL) |
| Control | 134 ± 10 | 24 ± 0.36 |
| Ajwaseeds | 130 ± 5.0 | 19 ± 1.4 |
| Diabetes | 328 ± 1.0 a*** | 11 ± 0.26 a*** |
| Diabetes + Metformin | 203 ± 21 b*** | 22 ± 0.87 b*** |
| Diabetes + Ajwa seeds | 164 ± 29 b*** | 31 ± 2.40 b***, c*** |
| Findings were described as mean ± SE (n = 5).aSignificant contrasted to the control rats. bSignificant contrasted to the diabetes rats.cSignificant contrasted to the Metformin rats.*** p<0.001. | ||
Impact of Ajwa seeds on serum indicators of liver injury: There was no significant difference in ALT, AST, ALP, and LDH levels between the Ajwa seeds rats and the control rats. The diabetes rats showed a significantly increased serum ALT, AST, ALP, and LDH contrasted to the control rats. Treatment of diabetes rats with Metformin and Ajwa seeds significantly decreased serum AST, ALP, and LDH contrasted to the diabetes rats. In comparison to the diabetes rats, Ajwa seed treatment substantially reduced serum ALT levels; however, Metformin therapy had no effect on serum ALT levels in diabetic rats. Ajwa seeds was found to significantly decreased serum insulin contrasted to the Metformin rats. There was no significant difference between serum TP in all the test rats (Table 2).
Table 2. Impact of Ajwa seeds on liver tissue indicators of liver injury
| Test Rats | ALT (U/L) | AST (U/L) | ALP (U/L) | LDH (U/L) | TP (g/dL) |
| Control | 18.9 ± 2.8 | 19.6 ± 1.5 | 520 ± 30 | 250 ± 20 | 5.2 ± 0.04 |
| Ajwa seeds | 12.3 ± 1.9 | 13.3 ± 2.4 | 346± 19 | 249 ± 29 | 5.6 ± 0.12 |
| Diabetes | 81.4 ± 13.2a*** | 122.3 ± 2.1a*** | 1169 ± 108a*** | 1496 ± 71a*** | 5.4 ± 0.49 |
| Diabetes + Metformin | 67.1 ± 6.2 | 29.8 ± 1.5b*** | 463 ± 39b*** | 209 ± 15b*** | 5.2 ± 0.06 |
| Diabetes + Ajwa seeds | 20.1 ± 1.1b***, c*** | 44.9 ± 1.7b***, c*** | 495 ± 95b*** | 119 ± 9b*** | 4.99 ± 0.31 |
| Findings are described as mean ± SE (n = 5).aSignificant contrasted to the control rats. bSignificant contrasted to the diabetes rats.cSignificant contrasted to the Metformin rats.***p<0.001. | |||||
Impact of Ajwa seeds on liver tissue histopathology (H & E): The control and Ajwa seeds liver sections showed normal central vein (CV) and normal hepatocytes with rounded, active, and centrally located nuclei. The diabetes liver section showed numerous histological changes mainly in CV regions mainly dilated CV, hepatocytes necrosis, and degenerated nuclei. The liver sections of rats treated with Metformin and Ajwa seeds showed apparent preservation of the hepatocyte’s morphology; besides, the CV was intact normal. More evident preservation was observed in the CV and hepatocytes of the Ajwa seeds rats contrasted to the diabetes and Metformin rats(Figure 1).
Figure1: Impact of Ajwaseeds on liver tissue histopathology (H & E)
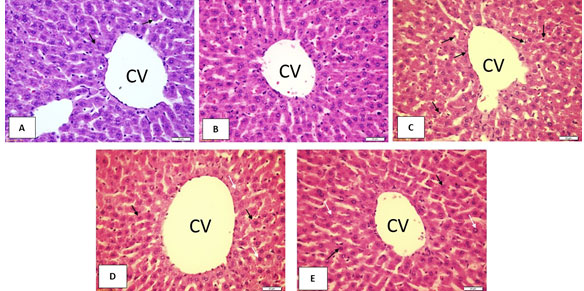
A: Control; B: Ajwa seeds; C: Diabetes; D: Diabetes + Metformin; E: Diabetes + Ajwa seeds
Impact of Ajwa seeds on liver tissue indicators of oxidative stress: There was no significant difference in SOD, CAT, MDA, and AGE levels between the Ajwa seeds rats and the control rats. Ajwa seeds significantly increased liver GSH content contrasted to the control rats. The diabetes ratshad a significantly higherliver contents ofMDA and AGEand significantly lower liver contents of GSH, SOD, and CAT contrasted to the control rats. In comparison to the diabetes rats, Metformin treatment significantly reduced MDA and AGE levels in the liver while significantly increasing SOD and CAT levels. In comparison to diabetes rats, Ajwa seeds treatment significantly lowered the liver content of MDA and AGE, whereas the liver content of SOD and CAT was significantly higher. Treatment of diabetes rats with Ajwa seeds significantly decreased liver content of AGE and significantly increased liver GSH, SOD, and CAT contrasted to the Metformin rats(Table 3).
Table 3. Impact of Ajwa seeds on liver tissue indicators of oxidative stress
| Test Rats | GSH (mg/g) | SOD (U/g) | CAT (U/g) | MDA (nmol/g) | AGE (ng/g) |
| Control | 8.7 ± 0.05 | 1261 ± 58 | 7.7 ± 0.44 | 29 ± 1.9 | 73.9 ± 5.1 |
| Ajwaseeds | 10.1 ± 0.49a* | 1268 ± 43 | 7.0± 0.67 | 29 ± 1.4 | 78.6± 8.6 |
| Diabetes | 6.4 ± 0.20a*** | 714 ± 8.0a*** | 0.93 ± 0.16a*** | 47.9 ± 1.2a*** | 172 ± 8.2a*** |
| Diabetes + Metformin | 7.22 ± 0.27 | 883 ± 24b* | 9.7 ± 0.29b*** | 33.1 ± 1.6b*** | 140.8 ± 7.8b* |
| Diabetes + Ajwa seeds | 10.83 ± 0.28b***, c*** | 1077 ± 50b***, c* | 7.4 ± 0.46b***, c** | 27.1 ± 0.91b*** | 86.1 ± 4.3b***, c*** |
| Findings are described as mean ± SE (n = 5).aSignificant contrasted to the control rats. bSignificant contrasted to the diabetes rats.cSignificant contrasted to the Metformin rats.*p <0.05, **p <0.01, ***p<0.001. | |||||
Impact of Ajwa seeds on liver tissue indicators of inflammation: There was no significant difference in TNF-α and NF-κB levels between the Ajwa seeds rats and the control rats. The diabetes rats showed significantly increased liver contents of TNF-α and NF-κB contrasted to the control rats. In comparison to the diabetes rats, treatment with Metformin and Ajwa seeds substantially reduced the liver content of TNF- and NF-B. In comparison to Metformin rats, diabetes rats treated with Ajwa seeds had significantly lower TNF- levels in their livers(Table 4).
Table 4. Effect of Ajwaseeds on liver tissueindicators of inflammation
| Test Rats | TNF-α (ng/g) | NF-κB (µg/g) |
| Control | 61 ± 7.2 | 1411 ± 109 |
| Ajwaseeds | 60 ± 2.1 | 1415 ± 53 |
| Diabetes | 152 ± 7.2a*** | 6730 ± 72a*** |
| Diabetes + Metformin | 125 ± 6.7b* | 1643 ± 90b*** |
| Diabetes + Ajwaseeds | 77 ± 4.8b***, c*** | 1488 ± 192b*** |
| Findings are described as mean ± SE (n = 5).aSignificant contrasted to the control rats. bSignificant contrasted to the diabetes rats.cSignificant contrasted to the Metformin rats.*p <0.05,*** p<0.001. | ||
Impact of Ajwa seeds on liver tissue immune expression of NF-κB protein: The control and Ajwa seeds liver sections showed slight NF-κB protein expression. The diabetes liver section showed marked positive NF-κB immuno-stained cells contrasted to the control section. The liver sections of rats treated with Metformin and Ajwa seeds showed apparent decrease in the expression of NF-κB protein contrasted to the diabetes rats (Figure 2).
Figure2: Impact of Ajwaseeds on liver tissue immunoexpression of NF-κB protein
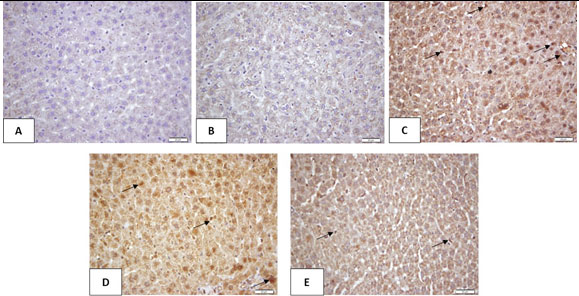
A: Control; B: Ajwa seeds; C: Diabetes; D: Diabetes + Metformin; E: Diabetes + Ajwa seeds
Impact of Ajwa seeds on liver tissue immune expression of caspase-3 protein: The control and Ajwa seeds liver sections showed slight caspase-3 protein expression. The diabetes liver section showed marked positive caspase-3 immuno-stained cells contrasted to the control section. The liver sections of rats treated with Metformin and Ajwa seeds showed apparent decrease in the expression of caspase-3protein contrasted to the diabetes rats. Ajwa seeds showed superior antiapoptotic effect contrasted to Metformin as shown in Figure 3.
Figure 3: Impact of Ajwa seeds on liver tissue immuno expression of caspase-3 protein
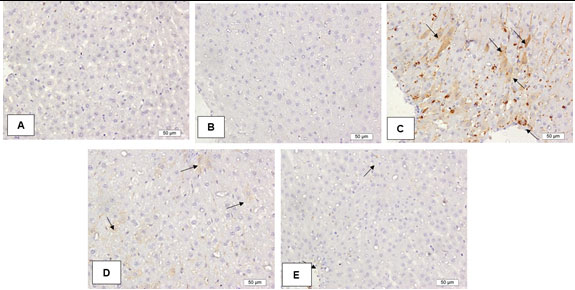
A: Control; B: Ajwa seeds; C: Diabetes; D: Diabetes + Metformin; E: Diabetes + Ajwa seeds
Diabetes is related to a number of liver disorders, including non-alcoholic fatty liver, acute liver failure, and elevated liver enzymes. Similarly, unregulated glycogen accumulation in the liver can exacerbate insulin resistance, which, when combined with hyperglycemia, can damage the liver and contribute to higher morbidity and mortality among diabetes patients(Mohamed et al., 2016).The results of the current study showed that consuming the Ajwa seed suspension protects rat’s liver from diabetes-induced damage. The findings of this study bolstered the potential of Ajwa seeds to reduce the elevated levels of liver enzymes associated with diabetes, and to improve liver tissue pathological features. Similar results previously demonstrated that date seeds suspension improved liver enzymes (ALT and AST) and liver histopathology (H & E) in diabetic rats (Abdelaziz et al., 2015).Furthermore, in rats with alloxan-induced diabetes, Ajwa seed aqueous extract was found to normalize liver enzymes (ALT, AST, and ALP) (Sarfraz et al., 2017).
Hyperglycemia cause oxidative stress, which causes liver damage and metabolic changes (increased gluconeogenesis and ketogenesis) that provoke more oxidative stress and inflammation leading to a rise in serum liver enzymes (Mohamed et al., 2016; Sarfraz et al., 2017).The ability of Ajwa seeds to reverse the raised in the serum liver enzymes and to retain the histopathological features of the liver may be attributed to its ability to lower the elevated serum glucose level which in turn reverse the oxidative stress state and prevent the hyperglycemia linked metabolic changes, (Sarfraz et al., 2017).
Numerous mechanisms are involved in hyperglycemia-induced tissue injuries including increased glucose flux through the polyol pathway, overproduction of intracellular AGE, increased AGE receptor expression, activation of protein kinase C isoforms, and induction of the hexosamine pathway. Several evidence suggest that all of these mechanisms are activated by a specific upstream incident, excess mitochondrial reactive oxygen species (ROS) formation(Brownlee, 2005; Giacco and Brownlee, 2010).
This study demonstrated the ability of Ajwa seeds to increase the concentrations of enzymatic (SOD and CAT) and non-enzymatic (GSH) antioxidants in the liver tissue of diabetic rats and their ability to reduce levels of oxidative stress products (MDA and AGE); these confirm their hepatoprotective action against the risk of destructive oxidative stress.By virtue of their antioxidant constituents, Ajwa seeds may be able to scavenge ROS and thus avoid diabetes-related hepatic oxidative stress, and liver damage(Habib et al., 2014; Habib and Ibrahim, 2011; Sarfraz et al., 2017).Several studies have shown that intracellular glucose overload reduces antioxidant enzyme activity (SOD and CAT) and increases lipid peroxidation (MDA)(Manna et al., 2010; Nain et al., 2012). The glycation of antioxidant enzymes may be the cause of this behavior(Yan and Harding, 1997). The current experiment showed that Ajwa seed-treated diabetic rats have a substantial increase in liver SOD and CAT levels.
These results may be attributed to Ajwa seeds’ ability to inhibit the glycation of the antioxidant enzymes and scavenge the ROS due to the plenty of powerful antioxidants, including flavonoids and phenolic active components (Habib et al., 2014).According to many studies, Ajwa seeds contain many antioxidants as they constitute a wealthy source of total polyphenols(Al-Farsi et al., 2007; Habib et al., 2014). Ajwa seeds have a significant polyphenol content, equivalent to 51 g/kg, higher than in the fruit. Besides that, catechins and flavanones, among the polyphenolic phytochemicals present in Ajwa seeds, possess a good absorption. This confirms a perfect bioavailability of Ajwa seeds’ polyphenols(Habib et al., 2014).TNF-α and other inflammatory cytokines are essential in the advancement of diabetes-induced liver inflammation(Chen et al., 2020; Soufi et al., 2012). Oxidative stress has also been shown to encourage the expression of NF-kB, which increases the production of pro inflammatory cytokines, including TNF-α (Arican et al., 2005; Chen et al., 2020).
Based on this evidence, targeted therapies that can tightly regulate blood glucose, minimize oxidative stress and inhibit pro-inflammatory cytokines are supposed to be effective in preventing diabetic complications(Chen et al., 2020).The present study findings demonstrated the ability of Ajwa seeds to decrease the concentrations of inflammatory mediators (TNF-α and NF-κB) and their ability to reduce the expression of NF-κBprotein in the hepatic tissue of hyperglycemic rats; these confirm their hepatoprotective action via inhibition of inflammation. A recently published study suggested that Ajwa seeds’ active constituents may prevent various inflammatory mediators (Bouhlali et al., 2020).
Studies have suggested that Ajwa seeds’ active constituents may prevent various inflammatory mediators. According to their research, rutin, quercetin, p-coumaric, and caffeic acids were the main polyphenols among the analyzed phenolic compounds abundant in Ajwa seeds. They found a significant correlation between the anti-inflammatory effect of Ajwa seeds and most of the fore mentioned polyphenols (Bouhlali et al., 2020).Ajwa seed extract was found to significantly lower the levels of many pro inflammatory cytokines, including TNF-α(Al-Rasheed et al., 2015; S Saryono et al., 2019). Ajwa seeds can also suppress the pro inflammatory mediator (TNF-α) and inhibit NF-kB translocation (Saryono et al., 2019).
STZ-induced liver injury in diabetic animals has accompanied increased hepatocyte apoptosis, which is proven by enhanced biochemical factors implicated in both the extrinsic and intrinsic mechanisms of apoptosis, as shown by the upregulation of Fas/FasL/caspase-3 and Bax/Bcl2 proteins expression (Rodríguez et al., 2018).In STZ-nicotinamide induced hyperglycemic rats, the hepatic expression levels of anti-apoptotic markers, p-PI3K and Bcl-2 were down-regulated, while that of pro-apoptotic markers, cytochrome c, and cleaved caspase-3 were upregulated (Asokan et al., 2019).The present study results showed that Ajwa seeds can lowered the hepatic protein expression of pro-apoptotic marker, caspase 3, which might be another hepatoprotective mechanism of Ajwa seeds.The inhibitory effect of Ajwafruit extract on the hepatic apoptotic cell death may be related to their antioxidant activity and down-regulating the pro-apoptotic factor caspase-3 (Elsadek et al., 2017).
CONCLUSION
Ajwa seeds suspension significantly lowered STZ-induced hyperglycemia by increasing insulin secretion. Ajwa seeds suspension dramatically protects the liver against STZ-induced damage in diabetic rats and significantly increased antioxidants factors in the liver. Moreover, Ajwa seeds suspension successfully ameliorated STZ-induced liver damage by suppressing liver inflammation, as indicated by the decreased TNF and NFKB levels. It also lowered the expression of NFKB as well as the pro apoptotic marker, caspase-3 proteins.
Conflict of interest: No conflict of interest has been reported.
Ethical Statement: The Biomedical Ethics Research Council, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia, approved the study’s procedure (346-19).
REFERENCES
Abdelaziz D, Ali S and Mostafa M (2015) Phoenix dactylifera seeds ameliorate early diabetic complications in streptozotocin-induced diabetic rats. Pharmaceutical Biology 53(6). Informa Healthcare: 792–799. DOI: 10.3109/13880209.2014.942790.
Afrin R, Arumugam S, Soetikno V, Soetikno V, Thandavarayan, RA, Pitchaimani, V, Karuppagounder, V. Sreedhar R, Harima M, Suzuki H, Miyashita S, Nomoto M, Suzuki K and WatanabeK (2015) Curcumin ameliorates streptozotocin-induced liver damage through modulation of endoplasmic reticulum stress-mediated apoptosis in diabetic rats. Free Radical Research 49(3). Informa Healthcare: 279–289. DOI: 10.3109/10715762.2014.999674.
Al-Alawi R, Al-Mashiqri J, Al-Nadabi J, Al-Shihi BI and Baqi Y (2017) Date Palm Tree (Phoenix dactylifera L.): Natural Products and Therapeutic Options. Frontiers in Plant Science 8. Frontiers Media S.A.: 845. DOI: 10.3389/fpls.2017.00845.
Al-Attar A and Alsalmi F (2019) Effect of Olea europaea leaves extract on streptozotocin induced diabetes in male albino rats. Saudi Journal of Biological Sciences 26(1). Elsevier B.V.: 118–128. DOI: 10.1016/j.sjbs.2017.03.002.
Al-Farsi M, Alasalvar C, Al-Abid M, Al-Shoaily K, Al-Amry M and Al-Rawahy F (2007) Compositional and functional characteristics of dates, syrups, and their by-products. Food Chemistry 104(3). Elsevier: 943–947. DOI: 10.1016/j.foodchem.2006.12.051.
Al-Harrasi A, Rehman N, Hussain J,Khan A, Al-Rawahi A, Gilani SA, Al-Broumi M and Ali L(2014) Nutritional assessment and antioxidant analysis of 22 date palm (Phoenix dactylifera) varieties growing in Sultanate of Oman. Asian Pacific Journal of Tropical Medicine 7(S1). Elsevier (Singapore) Pte Ltd: S591–S598. DOI: 10.1016/S1995-7645(14)60294-7.
Al-Rasheed N, Attia H, Mohamad R, Al-Rasheed NM, Al-Amin MA and Al-Onazi A(2015) Aqueous date flesh or pits extract attenuates liver fibrosis via suppression of hepatic stellate cell activation and reduction of inflammatory cytokines, transforming growth factor- β 1 and angiogenic markers in carbon tetrachloride-intoxicated rats. Evidence-based Complementary and Alternative Medicine 2015. Hindawi Publishing Corporation: 247357. DOI: 10.1155/2015/247357.
Al-Thobaiti S and Abu Zeid I (2019) Hepatoprotective and antioxidant effects of methanolic extracts of Balanites aegyptiaca against streptozotocin-induced liver damage in rats. Journal of Applied Sciences Research 13(6). American-Eurasian Network for Scientific Information (AENSI): 13–27. DOI: 10.22587/jasr.2019.15.6.2.
Arican O, Aral M, Sasmaz S and Ciragil P(2005) Serum levels of TNF-α, IFN-γ, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators of Inflammation 2005(5): 273–279. DOI: 10.1155/MI.2005.273.
Asokan S, Wang R, Hung T and Lin WT(2019) Hepato-protective effects of Glossogyne tenuifolia in Streptozotocin-nicotinamide-induced diabetic rats on high fat diet. BMC Complementary and Alternative Medicine 19(1). BioMed Central Ltd.: 117. DOI: 10.1186/s12906-019-2529-1.
Bouhlali E, Hmidani A, Bourkhis B, Khouya T, Ramchoun M, Filali-Zegzouti Y and Alem C(2020) Phenolic profile and anti-inflammatory activity of four Moroccan date (Phoenix dactylifera L.) seed varieties. Heliyon 6(2). Elsevier Ltd: e03436. DOI: 10.1016/j.heliyon.2020.e03436.
Brownlee M (2005) The pathobiology of diabetic complications: A unifying mechanism. In: Diabetes, June 2005, pp. 1615–1625. Diabetes. DOI: 10.2337/diabetes.54.6.1615.
Chaudhury A, Duvoor C, Reddy Dendi V, Kraleti S, Chada A, Ravilla R, Marco A, ShekhawatNS, Montales MT, Kuriakose K, Sasapu A, Beebe A, Patil, N, Musham CK, Lohani GP and Mirza W (2017) Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Frontiers in Endocrinology 8. Frontiers Media SA: 6. DOI: 10.3389/fendo.2017.00006.
Chen L, Yao M, Fan X, Lin X, Arroo R, Silva A, Sungthong B, Dragan S, Paoli P, Wang S, Teng H and Xiao J (2020) Dihydromyricetin Attenuates Streptozotocin-induced Liver Injury and Inflammation in Rats via Regulation of NF-κB and AMPK Signaling Pathway. eFood 1(2). Atlantis Press: 188–195. DOI: 10.2991/efood.k.200207.001.
Dewidar B, Kahl S, Pafili K and Roden M (2020) Metabolic liver disease in diabetes – From mechanisms to clinical trials. Metabolism: Clinical and Experimental. W.B. Saunders. DOI: 10.1016/j.metabol.2020.154299.
El-Sayed M, Al-Massarani S, El Gamal A, El-Shaibany A and Al-Mahbashi HM (2020) Mechanism of antidiabetic effects of Plicosepalus acaciae flower in streptozotocin-induced type 2 diabetic rats, as complementary and alternative therapy. BMC Complementary Medicine and Therapies 20(1). BioMed Central Ltd: 290. DOI: 10.1186/s12906-020-03087-z.
Elsadek B, El-Sayed E, Mansour A and Elazab A(2017) Abrogation of carbon tetrachloride-induced hepatotoxicity in Sprague-Dawley rats by Ajwa date fruit extract through ameliorating oxidative stress and apoptosis. Pakistan Journal of Pharmaceutical Sciences 30(6): 2183–2191. Available at: https://pubmed.ncbi.nlm.nih.gov/29175788/ (accessed 22 March 2021).
Eoin L (2016) Systematics: Blind dating. Nature Plants 2(5). Springer Science and Business Media LLC: 16069. DOI: 10.1038/nplants.2016.69.
Garcia-Compean D, Jacquez-Quintana J, Gonzalez-Gonzalez J and Maldonado-Garza H(2009) Liver cirrhosis and diabetes: Risk factors, pathophysiology, clinical implications and management. World Journal of Gastroenterology. Baishideng Publishing Rats Inc. DOI: 10.3748/wjg.15.280.
Giacco F and Brownlee M (2010) Oxidative stress and diabetic complications. Circulation Research. NIH Public Access. DOI: 10.1161/CIRCRESAHA.110.223545.
Habib H and Ibrahim W (2011) Effect of date seeds on oxidative damage and antioxidant status in vivo. Journal of the Science of Food and Agriculture 91(9). J Sci Food Agric: 1674–1679. DOI: 10.1002/jsfa.4368.
Habib H, Platat C, Meudec E, Cheynier V and Ibrahim WH (2014) Polyphenolic compounds in date fruit seed (Phoenix dactylifera): Characterisation and quantification by using UPLC-DAD-ESI-MS. Journal of the Science of Food and Agriculture 94(6). John Wiley and Sons Ltd: 1084–1089. DOI: 10.1002/jsfa.6387.
Harrison S (2006) Liver disease in patients with diabetes mellitus. Journal of Clinical Gastroenterology. J Clin Gastroenterol. DOI: 10.1097/01.mcg.0000190774.91875.d2.
Hasan M and Mohieldein A (2016) In vivo evaluation of anti diabetic, hypolipidemic, antioxidative activities of saudi date seed extract on streptozotocin induced diabetic rats. Journal of Clinical and Diagnostic Research 10(3). Journal of Clinical and Diagnostic Research: FF06-FF12. DOI: 10.7860/JCDR/2016/16879.7419.
Heindel J, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, Nadal A, Palanza P, Panzica G, Sargis R, Vandenberg LN and vom Saal F (2017) Metabolism disrupting chemicals and metabolic disorders. Reproductive Toxicology 68. Elsevier Inc.: 3–33. DOI: 10.1016/j.reprotox.2016.10.001.
Khan F, Khan T, Kalamegam G, Pushparaj PN, Chaudhary A, Abuzenadah A, Kumosani T, Barbour E and Al-Qahtani M (2017) Anti-cancer effects of Ajwa dates (Phoenix dactylifera L.) in diethylnitrosamine induced hepatocellular carcinoma in Wistar rats. BMC Complementary and Alternative Medicine 17(1). BioMed Central Ltd.: 418. DOI: 10.1186/s12906-017-1926-6.
Kooti W, Farokhipour M, Asadzadeh Z, Ashtary-Larky D and Asadi-Samani M (2016) The role of medicinal plants in the treatment of diabetes: a systematic review. Electronic physician 8(1). Mehr Publishing Rats: 1832–1842. DOI: 10.19082/1832.
Manna P, Das J, Ghosh J, and Sil PC (2010) Contribution of type 1 diabetes to rat liver dysfunction and cellular damage via activation of NOS, PARP, IκBα/NF-κB, MAPKs, and mitochondria-dependent pathways: Prophylactic role of arjunolic acid. Free Radical Biology and Medicine 48(11). Free Radic Biol Med: 1465–1484. DOI: 10.1016/j.freeradbiomed.2010.02.025.
Meng XM, Ma XX, Tian YL, Jiang Q, Wang LL, Shi R, Ding L and Pang SG (2017) Metformin improves the glucose and lipid metabolism via influencing the level of serum total bile acids in rats with streptozotocin-induced type 2 diabetes mellitus. European review for medical and pharmacological sciences 21(9): 2232–2237. Available at: https://pubmed.ncbi.nlm.nih.gov/28537659/ (accessed 21 March 2021).
Meo S, Usmani A and Qalbani E (2017) Prevalence of type 2 diabetes in the Arab world: impact of GDP and energy consumption. European review for medical and pharmacological sciences 21(6): 1303–1312. Available at: https://pubmed.ncbi.nlm.nih.gov/28387897/ (accessed 19 March 2021).
Meo S, Sheikh S, Sattar K, Akram A, Hassan A, Meo AS, Usmani AM, Qalbani E and Ullah A (2019) Prevalence of Type 2 Diabetes Mellitus Among Men in the Middle East: A Retrospective Study. American Journal of Men’s Health 13(3). SAGE Publications Inc.: 1–9. DOI: 10.1177/1557988319848577.
Mohamed J, Nazratun Nafizah A, Zariyantey A and Budin SB(2016) Mechanisms of diabetes-induced liver damage: The role of oxidative stress and inflammation. Sultan Qaboos University Medical Journal. Sultan Qaboos University. DOI: 10.18295/squmj.2016.16.02.002.
Mrabet A, Jiménez-Araujo A, Guillén-Bejarano R, Rodríguez-Arcos R and Sindic M (2020) Date seeds: A promising source of oil with functional properties. Foods. MDPI Multidisciplinary Digital Publishing Institute. DOI: 10.3390/foods9060787.
Nain P, Saini V, Sharma S and Nain J (2012) Antidiabetic and antioxidant potential of Emblica officinalis Gaertn. leaves extract in streptozotocin-induced type-2 diabetes mellitus (T2DM) rats. Journal of Ethnopharmacology 142(1). J Ethnopharmacol: 65–71. DOI: 10.1016/j.jep.2012.04.014.
Naseri R, Navabi S, Samimi Z, Mishra AP, Nigam M, Chandra H, Olatunde A, Tijjani H, Morais-Urano RP and Farzaei MH (2020) Targeting Glycoproteins as a therapeutic strategy for diabetes mellitus and its complications. DARU, Journal of Pharmaceutical Sciences. Springer. DOI: 10.1007/s40199-020-00327-y.
Nematallah K, Ayoub N, Abdelsattar E, Meselhy MR, Elmazar MM, El-Khatib AH, Linscheid MW, Hathout RM, Godugu K, Adel A and Mousa SA (2018) Polyphenols LC-MS2 profile of Ajwa date fruit (Phoenix dactylifera L.) and their microemulsion: Potential impact on hepatic fibrosis. Journal of Functional Foods 49. Elsevier Ltd: 401–411. DOI: 10.1016/j.jff.2018.08.032.
Rodríguez V, Plavnik L and Tolosa de Talamoni N (2018) Naringin attenuates liver damage in streptozotocin-induced diabetic rats. Biomedicine and Pharmacotherapy 105. Elsevier Masson SAS: 95–102. DOI: 10.1016/j.biopha.2018.05.120.
Sarfraz M, Khaliq T, Khan J and Aslam B(2017) Effect of aqueous extract of black pepper and ajwa seed on liver enzymes in alloxan-induced diabetic Wister albino rats. Saudi Pharmaceutical Journal 25(4). Elsevier B.V.: 449–452. DOI: 10.1016/j.jsps.2017.04.004.
Saryono, Dardjito E, Proverawati A, Sumeru A, Setiyani R, Upoyo AS and Kamaludin R (2019) Date seeds (Phoenix dactylifera L.) consumption as anti-inflammatory and immunostimulant: A systematic review. In: IOP Conference Series: Earth and Environmental Science, 5 April 2019, p. 012038. Institute of Physics Publishing. DOI: 10.1088/1755-1315/250/1/012038.
Saryono S, Taufik A, Proverawati A and Efendi F(2019) Dietary supplementation of Phoenix dactylifera L. Seeds decreases pro-inflammatory mediators in CCl4-induced rats. Journal of HerbMed Pharmacology 8(3). Nickan Research Institute: 212–217. DOI: 10.15171/jhp.2019.31.
Soufi F, Vardyani M, Sheervalilou R, Mohammadi M and Somi MH (2012) Long-term treatment with resveratrol attenuates oxidative stress pro-inflammatory mediators and apoptosis in streptozotocin-nicotinamide-induced diabetic rats. General physiology and biophysics 31(04): 431–438. DOI: 10.4149/gpb_2012_039.
Temitope Idowu A, Osarumwense Igiehon O, Ezekiel Adekoya A and Idowu S (2020) Dates palm fruits: A review of their nutritional components, bioactivities and functional food applications. AIMS Agriculture and Food 5(4). American Institute of Mathematical Sciences (AIMS): 734–755. DOI: 10.3934/agrfood.2020.4.734.
Yan H and Harding J (1997) Glycation-induced inactivation and loss of antigenicity of catalase and superoxide dismutase. Biochemical Journal 328(2). Portland Press Ltd: 599–605. DOI: 10.1042/bj3280599.
Zhang M, Lv X, Li J, Xu ZG and Chen L (2008) The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Experimental diabetes research 2008. Exp Diabetes Res: 704045. DOI: 10.1155/2008/704045.


