1Department of Biotechnology, Sri Kaliswari College, Sivakasi, Tamil Nadu, India.
2Department of Life sciences, Kristu Jayanti College, Bangalore, Karnataka, India.
3Department of Physics, Kamaraj College of Engineering and Technology, Virudhunagar, Tamil Nadu, India.
Correspondin gauthor email: smileeyes.2320@gmail.com
Article Publishing History
Received: 19/10/2021
Accepted After Revision: 19/12/2021
The use of nanoparticles as drug carriers has been investigated, and it offers various benefits, including the controlled and targeted release of loaded or associated drugs, as well as enhanced drug bioavailability. They do, however, have certain disadvantages, such as in vivo toxicity, which affects all organs, including healthy ones, and overall disease treatment improvement, which might be undetectable or limited. Silver nanoparticles are being studied more and more due to their unique physical, chemical, and optical properties, which allow them to be used in a variety of applications, including drug delivery to specific targets in the body.
Given the constraints of traditional cancer treatment, such as low bioavailability and the resulting usage of high doses that produce side effects, attempts to address these challenges are essential. In this work, Biocompatible Silver nanoparticles (AgNps) loaded with tamoxifen have been prepared using the gelation process. Tamoxifen-loaded green synthesized AgNps are reported to be amorphous. The phytochemicals present in the extract of Hemionitis arifolia leaf were responsible for the reduction of silver nitrate to AgNPs. The functional groups existing in the particles were identified with FT-IR analysis. XRD analysis state that the particles were crystalline in nature and arranged in quartzite crystal.
Particle size and shape were illustrated from SEM analysis and revealed that the particles were amorphous in nature. UV-visible spectrophotometer showed the band around 440nm which was identified as “surface Plasmon resonance band”. The synthesized AgNps loaded with tamoxifen were significantly effective against Human breast cancer cells. The silver nanoparticle loaded with tamoxifen was found to be inducing apoptotic signals in the selected cells. It inhibits the breast cancer cells even at the lower concentration of AgNPs and TAM-AgNPs. Further apoptotic studies (AO/EtBr and DAPI) reveal that cell death is due to the fragmentation of nuclear material of the treated cells.
Apoptosis, Breast Cancer, Hemionitis Arifolia, Silver Nanoparticles, Tamoxifen.
Varadharajaperumal P, Muthuswamy S, Thiruvengadam S, Muthuswamy S, Mahalingam S. Biosynthesised Drug-Loaded Silver Nanoparticles: A Vivid Agent For Drug Delivery On Human Breast Carcinoma. Biosc.Biotech.Res.Comm. 2021;14(4).
Varadharajaperumal P, Muthuswamy S, Thiruvengadam S, Muthuswamy S, Mahalingam S. Biosynthesised Drug-Loaded Silver Nanoparticles: A Vivid Agent For Drug Delivery On Human Breast Carcinoma. Biosc.Biotech.Res.Comm. 2021;14(4). Available from: <a href=”https://bit.ly/31eKDO1“>https://bit.ly/31eKDO1</a>
Copyright © Varadharajaperumal et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Cancer is an aggressive phase of cell proliferation in which the cells have a relatively uncontrolled division that results in a gradual increase in dividing cell count (Devi et al. 2012). Cancer is one of the world’s leading deaths with an estimated 13.1 million deaths expected in 2030. It is the second most prevalent condition for overall deaths in the world after coronary diseases. There are nearly 23% of deaths in the US and 7% in India. Various cancers are found based on their tissue location (Parveen et al. 2015).
One of the most prevalent cancers in women is breast cancer; about 1.7 million new women are diagnosed with breast cancer worldwide. While substantial progress has been made in breast cancer care in recent decades, existing approaches to therapy remain constrained by a non-specific systemic delivery, inadequate drug concentrations reaching the tumor, and multidrug resistance. The advent of nanotechnology has revolutionized the arena of cancer diagnosis and treatment and nanotechnology in medicine helps to resolve chemotherapy limitations (Su et al. 2021).
Nanoparticles with optimized surface properties can more efficiently migrate within tumor cells delivering a high amount of drugs with significantly reduced toxicity. The applications of Nanotechnology could revolutionize the paradigm of breast cancer treatment in the future (Varadharajaperumal et al. 2017). Metallic nanoparticles have been intrigued for over a century and are now used widely in biomedical sciences and engineering (Pradeepa et al. 2017).
Among metal nanoparticles, silver nanoparticles (AgNps), in particular, are attracted to the intensive research interest for important applications in medical industries, catalysis, and surface-enhanced scattering (Chahardoli et al. 2017). AgNps have developed as a superior product in the field of nanotechnology (Nqakala et al. 2021).
AgNps have been used as vehicles for the controlled release of drugs as it is acting as a carrier of drugs and deliver them on a selective basis. AgNps are magic “bullets” that go directly to cells of a particular tissue (Pradeepa et al. 2017). Synthesis of nanoparticles by various physical and chemical methods like laser ablation, lithography, chemical vapor deposition, sol-gel technique, and electro-deposition is very expensive and highly toxic.
Bio-material scientists also look forward to improving the synthesis of metal nanoparticles with simple, nontoxic additives, environmentally benign solvents, and renewable materials (Emmanuel et al. 2015). Green synthesis or biological synthesis of nanoparticles is an alternative for the physical and chemical methods of synthesis of nanoparticles (Singh et al. 2017). Biogenic nanoparticles are the organic synthesis of nanoparticles by biological approaches such as the use of enzymes, microbes, fungus, plant, or plant extracts (Soni et al. 2021).
In particular, in recent years, the biological synthesis of plant-based nanoparticles has received far more attention as it is cost-effective and also overcomes the downside of retaining pure microbial cultures as needed in microbial methods. Therefore, green synthesis is the latest approach used for large-scale nanoparticle synthesis (Iravani 2011). Besides, biologically synthesized AgNPs have been tested for possible cytotoxic effects in human breast cancer cells (MCF-7) using cell viability, membrane integrity, ROS generation, and DNA fragmentation (Gurunathan et al. 2013).
In our research, the Green Nanoparticle Synthesis was performed using the leaf extract of Hemionitis Arifolia. Hemionitis arifolia (Burm.f.) T.moore. of family Hemionitidaceae is the endemic and widely distributed species on western ghats of India. It has been made use of for the treatment of burns, menstrual disorders, antifertility, and anti-flatulence. Hemionitis arifolia frond juice was evaluated for its hypoglycaemic and anti-diabetic properties using rats and has been used to cure burns and a folklore anti- diabetes fern. The medicinal significance of Hemionitis arifolia is due to the presence of Alkaloids, Flavonoids, Phenols, Tannins, and Saponins (Sureshkumar et al. 2021).
Tamoxifen is an anti-cancer drug that is widely used for breast cancer, carcinoma, osteosarcoma, and sarcoma of soft tissues. The effectiveness of tamoxifen in the treatment of different forms of cancers is considerably limited by its significant side effects. The initial side effects due to tamoxifen administration include less severe symptoms such as nausea, fatigue, myelo-repression, and arrhythmia, which are typically reversible.
Nevertheless, cardiomyopathy associated with tamoxifen and congestive heart failure has raised considerable concern among health practitioners. The development of nanoparticle-based therapeutic delivery systems is a highly deliberated approach to improving effectiveness and reducing the adverse effects induced by anti-cancer agents such as Tamoxifen (Pradeepa et al. 2017; Sureshkumar et al. 2021).
The objective of the study was to explore a novel Tamoxifen delivery system based on green synthesized AgNPs from Hemionitis arifolia leaf extract that is environmentally friendly and to characterize the silver nanoparticles using UV Visible Spectroscopy, SEM, XRD, EDAX, and FTIR. This form of Tamoxifen delivery system could offer a fast and convenient way to enable the controlled release of drugs and enrich its chemotherapeutic efficiency. To be the best of my knowledge, no study has been carried out on Tamoxifen-loaded AgNPs for stimuli-sensitive drug delivery systems. In this study, Tamoxifen was loaded on green synthesized AgNps to construct drug-loaded nanoparticles as a drug delivery system in MCF-7 breast cancer cells.
Material and Methods
MTT-3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-tetrazolium bromide, Acridine orange, ethidium bromide, DAPI (4′, 6-diamidino-2-phenylindole, dihydrochloride) stain, and dimethyl sulfoxide (DMSO) were purchased from Hi-media laboratories Pvt. Ltd. DMEM medium, fetal bovine serum, penicillin, streptomycin, Chitosan (purified viscosity grade 50 cps; MW150 kDa; deacetylation degree 85%), Fluorescent Isothiocyanate (FITC), Propidium iodide (PI) were purchased from Sigma–Aldrich (USA).
The Breast Cancer Cell line (MCF-7) was procured from National Center for Cell Science (NCCS, Pune) and was maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) with 1% antibiotic-antimycotic solution. Cells were grown to 100% confluence at 37°C, with a humidity of 5% (v/v) CO2 atmosphere. A known number of cells were seeded into tissue culture plates and maintained for further studies.
The leaves of Hemionitis arifolia were washed several times with water to remove the dust particles and shade dried at room temperature. The dried leaves were made into a fine powder. The leaf powder was weighed and 50g was taken for synthesis purposes. The powder was packed using Whatman No. 1 filter paper (pore size 25μm) (Varadharajaperumal et al. 2017).
The plant aqueous extract was obtained from 5g leaves shade-dried powder dissolved in 100ml of DDW (Double Distilled Water) with boiling 60°C at 20 mins. The extract was cooled to room temperature and filtered using a Buchner funnel (Varadharajaperumal et al. 2017). Silver nitrate was used as a precursor material for nanoparticle synthesis. Silver nitrate was dissolved in de-ionized water under constant stirring. The 50% and 25% of plant extract were prepared with de-ionized water and the volume was made up to 250ml. 50ml of Silver nitrate solution was added along with 20ml of Hemionitis arifolia leaves extract under constant stirring.
The pH of the mixture was maintained at 6. This mixture of the solution was kept under vigorous stirring at 60ºC for 4-5h in a magnetic stirrer. After this process, a green-yellow precipitate was obtained. This mixture was centrifuged at 2500 rpm for 15 min and the green precipitate was discarded. The supernatant was stirred again at 2500 rpm for 15 min along with distilled water twice. A dark yellow-brown color solid precipitate was obtained. The precipitate was washed with methanol and air-dried (Varadharajaperumal et al. 2017).
UV–Vis spectral analysis was done by using Shimadzu UV visible spectrophotometer (UV-1800, Japan). UV–Visible absorption spectrophotometer with a resolution of 1 nm between 200 and 800 nm was used. One milliliter of the sample was pipetted into a test tube and subsequently analyzed at room temperature. Fourier transforms infrared (FT-IR) spectroscopy of AgNP was performed by using a Nicolet 5700 instrument (Nicolet Instrument, Thermo Company, USA) with the KBr pellet method.
Each KBr disk was scanned over a wavenumber region of 500-4000 cm-1. The method of X-ray diffraction (XRD) was used to investigate the crystalline structure of AgNP nanoparticles. The XRD analysis was conducted with a Philips PW 17291 powder X-ray diffractometer with a voltage of 40kV and a current of 25mA. The scanning rate employed was 1min−1 over the 10-80o 2θ range (Varadharajaperumal et al. 2017).
The samples were placed on a polycarbonate substrate and the excess water was left to dry at room temperature. They were then dried in a critical point dryer using carbon dioxide, and sputter-coated with gold in a metallizer, and examined under a scanning electron microscope (JSM5600LV, JEOL, Japan) operating at an accelerating voltage of 20 kV (Varadharajaperumal et al. 2017).
Tamoxifen conjugated Silver NPs were prepared by a co-precipitation method with minor modifications. In general, Tamoxifen is virtually insoluble in water but it is soluble in ethanol. The required amount of Tamoxifen was dissolved in 3 ml of ethanol. Then, 100mg of Silver NPs were dissolved in 30 ml of dimethyl sulfoxide (DMSO) and de-ionized water under ultrasonication. Subsequently, the Tamoxifen solution was added to the above mixture and the ultrasonication was continued and allowed to stir for 24 h at room temperature.
The pH of the solution was adjusted to 10 by adding KOH solution. The resulting black-colored precipitate was washed with distilled water to remove any unbound drug or any other organic impurities. The resultant drug-loaded Silver NPs were collected using a lyophilizer (Pradeepa et al. 2017).
Tamoxifen-loaded AgNPs (3mg) were dispersed into 6ml of phosphate buffer solution (PBS) and centrifuged at 12,000 pm for 30min. The supernatant was collected to measure the ultraviolet absorption at 280nm. The loading efficiency and encapsulation efficiency of the Tamoxifen-loaded Silver NPs were calculated as follows.
Loading efficiency = W0/W x 100% Encapsulation efficiency = W0/W1 x 100%
Where W0 is the amount of Tamoxifen contained in the Silver NPs, W is the amount of Silver NPs, and W1 is the amount of Tamoxifen added into the system. The release behavior of Tamoxifen from nanoparticles was investigated. Tamoxifen-loaded AgNPs were dispersed in PBS (pH 7.4, 5 ml) and transferred into a dialysis bag.
The dialysis bag was then immersed in 95 ml of PBS at pH 4.0 and 7.4. The release medium was continuously agitated with a stirrer at 50 g and 37 ºC. At predetermined time intervals, 2 ml of the external medium was collected and replaced with the same fresh PBS. The amount of released Tamoxifen in the medium was then determined at 254 nm (Pradeepa et al. 2017).
To evaluate the cytotoxicity of Tamoxifen-loaded Silver nanoparticles the following experiments were carried out quadruplicate wells of confluent monolayers of MCF- 7 cells were grown in 96-well tissue culture plates. Cells were incubated with different concentrations of silver nanoparticles. Then, we examined cell viability, as the ability of the cells to cleave the tetrazolium salt MTT [3-(4, 5-dimethylthiazol-2ol)-2,5diphenyl tetrazolium bromide), Sigma Chem. Co. St. Louis, USA], by the mitochondrial enzyme succinate dehydrogenase which develops a formazan crystal. Each concentration was replicated three times.
The 50% inhibitory concentration (IC50) was needed as the test compound concentration required for reduction of cell viability by 50% was calculated by regression analysis. In the second experiment, plating efficiency was checked with the subtoxic dose of NPs. MCF- 7 cells were used as a control for the anti-proliferation assay. The MCF- 7 cells that were grown on coverslips (1×105 cells/coverslip) were incubated for 6-24h with Tamoxifen-loaded Silver nanoparticles at the IC50 concentration, and they were then fixed in ethanol: acetic acid solution (3:1; v/v). The coverslips were gently mounted on glass slides for the morphometric analysis.
Three monolayers per experimental group were photomicrographed. The morphological changes of the MCF- 7 cells were analyzed using Nikon (Japan) bright-field inverted light microscopy at 40 x magnifications. Approximately 1μL of a dye mixture of 100 mg/mL acridine orange (AO) and 100 mg/mL ethidium bromide (EtBr) in distilled water was mixed with 9 mL of cell suspension (1×105 cells/mL) on clean microscope coverslips.
The MCF- 7 cells were collected, washed with phosphate-buffered saline (PBS) (pH 7.2), and stained with 1 mL of AO/EtBr. After incubation for 2 min, the cells were washed twice with PBS (5 min each) and visualized under a fluorescence microscope (Nikon Eclipse, Inc, Japan) at 400x magnification with an excitation filter at 480 nm. The percentage of apoptotic cells was determined using the following formula
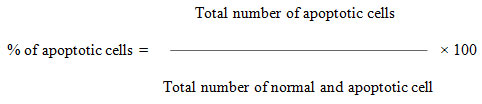
MCF-7 cells were grown (1× 105 cells/coverslip) and incubated with Tamoxifen-loaded Silver nanoparticles at their IC50 concentration and then they were fixed with methanol: acetic acid (3:1, v/v) before washing with PBS. The washed cells were then stained with 1 mg/ml DAPI (4, 6-diamidino-2-phenylindole, dihydrochloride) for 20 min in the dark. Stained images were recorded with a fluorescent microscope with an appropriate excitation filter (Pradeepa et al. 2017).
RESULTS AND DISCUSSION
Over the last few decades, several metallic nanoparticles using biomaterials have been extensively developed for use as drug delivery systems (DDS) and other medical applications (Guo et al. 2011). Silver is a versatile metal that has been used in a drug delivery system as nanoparticles because of its biodegradable, biocompatible, and low toxicity (Dodane et al. 1998).
The positive amine group of silver gives stability to the nanoparticles with anionic material such as gene, drug, protein, and small molecule by electrostatic interaction. Silver nanoparticles, synthesized from natural plant extracts, are cost-effective, eco-friendly, stable, and safe in cancer treatment (Singh et al. 2017; Bharadwaj et al. 2021).
The AgNps were prepared by adding 25mL of an aqueous extract with 475mL of 1M AgNo3. The formation of brown color determined the synthesize of Silver nanoparticles in that solution. The notable color change was observed by visual observation in the Erlenmeyer flask which contains AgNO3 solution with Hemionitis arifolia plant extract. The color of the AgNO3/plant extract solution changed from colorless to light brown and eventually to dark brown.
This color change indicates the formation of AgNPs in the solution. Plant extract without AgNO3 did not show any color changes. The color variation was interpreted as an indication that AgNPs were formed as a result of the reduction of Ag+ ions (Bharadwaj et al. 2021). The formation of Ag Nanoparticles was further confirmed by using UV-visible spectroscopy. The reduction of silver nitrate to silver is examined by the UV-vis spectrum (Figure 1).
Figure 1: UV-Visible spectroscopic analysis of Silver nanoparticles
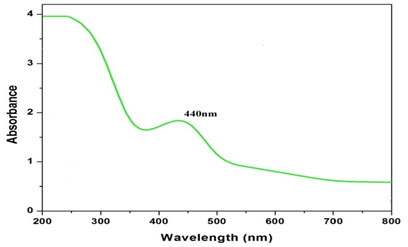
The color change, i.e. light yellow to dark brown, was due to the phenomenon of plasmon resonance, which was the collection of electron oscillation. The band was observed around 440nm which was identified as a “surface plasmon resonance band” and this band was attributed to excitation of valence electrons of AgNPs arranged in the nanoparticles. The shape of the band was symmetrical, suggesting uniform scattering of spherical shape that also confirms that particle existence and stability (Ma et al. 2021).
Thus, from this result, it was concluded that the synthesized AgNPs showed high aqueous stability since a minor reduction in absorbance was observed at 440 nm. A similar type of analysis with almost the same range of results was obtained previously in recent studies of green synthesis of AgNPs using extracts of different plants, such as pomegranate leaves, Benincasa hispida, Prosopis juliflora, Allium cepa, Parkia speciosa and Salvia hispanica (Bharadwaj et al. 2021).
This suggests that the phytochemicals present in Hemionitis arifolia extracted successfully act as reducing and capping agents. Possible biomolecules responsible for the reduction of silver nanoparticle and capping agent of bio-reduced silver NPs through particular bond vibrations peaks coming at defined wave numbers were identified through the FT-IR spectrum (Figure 2).
Figure 2: Fourier Transform – Infrared Spectroscopic analysis of Silver nanoparticles
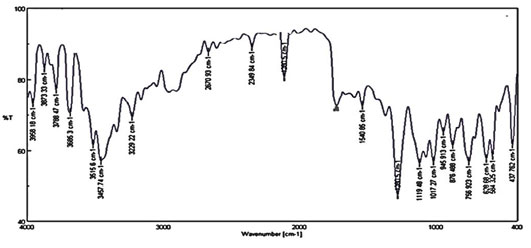
The peak found around 2670.93cm-1 was C=O. The peak found around 1612.2cm-1 was C=C. The peak found around 1522.52cm-1 was a carboxylic group and N-H binding. The peak found around 2349.84 cm-1 was C-O strong bond. The peak found around 1540.78cm-1 was CH3. The peak found around 1280.5 cm-1 was O-H deformation. The absorbance peaks located between 1000 and 1600 cm-1 were assigned to the stretching vibrations of hydroxyl groups (O-H) and amine (N-H). The O-H stretching vibration was characteristic of Polyphenols and N-H stretching was attributed to the presence of amino acids and peptides (David et al. 2014).
The peak found around 1119.48 cm-1, was C-O-C. The peak found around 1017.27 cm-1 was PO4-3 stretching. The peak found around 756.923 cm-1 was CH (Anandalakshmi et al. 2016). Through this, the loaded nanoparticles were surrounded by proteins and metabolites such as terpenoids having functional groups. From the analysis of FT-IR studies, it was confirmed that the carbonyl groups from the amino acid residues and proteins have the stronger ability to bind metal indicating that the proteins could form the metal nanoparticles (Hamouda et al. 2019).
X-ray powder diffraction (XRD) is a prompt analytical technique predominantly used for phase identification of crystalline material. It provides information on unit cell dimensions and is used for the identification of unknown crystalline materials (e.g. minerals, inorganic compounds). In the present study, the XRD pattern (Fig.3) of silver nanoparticles showed that an amorphous pattern of diffraction with the peaks appeared at 2θ values (111), (142), (200) of and (220).
Figure 3: X-Ray power Diffraction analysis of Silver nanoparticles
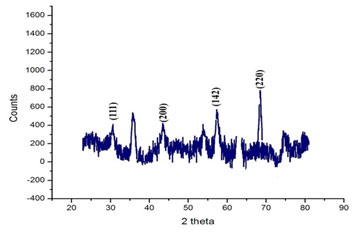
This result corroborated with the XRD analysis reported in previous studies (Garibo et al. 2020). The diffraction patterns showed good agreement with JCPDS(no. 04-0783). Thus, XRD patterns clearly showed the crystalline AgNPs formed by the complete reduction of Ag+ ions by the aqueous extract of Hemionitis arifolia. The other unassigned peak ensued the crystallization of silver nanoparticles along with the organic moieties or impurities that bound to the surface of nanoparticles (Pirtarighat et al. 2019).
FE-SEM images (Fig.4) of synthesized silver nanoparticles at different magnifications provide information on the morphology and particle size of the material. In the present exploration, the size of the particles was 30 nm in diameter and the morphology of particles was nearly like structure due to the electrostatic attraction. The findings clearly show that the synthesised AgNPs material contains fine grain-like particles that agglomerated to form crystals with an almost uniform nearly spherical and a smooth surface. A similar type of findings was previously reported in previous studies (Vinay et al. 2019; Garibo et al. 2020).
Figure 4: Scanning Electron Microscope Analysis of Silver nanoparticles
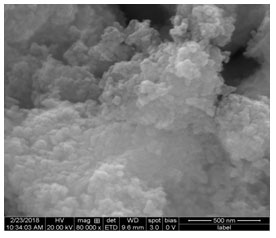
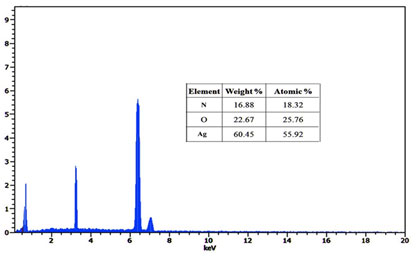
The Energy Dispersive X-ray Spectroscopy attachment present with the SEM was known to provide the chemical composition and densely agglomerated area of the Ag nanoparticles at specific locations (Fig.5). Further, the EDX spectrum shows that the Ag atom exhibited a strong signal, along with the Ag atom the small-signal was exhibited from N and O atoms respectively. Ag NPs show a typical absorption peak at approximately 55.2keV due to the SPR phenomenon. Strong signals from the silver observed in EDX spectra confirmed the presence of elemental silver (Vinay et al. 2019; Gomathi et al. 2020).
Figure 5. Energy-dispersive X-ray Spectroscopy of Silver nanoparticles
The drug loading on AgNPs is done and the amount of drug-loaded is shown in table 1. The result indicates that the amount of drug loading depends on the ratio of drug and carrier. The percentage of drug loading increases as the amount of AgNPs is increased and decreases when the amount of drug is increased with a given amount of AgNPs. The amount of drug-loaded per mg of AgNPs has been calculated.
The amount of drug-loaded per mg of AgNPs decreases with an increase in the AgNPs and it increases with an increase in the amount of drug. At a ratio of 2:1, the maximum amount of drug is loaded per mg of AgNPs. When the amount of drug is increased, there is no further increase in the amount of drug-loaded per mg of AgNPs. 0.7 mg of Tamoxifen is the maximum of the drug-loaded per mg of AgNPs (Vinay et al. 2019; Gomathi et al. 2020).
Table 1. Drug loading percentage for different ratios of tamoxifen and AgNPs
| S.No | Tamoxifen: AgNPs | Drug loading (%) | Mg of Tamoxifen/mg of AgNPs |
|
|
1.1 | 50.6±0.2 | 0.51 |
|
|
1.2 | 65.14±0.5 | 0.33 |
|
|
1.4 | 71.35±0.6 | 0.31 |
|
|
2.1 | 28.5±0.7 | 0.70 |
|
|
4.1 | 15.5±0.8 | 0.62 |
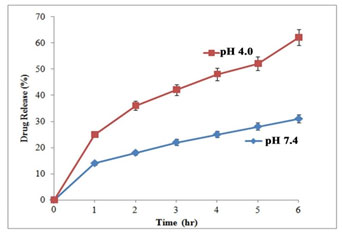
The pH of the medium and release time plays important role in drug release (Fig 6). The slow and sustained drug release was at pH 7.4 with a release ratio of about 26± 0.58% within 48 h. On the other hand, at lower pH Tamoxifen release rate was considerably faster, with approximately 53 ± 0.63% (pH 4.0) of the drug released within 48h. At lower pH, protonation of the drug occurred which released chemisorbed drug molecules into the medium.
The surface charge of AgNps turned positive at lower pH, which facilitated the drug release process and blunted the electrostatic interaction of the drug. These results validate drug release behavior from the novel Tamoxifen Delivery System (Vinay et al. 2019; Gomathi et al. 2020).
Figure 6: In vitro drug release profile of tamoxifen at different pH
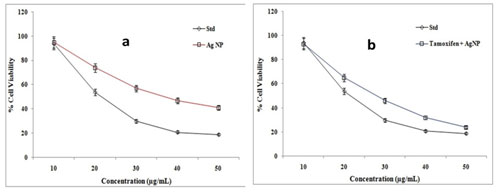
The anti-proliferative potential of the AgNPs and Tamoxifen loaded AgNPs were evaluated by MTT assay (Fig.7). The cell proliferation was inhibited by the nanoparticles in a dose-dependent and time-dependent manner. The IC50 value was calculated from the growth curve obtained by MTT assay. The IC50 value for AgNps, Tamoxifen, and Tamoxifen loaded AgNPs were 33±1.0, 15±0.5, and 24±1.0 respectively. The Tamoxifen loaded AgNPs showed the greatest effectiveness against MCF- 7 cells (Vinay et al. 2019; Gomathi et al. 2020).
Figure 7: MTT analysis of green synthesized AgNPs (a) and tamoxifen
loaded AgNPs (b) on MCF-7 cells
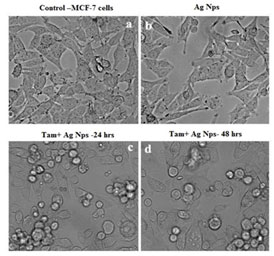
The phase-contrast micrographs supported the MTT results as there was a decrease in cell density when treated with different doses of chitosan nanoparticles (Fig.8). Significant numbers of cells were found to be formless, detached, and floating at higher concentrations of silver in the treated cells.
Figure 8: Morphometric analysis of tamoxifen loaded AgNPs on MCF-7 cells; (a). Control (b). Silver Nanoparticles (c).
Tamoxifen with Silver Nanoparticles (24h) (d). Tamoxifen with Silver Nanoparticles (48h)
Based on the overall cell morphology and the cell membrane integrity, necrotic and apoptotic cells were distinguished from one another using fluorescence microscopy (Nikon Eclipse, Inc., Japan). The apoptotic bodies were detected as orange-colored whereas the necrotic cells were observed to be red. A fluorescence microscopic analysis demonstrated that untreated MCF- 7 cells were stained with a uniform green fluorescence (Fig. 9).
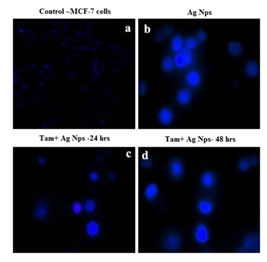
Similarly nuclear dye DAPI also exhibits nuclear fragmentation in treated cells (Fig. 10). The loss of cell adherence property and severe membrane disintegration were found in MCF- 7 cells treated with Silver nanoparticles. This further confirms the particle-induced detachment of cells through activating membrane precipitating proteins. The overall results clearly shows that green synthesized metal nanoparticles can potentially inhibit the proliferation of MCF-7 cells and trigger apoptosis through Bax/Bcl2 and caspase–cascade mediated mitochondrial dysfunction. This research concludes that biogenic metal nano-drug formulations can be utilized for cancer chemotherapy (Jeyaraj et al. 2015; Hamouda et al. 2019; Gomathi et al. 2020).
Figure 9: Apoptotic analysis of tamoxifen loaded AgNPs on MCF-7 cells; (a). Control (b). Silver Nanoparticles (c).
Tamoxifen with Silver Nanoparticles (24h) (d). Tamoxifen with Silver Nanoparticles (48h)
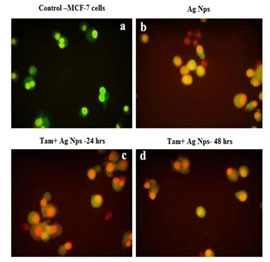
Figure 10: Nuclear fragmentation analysis tamoxifen loaded AgNPs on MCF-7 cells; (a). Control (b). Silver Nanoparticles (c).
Tamoxifen with Silver Nanoparticles(24h) (d). Tamoxifen with Silver Nanoparticles (48h)
CONCLUSION
The findings of the present study are in agreement with the hypothesis that the mechanisms of silver nanoparticles toxicity might be related to the proliferative potential of the cell. The antitumor mechanism of Tam-Ag nanoparticles is related to their membrane disrupting and apoptosis-inducing activities. Based on these findings, we confirm that tamoxifen mediated silver nanoparticles act as an effective anti-cancer agent against all highly proliferating cells, especially the cancerous cells. In conclusion, although the application of silver nanoparticles in cancer therapy looks intriguing and exciting, specific tumor cell targeting will be essential. Therefore, further research is needed to be carried out to explain our mechanism of action.
ACKNOWLEDGEMENTS
The instrumentation facilities for this study was provided by the Department of Biotechnology, Sri Kaliswari College, Sivakasi, Tamil Nadu, India.
Conflict of Interests: Authors declare no conflicts of interests to disclose.
REFERENCES
Anandalakshmi, K., Venugobal, J., and Ramasamy, V. (2016). Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Applied nanoscience, 6(3), 399-408.
Bharadwaj, K. K., Rabha, B., Pati, S et al. (2021). Green Synthesis of Silver Nanoparticles Using Diospyros malabarica Fruit Extract and Assessments of Their Antimicrobial, Anticancer and Catalytic Reduction of 4-Nitrophenol (4-NP). Nanomaterials, 11(8), 1999.
Chahardoli, A., Karimi, N. and Fattahi, A., (2017). Biosynthesis, characterization, antimicrobial and cytotoxic effects of silver nanoparticles using Nigella arvensis seed extract. Iranian journal of pharmaceutical research: IJPR, 16(3), p.1167.
David, L., Moldovan, B., Vulcu, A et al. (2014). Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Colloids and Surfaces B: Biointerfaces, 122, 767-777.
Devi, J.S., Bhimba, B.V. and Ratnam, K., (2012). In vitro anticancer activity of silver nanoparticles synthesized using the extract of Gelidiella sp. Int J Pharm Pharm Sci, 4(4), pp.710-715.
Dodane, V. and Vilivalam, V.D., (1998). Pharmaceutical applications of chitosan. Pharmaceutical Science & Technology Today, 1(6), pp.246-253.
Emmanuel, R., Palanisamy, S., Chen, S.M et al. (2015). Antimicrobial efficacy of green synthesized drug blended silver nanoparticles against dental caries and periodontal disease causing microorganisms. Materials Science and Engineering: C, 56, pp.374-379.
Gomathi, A. C., Rajarathinam, S. X., Sadiq, A. M et al. (2020). Anticancer activity of silver nanoparticles synthesized using aqueous fruit shell extract of Tamarindus indica on MCF-7 human breast cancer cell line. Journal of Drug Delivery Science and Technology, 55, 101376.
Guo, M., Que, C., Wang, C et al. (2011). Multifunctional superparamagnetic nanocarriers with folate-mediated and pH-responsive targeting properties for anticancer drug delivery. Biomaterials, 32(1), pp.185-194.
Gurunathan, S., Han, J.W., Dayem, A.A et al. (2013). Green synthesis of anisotropic silver nanoparticles and its potential cytotoxicity in human breast cancer cells (MCF-7). Journal of Industrial and Engineering Chemistry, 19(5), pp.1600-1605.
Hamouda, R. A., Hussein, M. H., Abo-Elmagd, R. A et al. (2019). Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Scientific reports, 9(1), 1-17.
Iravani, S., (2011). Green synthesis of metal nanoparticles using plants. Green Chemistry, 13(10), pp.2638-2650.
Jeyaraj, M., Renganathan, A., Sathishkumar, G et al. (2015). Biogenic metal nanoformulations induce Bax/Bcl2 and caspase mediated mitochondrial dysfunction in human breast cancer cells (MCF 7). RSC Advances, 5(3), pp.2159-2166.
Ma, Z., Liu, J., Liu, Y. et al. (2021). Green synthesis of silver nanoparticles using soluble soybean polysaccharide and their application in antibacterial coatings. International Journal of Biological Macromolecules, 166, 567-577.
Nqakala, Z. B., Sibuyi, N. R., Fadaka, A. O et al. (2021). Advances in Nanotechnology towards Development of Silver Nanoparticle-Based Wound-Healing Agents. International Journal of Molecular Sciences, 22(20), 11272.
Parveen, A. and Rao, S., (2015). Cytotoxicity and genotoxicity of biosynthesized gold and silver nanoparticles on human cancer cell lines. Journal of Cluster Science, 26(3), pp.775-788.
Pirtarighat, S., Ghannadnia, M., and Baghshahi, S. (2019). Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. Journal of Nanostructure in Chemistry, 9(1), 1-9.
Pradeepa, V., Sriram, T and Sujatha, M., (2017). Studies on Drug Delivery Efficacy of Silver Nanoparticles Synthesized using Human Serum Albumin as Tamoxifen Carriers in MCF-7 Cell Line. International Journal of Science and Research (IJSR) 6,PP. 1881-1888.
Singh, J., Singh, T. and Rawat, M., (2017). Green synthesis of silver nanoparticles via various plant extracts for anti-cancer applications. Nanomedicine, 7(3), pp.1-4.
Soni, V., Raizada, P., Singh, P. et al. (2021). Sustainable and green trends in using plant extracts for the synthesis of biogenic metal nanoparticles toward environmental and pharmaceutical advances: A review. Environmental Research, 111622.
Su, Z., Dong, S., Zhao, S. C et al. (2021). Novel nanomedicines to overcome cancer multidrug resistance. Drug Resistance Updates, 58, 100777.
Sureshkumar, J., Ayyanar, M., and Silambarasan, R. (2021). Ethnomedicinal uses, phytoconstituents and pharmacological importance of pteridophytes used by Malayalis in Kolli hills, India: A quantitative survey. Journal of Herbal Medicine, 25, 100418.
Varadharajaperumal, P., Subramanian, B. and Santhanam, A., (2017). Biopolymer mediated nanoparticles synthesized from Adenia hondala for enhanced tamoxifen drug delivery in breast cancer cell line. Advances in Natural Sciences: Nanoscience and Nanotechnology, 8(3), p.035011.
Vinay, S. P., Nagarju, G., Chandrappa, C. P et al. (2019). Enhanced photocatalysis, photoluminescence, and anti-bacterial activities of nanosize Ag: green synthesized via Rauvolfia tetraphylla (devil pepper). SN Applied Sciences, 1(5), 1-14.


