1Department of Environmental Health, Health Promotion Research Center, School of Public Health, Zahedan University of Medical Sciences, Zahedan, Iran
2Department of Environmental Health, Faculty of Heatlth School, Saveh University of Medical Sciences, Saveh, Iran
Article Publishing History
Received: 11/08/2016
Accepted After Revision: 17/09/2016
Dyes are the main pollutants existing in wastewater of textile industries. This paper presents the sorption studies of Reactive Blue 19 (RB19) dye by the Lemna minor. The effect of different parameters like pH, adsorbent dose, contact time, temperature and initial dye concentrations were investigated. The biosorption data have been analysed using Langmuir, Freundlich and Temkin isotherms. The equilibrium uptake capacity was increase from 7.12 mg/g to 46.51 mg/g, when increasing the dye concentration from 25 mg/L to 200 mg/L. The equilibrium data were best represented by the Langmuir isotherm. The adsorption kinetics were found to follow the pseudo-second-order kinetic model. Thermodynamic parameters such as ΔGo, ΔHo and ΔSo have also been evaluated and sorption process was feasible, spontaneous and exothermic in nature. The results indicate that L. minor is a suitable adsorbent for the adsorption of textile dyes.
Adsorption, Lemna Minor, Reactive Blue 19, Thermodynamics, Isotherms, Kinetics.
Balarak D, Mostafapour F. K, Azarpira H. Biosorption of Reactive Blue 19 Dye Using Lemna Minor: Equilibrium, Kinetic and Thermodynamic Studies. Biosc.Biotech.Res.Comm. 2016;9(3).
Balarak D, Mostafapour F. K, Azarpira H. Biosorption of Reactive Blue 19 Dye Using Lemna Minor: Equilibrium, Kinetic and Thermodynamic Studies. Biosc.Biotech.Res.Comm. 2016;9(3). Available from: https://bit.ly/364IH8h
Introduction
The one of important issues caused by industrialization is the environmental problem which it is made by various contaminants such as dyes, heavy metal, organic pollutants, etc (Cengiz et al., 2012 and Balarak et al., 2016). Various industries such as paper, textile, plastic and food, etc apply the synthetic dyes and produce colorful wastewater which can create problematic condition to environment and human health (Li et al., 2011 and Robinson et al., 2002). Previous studies have indicated that the textile industries are considered as the largest dyes consumer (Ozcan et al., 2007 and Tan et al., 2010). Aesthetic problems are the adverse effect of using the dye which it can occur by discharging the dyes into water body (even in low concentration). Also, they have been shown the toxic to aquatic life The literature review shows the dyes can be toxic due to presence of carcinogen compounds in their structure (Couto, 2009, Ali et al., 2012, Yang et al., 2013 and Balarak et al., 2015).
Therefore the dye removal from water and wastewater is important subject. There are many techniques to remove the dyes from wastewater such as chemical coagulation/flocculation, ozonation, oxidation processes, chemical precipitation, adsorption, ion exchange, reverse osmosis and ultra filtration, etc (Deniz et al., 2011 and Oei et al., 2009). The adsorption and specially adsorption onto activated carbon is promising and efficient in dye removal, however the high cost of activated carbon limits the use of it as adsorbent (Demirbas et al., 2009). Today, various researches are performing to discover the low-cost alternative adsorbent to remove the dyes from wastewaters. Many natural adsorbent such as Canola, Azolla, Wood, Coal, Rice straw have been applied to remove the pollutants (Safa et al., 2011, Zazouli et al., 2014, Ferdag et al., 2009). L. minor is one of the plants which has been used for removal of dyes in several earlier studies (Khataee et al., 2012 and Kiliç et al., 2010). The characteristics of Lemna such as its small size, high multiplication rates and vegetative propagation make it as a proper system to assess the aquatic pollutants (Diyanati et al., 2013 and Zazouli et al., 2014).
Since L. minor can be found widely in Mazandaran and as there are very few studies on L. minor in kinetic analysis of pollution abatement, therefore, the present study was carried out to investigate the RB19 dye removal by dried L. minor. The effects of various parameters including contact time, pH, adsorbent dose, temperature and dye concentration were assessed along with also the isotherm and kinetic studies which were also performed.
Material And Methods
Preparation of adsorbent: L. minor was collected from rice fields of Sari, Iran. It was sun dried then crushed and finally sieved to particle sizes in the range of 1–2 mm. The biomass was treated with 0.1 M HCl for 5 h followed by washing with distilled water and then dried in shade. The resultant biomass was subsequently used in absorption experiments (Padmesh et al., 2006).
Instruments used for characterization: The surface images of dried L. minor before and after adsorption process were captured by scanning electron microscopy (SEM). The SEM used was a Philips XL30. The specific surface area of dried L. minor before use was determined by the BET-N2 method using an ASAP 2000 apparatus based on nitrogen adsorption–desorption isotherms at 77K.
The RB19 dye was obtained from Merck Company (Germany). All chemicals used in this work were analytical reagent grade and used without further purification. Stock solution was prepared by dissolving accurately weighed dye in double distilled water. Experimental solutions of the desired concentrations were obtained by diluting the stock solution by using distilled water. RB19 (C22H16N2Na2O11S3) is a Azo dye, molecular weight of 626.5 g/mol, and its chemical structure is shown in Fig. 1.
 |
Figure 1: The chemical structure of Reactive Blue 19 |
Adsorption Experiments
Various experimental conditions which may influence the biosorption of RB19 on dried L. minor including contact time, pH, adsorbent dose, temperature and initial RB19 concentration, were tested using batch experiments. Batch adsorption experiments were carried out by adding a fixed amount of sorbent (0.35 g) into 100 mL of different initial dye concentrations of solution.
To study the effect of adsorbent dose 25 mg/L dye solution was prepared from the stock solution and the different amount of sorbent was added (0.05- 0.5 g) to the 100 mL of dye solution and the system is agitated in a shaker for the equilibrium time of 75 min. To study the effect of pH on equilibrium uptake capacity of L. minor was measured by adding a fixed amount of sorbent (0.35 g) into 100 mL of 25 mg/L RB19 dye solution having different pH such as 3-11. The pH of the dye solution was varied by using 0.1 M HCL and 0.1M NaOH. The initial and equilibrium dye concentrations were determined by absorbance measurement using UV spectrophotometer (DR 5000) at maximum 592 nm (Gök et al., 2007). All batch experiments were carried out in triplicate. The amount of adsorption at equilibrium, qe (mg/g), was calculated by (Deniz et al., 2011):
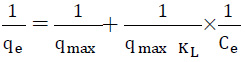
Where C0 and Ce (mg/L) are the liquid-phase concentrations of dye at initial and equilibrium respectively. V is the volume of the solution (L) and M is the mass of biomass used (g).
![]()
Results And Discussion
Characterizations Of Dried Lemna Minor
Dried L. minor was also examined before and after use using environmental scanning electron microscopy. Fig. 2(a) clearly shows the pore textural structure of dried L. minor before use. However, as show in Fig. 2(b) clear pore textural structure is not observed on the surface of dried L. minor after use which could be due to either agglomeration on the surface or the incursion of RB19 into the pores of dried L. minor.
 |
Figure 2: The scanning electron microscopy (SEM) image of dried L. minor: (a) before RB19 adsorption; (b) after RB19 adsorption (magnification = 500). |
The specific surface area is related to the number of active adsorption sites of dried L. minor. For example, Chandrasekhar and Vilar claimed that adsorption increased with the specific surface area and pore volume of the sorbent (Chandrasekhar et al., 2006 and Vilar et al., 2007). The surface area of dried L. minor was 30 m2/gr.
Effect of Contact Time and pH: The experiments were performed to determine the optimum contact time in a range of 10-150 min. As shown in Fig. 3, the adsorption of RB19 on dried L. minor increased rapidly within the 30 min and then slowed from 45 min to 60 min and reaching equilibrium after 75 min. The initial high adsorption rate of RB19 on dried L. minor within the first 30 min was attributed to the high availability of binding sites on the surface of dried L. minor, and the subsequent lower biosorption rate after 30 min was decreased availability of binding sites on the surface of dried L. minor due to absorption of initial RB19 molecules (Padmesh et al., 2006). Similar results were observed for biosorption of reactive dyes on quaternized wood (Low et al., 2000) and the biosorption of bromophenol blue dye on Fungi (Zeroual et al., 2006). To ensure adsorption reached equilibrium, contact time of 75 min was selected for the remaining experiments.
 |
Figure 3: Effect of contact time on RB19 biosorption (C0 = 25 mg/L, adsorbent dose 3.5 g/L, pH 3) |
Fig. 4. shows the plot of effect of solution pH on the equilibrium uptake of RB19 using L. minor. The dye uptake capacity was found to be more at pH 3. This may be due to two sulfonate groups of RB19 are easily dissociated at pH 3. Therefore pH 3 was taken as optimum value. The dye uptake capacity is decreased with increase the pH of the solution. This can be due to the surface charge of the L. minor. At low pH the active site on the sorbent is positively charged and can absorb the RB19 dye, due to opposite charge attraction between negatively charged dye anions and positively charged adsorption sites. At high pH the surfaces are probably negatively charged which repulse negatively charged dye anions (Mane et al., 2007). The similar types of results were obtained for sorption of Brilliant Green dye from aqueous solutions onto rice husk and methylene blue onto Canola (Mane et al., 2007 and Balarak et al., 2015).
 |
Figure 4: Effect of pH on RB19 removal efficiency (C0 = 25 mg/L, adsorbent dose of 3.5 g/L, contact time = 75 min) |
Effect of Adsorbent Dose: The effect of adsorbent dose on removal of RB19 was studied to determine an optimum biosorbent dose. The tested biosorbent dosages varied from 0.5 to 5 g/L using an initial RB19 concentration of 25 mg/L and contact time of 75 min. As shown in Fig. 5, the biosorption capacity of RB19 on the biomass decreased from 19.6 to 4.97 mg/g, while the RB19 removal percent increased from 39.2% to 99.4%, when biosorbent dosage increased from 0.5 to 5 g/L. Lower biosorption capacity of RB19 at a higher dosage of biomass is probably due to the decrease of the surface area of the biomass by the overlapping or aggregation during the sorption (Dogan et al., 2008 and Mehmet et al., 2004). However, the higher the dose of the biosorbent in the solution, the greater the availability of active sites for RB19, leading to the higher RB19 removal (Ho et al., 2005 and Khattri et al., 2009). The removal of 99.4% RB19 was observed when biomass dosage increased to 3.5 g/L and remained approximately constant with further increases in biosorbent dosage. On the basis of both biosorption capacity and the removal percentage, an optimum biosorbent dosage of 3.5 g/L (0.35 g/100 mL) was selected for all further experiments.
 |
Figure 5: Effect of biomass dose on RB19 biosorption (C0 = 25 mg/L, pH=3 Contact time=75 min) |
Isotherm studies: Isotherms are the equilibrium relations between the concentration of the adsorbate on the solid phase and its concentration in the liquid phase (Balarak et al., 2015). The equilibrium biosorption data have been analysed using Langmuir, Freundlich and Temkin isotherms. Analysis of such isotherms is important in order to develop an equation that accurately represents the results and could be used for design purposes.
The Langmuir isotherm model assumes the uniform energies of adsorption onto the adsorbent surfaces. Furthermore, the Langmuir equation is based on the assumption of the existence of monolayer coverage of the adsorbate at the outer surface of the adsorbent where all sorption sites are identical. The Langmuir equation is given as follows (Garg et al., 2003 and Zazouli et al., 2014):
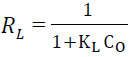
Where qe (mg/g) and Ce (mg/L) are the amount of adsorbed dye per unit mass of sorbent and unadsorbed dye concentration in solution at equilibrium, respectively. Qmax is the maximum amount of the adsorbed dye per unit mass of sorbent to form a complete monolayer on the surface bound at high Ce (mg/g), and KL (L/mg) is a constant related to the affinity of the binding sites. The plots of Ce/qe vs. Ce for the biosorption of RB19 onto L. minor give a straight line of slope (1/qmax) and intercept (1/qmax KL). The essential features of Langmuir can be expressed in terms of dimensionless constant separation factor RL which is calculated using the following equation equation (Gulnaz et al., 2011):
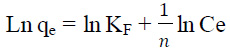
Values of RL indicate the shapes of isotherms to be either unfavorable (RL > 1), linear (RL = 1), favorable (0 < RL < 1).
The Freundlich isotherm model equation is expressed as (Diyanati et al., 2013 and Ong et al., 2007):
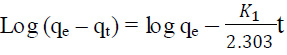
Where qe is the equilibrium dye concentration on the adsorbent (mg/g); Ce, the equilibrium dye concentration in solution (mg/L); and KF is the Freundlich constant. In this function, it is assumed that the sorbent has a surface with a non-uniform distribution of sorption heat. This equation was primarily proposed on a purely empirical basis for adsorption phenomena occurring on gas–solid interfaces, although it can be theoretically derived for an adsorption model in which the heat of adsorption varies exponentially with surface coverage. The slope of plot 1/n ranging 0 and 1, is a measure of adsorption intensity or surface heterogeneity, becoming more heterogeneous as its value gets closer to zero (Balarak et al., 2016).
The Temkin isotherm model suggests an equal distribution of binding energies over the number of the exchanging sites on the surface. The linear form of the Temkin isotherm equation is represented by the following equation (Cicek et al., 2007 and Balarak et al., 2016):
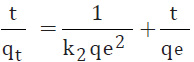
Where B = RT/b, T is the absolute temperature in K, R the universal gas constant (8.314 J/K mol), A the equilibrium binding constant and the constant B is related to the heat of adsorption.
The results of the isotherm constants are displayed in Table 1. As shown in Table 1 that the correlation coefficients for the Langmuir isotherm model were close to 1.0 for all temperatures (fig. 6). The correlation coefficients for Temkin isotherm were low and it can be said that the experimental data was not fitted better to the Temkin isotherm model. The results indicated that the surface of L. minor is homogeneous in nature and did not possess equal distribution of binding energies on the available binding sites. Finally the correlation coefficients for the Freundlich and Temkin isotherm models were lower than that of the Langmuir isotherm model.
| Table 1: Adsorption isotherm constants for the adsorption of RB19 onto L. minor at various temperatures | |||||||||||
| Tempkin model | Freundlich model | Langmuir model | |||||||||
| R2 | A | B | R2 | KF (L/g) | n | R2 | RL | KL(L/mg) | qm (mg/g) | qe exp (mg/g) | Temp (K) |
| 0.839 | 0.64 | 21.25 | 0.921 | 14.25 | 2.74 | 0.998 | 0.271 | 0.027 | 23.31 | 22.44 | 273 |
| 0.799 | 0.45 | 28.93 | 0.904 | 10.44 | 2.17 | 0.999 | 0.208 | 0.038 | 26.72 | 24.95 | 298 |
| 0.824 | 0.26 | 36.78 | 0.936 | 6.79 | 1.65 | 0.998 | 0.163 | 0.051 | 29.53 | 27.38 | 323 |
 |
Figure 6: Langmuir plots for the RB19 dye adsorption onto L. minor biomass |
Biosorption kinetics: In order to analyzed the biosorption kinetics of RB19 dye, the first-order, pseudo-second-order and intraparticle diffusion models were applied to data obtained from the experiments. The first-order rate expression given as (Mittal et al., 2010):
![]()
Where qe and qt are the amounts of dye (mg/g) adsorbed at equilibrium and time t, respectively, and k1 is the rate constant of adsorption (min−1) biosorption.
The pseudo-second-order kinetic model is expressed as (Pavan et al., 2008):
![]()
where qe is the biosorbed dye amount at equilibrium (mg/g) for the pseudo-second-order biosorption, qt is the amount of dye biosorbed at time t (mg/g) and k2 is the pseudo-second-order kinetic rate constant (g/mg min).
The intraparticle diffusion equation can be written as follows (Dizge et al., 2008):
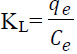
where C is the intercept, and kdif is the intraparticle diffusion rate constant (mg g−1 min−1/2). The results of the kinetic parameters for biosorption are given in Table 2. The correlation coefficients for the pseudo-second-order kinetic model were close to 1.0 for all concentration
(fig. 7). The correlation coefficients for the first-order kinetics and intraparticle diffusion equation models were lower than that the pseudo-second-order. These results indicate that the biosorption of RB19 dye by L. minor follows the pseudo-second-order kinetics model, which relies on the assumption that the rate-limiting step may be biosorption involving valence forces through the sharing or exchange of electrons between biosorbent and sorbate (Cicek et al., 2007). Waranusantigul suggested that the removal of reactive dyes onto duck weed obeyed the pseudo-second-order kinetic models (Waranusantigul et al., 2003).
| Table 2: Kinetic parameters for the adsorption of RB19 onto L. minor at various concentration | ||||||||||
| Con(mg/L) | qe exp (mg/g) | Pseudo-first order | Pseudo-second order | Intraparticle diffusion | ||||||
| K1 | qe | R2 | K2 | qe | R2 | kdif | C | R2 | ||
| 25 | 7.12 | 0.0176 | 4.42 | 0.892 | 0.0098 | 7.76 | 0.999 | 0.471 | 4.46 | 0.812 |
| 50 | 13.75 | 0.0254 | 9.18 | 0.861 | 0.0071 | 12.95 | 0.998 | 0.583 | 3.24 | 0.798 |
| 100 | 25.54 | 0.0417 | 18.27 | 0.883 | 0.0052 | 24.84 | 0.999 | 0.772 | 2.97 | 0.826 |
| 200 | 46.51 | 0.0652 | 33.62 | 0.913 | 0.0027 | 48.17 | 0.998 | 0.942 | 1.78 | 0.845 |
 |
Figure 7: Pseudo-second-order kinetic plots for RB19 adsorption onto L. minor biomass |
THERMODYNAMIC STUDIES
The feasibility of the adsorption process was evaluated by the thermodynamic parameters including free energy change (∆Go), enthalpy (∆Ho), and entropy (∆So). (∆Go) was calculated from the following equation(Zazouli
et al., 2015 and Balarak et al., 2016):
where R is the universal gas constant (8.314 J/mol K), T is the temperature (K), and KL is the distribution coefficient. The Kd value was calculated using following equation:
where qe and Ce are the equilibrium concentration of RB19 dye on adsorbent (mg/L) and in the solution (mg/L), respectively. enthalpy change (∆Ho), and entropy change (∆So) of adsorption were estimated from the following equation:
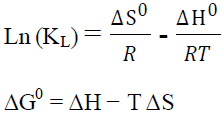
The values of ∆Go, ∆Ho, and ∆So for the adsorption of RB19 dye onto L. minor at different temperatures are given in Table 3. The negative values of ∆Go in the temperature range of 273–323 K indicated that the adsorption process was feasible and spontaneous. In addition, the decrease in the values of ∆Go with the increasing temperature indicates the spontaneity of the process at higher temperatures. Generally, the change of standard free energy for physiosorption is in the range of -20 to 0 kJ/mol and for chemisorption varies between – 80 and -400 kJ/mol. In the present study, the overall ∆G˚ has values ranging from – 4.5 to – 8.28 kJ mol-1.
| Table 3: Thermodynamic parameters for the adsorption of AB19 on L. minor | |||
| T (K) | ΔG0 (kJ/mol) | ΔH0 (kJ/mol) | ΔS0 (kJ/mol K) |
| 273 | −4.58 | 31.56 | 0.584 |
| 298 | −6.66 | ||
| 323 | −8.28 | ||
These results correspond to a spontaneous physical adsorption, indicates that this system does not gain energy from external resource. The endothermic nature was also confirmed from the positive values of enthalpy change (∆Ho), while good affinity of RB19 towards the adsorbent materials is revealed by the positive value of ∆So. This phenomenon had also been observed in the adsorption of acid orang dye by cashew nut shell (Kumar et al., 2010) and acidic dyes by Paenibacillus macerans (Ferdag et al., 2009).
Conclusion
This study shows that L. minor is effective adsorbent for the removal of RB19 dye from aqueous solution. The adsorbent was the most effective at pH = 3. Adsorption of RB19 onto L. minor increased with the increase in the adsorbent dose and the optimum adsorbent dosage was found to be 3.5 g/L. The equilibrium between the dye and the adsorbent in the solution was established within 75 min. The best correlation was obtained using the pseudo-second-order kinetic model. Equilibrium data were also fitted well to the Langmuir isotherm model. Thermodynamic analyses indicated that the adsorption of RB19 dyes onto L. minor was endothermic and spontaneous. The value of ΔHo was positive, indicating that the adsorption reaction was endothermic. The positive value of ΔSo reflects the affinity of L. minor for AB19 and suggests some structural changes in AB19 and L. minor.
References
Cengiz S, Tanrikulu F, Aksu S. (2012). An alternative source of adsorbent for the removal of dyes from textile waters: Posidonia oceanica (L). Chemical Engineering Journal. 189–190; 32–40.
Balarak D, Mahdavi Y , Bazrafshan E , Mahvi AH. (2016). Kinetic, isotherms and thermodynamic modeling for adsorption of acid blue 92 from aqueous solution by modified Azolla filicoloides. Fresenius Environmental Bulletin. 25; 1321-1330.
Li Q , Yue Q, Su Y, Gao B. (2011). Equilibrium and a two-stage batch adsorber design for reactive or disperse dye removal to minimize adsorbent amount. Bioresource Technology. 102; 5290–5296.
Robinson T, Chandran B, Nigam P. (2002). Removal of dyes from a synthetic textile dye effluent by adsorption on apple pomace and wheat straw. Water Research. 36; 2824–2830.
Ozcan A, Omeroglu C, Erdogan Y. (2007). Modification of bentonite with a cationic surfactant: An adsorption study of textile dye Reactive Blue 19. Journal of Hazardous Materials. 140;173–179.
Tan C-y, Li G, Lu X-Q, Chen Z-l. (2010). Biosorption of Basic Orange using dried A. filiculoides. Ecol Engin. 36:1333–40.
Balarak D, Jaafari J, Hassani G, Mahdavi Y, Tyagi I, Agarwal S, Gupta VK. (2015). The use of low-cost adsorbent (Canola residues) for the adsorption of methylene blue from aqueous solution: Isotherm, kinetic and thermodynamic studies.Colloids and Interface Science Communications. Colloids and Interface Science Communications. 7;16–19.
Ali NF, El-Mohamedy RSR. (2012). Microbial decolourization of textile waste water. Journal of Saudi Chemical Society. 16;117–123.
Yang Y, Wei B, Zhao Y, Wang J. (2013). Construction of an integrated enzyme system consisting azoreductase and glucose 1-dehydrogenase for dye removal. Bioresource Technology. 130; 517–521.
Couto SR. Dye removal by immobilised fungi. (2009). Biotechnology Advances. 27; 227–235.
Deniz F, Karaman S. (2011). Removal of Basic Red 46 dye from aqueous solution by pine tree leaves. Chemical Engineering Journal. 170; 67–74.
Oei BC, Ibrahim S, Wang S, Ang HM. (2009). Surfactant modified barley straw for removal of acid and reactive dyes from aqueous solution. Bioresource Technology. 100; 4292–4295.
Demirbas A. (2009). Agricultural based activated carbons for the removal of dyes from aqueous solutions: A review.Journal of Hazardous Materials.167;1–9.
Safa Y, Bhatti HN. (2011). Adsorptive removal of direct textile dyes by low cost agricultural waste: Application of factorial design analysis.Chemical Engineering Journal. 167;35-41.
Zazouli MA, Bazrafshan E, Mahdavi Y, Balarak D. (2014). Phytodegradation potential of bisphenolA from aqueous solution by Azolla filiculoides: journal Iranian journal of environmental health science and engineering.10:14-20.
Ferdag C, Necip AO. (2009). Biosorption of acidic dyes from aqueous solution by Paenibacillus macerans: Kinetic, thermodynamic and equilibrium studies. Chemical Engineering Journal.150:122-30.
Khataee AR, Movafeghi A, Torbati S, SalehiLisar SY, Zarei M. (2012). Phytoremediation potential of duckweed (Lemna minor ) in degradation of Acid Blue 92: Artificial neural network modeling. Ecotoxicology and Environmental Safety. 80; 291–298.
Kiliç NK, Duygu E, Dönmez G. (2010). Triacontanol hormone stimulates population, growth and Brilliant Blue R dye removal by common duckweed from culture media. Journal of Hazardous Materials. 182: 525-30.
Diyanati RA, Yazdani J, Balarak D. (2013). Effect of sorbitol on phenol removal rate by Lemna minor. Mazandaran university of medical science. 22;58-64.
Zazouli MA, Balarak D, Karimzadeh F, Khosravi F. (2014). Removal of Fluoride from Aqueous Solution by Using of Adsorption onto Modified Lemna Minor: Adsorption Isotherm and Kinetics Study. Journal of Mazandaran University Medical Science. 24;41-8.
Padmesh TVN, Vijayaraghavan k, Sekaran G, Velan M. (2006). Application of Azolla rongpong on biosorption of acid red 88, acid green 3, acid orange 7 and acid blue 15 from synthetic solutions.Chemical Engineering Journal. 122:55–63.
Gök O, Özcan S, Özcan A. (2007). Adsorption behavior of a textile dye of Reactive Blue 19 from aqueous solutions onto modified bentonite. Journal of Hazardous Materials. 140; 173-179.
Chandrasekhar S, Pramada PN. (2006). Rice husk ash as an adsorbent for methylene blue-effect of ashing temperature. Adsorption. 12, 27–43.
Vilar VJP, Botelho CM, Boaventura RUR. (2007). Methylene blue adsorption by algal biomass based materials: biosorbents characterization and process behaviour. Journal of Hazardous. Materials. 147, 120–132.
Kaushik P, Malik A. (2009). Fungal dye decolourization: recent advances and future potential. Environment international. 35, 127–141.
Padmesh TVN, Vijayaraghavan K, Sekaran G, Velan M. (2006). Biosorption of Acid Blue 15 using fresh water macro alga Azolla filiculoides: batch and column studies. Dyes Pigments. 71, 77–82.
Low KS, Lee CK, Tan BF. (2000). Quaternized wood as sorbent for reactive dyes. Applied Biochemistry and Biotechnology. 87;233–245.
Zeroual Y, Kim BS, Kim CS, Blaghen M, Lee KM. (2006). Biosorption of bromophenol blue from aqueous solutions by Rhizopus stolonifer biomass. Water Air and Soil Pollution. 177;135–146.
Mane VS ,Mall ID. (2007). Kinetic and equilibrium isotherm studies for the adsorptive removal of Brilliant Green dye from aqueous solution by Rice husk ash, J. Environ. Manage. 84; 390–400.
Dogan M, Abak H, Alkan M. (2008). Biosorption of methylene blue from aqueous solutions by Hazelnut shells: equilibrium, parameters and isotherms. Water Air and Soil Pollution. 192, 141–153.
Mehmet D, Mahir A, Aydın T. (2004). Kinetics and mechanism of removal of methylene blue by adsorption onto Perlite. Journal of Hazardous Materials. 109:141-48.
Ho YS, Chiang TH, Hsueh YM. (2005). Removal of basic dye from aqueous solution by Tree fern as a adsorbent. Process Biochemistry. 40, 119–124.
Khattri SD, Singh MK. (2009). Removal of malachite green from dye wastewater using neem Sawdust by adsorption. Journal of Hazardous Materials.167;1089-94.
Garg VK, Gupta R, Yadav AB. (2003). Dye removal from aqueous solution by adsorption on treated Sawdust. Bioresource Technology. 89; 121–4.
Zazouli MA, Mahvi AH, Dobaradaran S, Barafrashtehpour M, Mahdavi Y, Balarak D. (2014). Adsorption of fluoride from aqueous solution by modified Azolla Filiculoides. Fluoride. 47;349-58.
Gulnaz O, Sahmurova A, Kama A. (2011). Removal of Reactive Red 198 from aqueous solution by Potamogeton crispus. Journal of Chemical Engineering. 174: 579–85.
Diyanati RA, Yousefi Z, Cherati JY, Balarak D. (2013). The ability of Azolla and Lemna minor biomass for adsorption of phenol from aqueous solutions. Journal of Mazandaran University Medical Science. 23;21-8.
Ong ST, Lee CK, Zainal Z. (2007). Removal of basic and reactive dyes using ethylenediamine modified Rice hull. Bioresource Technology. 98(15): 2792-9.
Balarak D, Mahdavi Y, Maleki A, Daraei H and Sadeghi S. (2016). Studies on the Removal of Amoxicillin by Single Walled Carbon Nanotubes. British Journal of Pharmaceutical Research. 10;1-9.
Cicek F, Ozer D, Ozer A. (2007). Low cost removal of reactive dyes using Wheat bran. Journal of Hazardous Materials.146;408-16.
Balarak D, Kord Mostafapour F, Joghataei A. (2016). Experimental and Kinetic Studies on Penicillin G Adsorption by Lemna minor. British Journal of Pharmaceutical Research. 9;1-10.
Mittal A, Jain R, Mittal J. (2010). Adsorptive removal of hazardous dye quinoline yellow from wastewater using Coconut husk as potential adsorbent. Fresenius Environmental Bulletin. 19;1171-9.
Pavan FA, Mazzocato AC, Gushikem Y. (2008). Removal of methylene blue dye from aqueous solutions by adsorption using yellow passion fruit peel as adsorbent. Bioresource Technolology. 99;3162-5.
Dizge N, Aydiner C, Demirbas E. (2008). Adsorption of reactive dyes from aqueous solutions by Fly ash: Kinetic and equilibrium studies. Journal of Hazardous Materials. 150(3):737-46.
Waranusantigul P, Pokethitiyook P, Kruatrachue M, Upatham ES. (2003). kinetics of basic dye (methylene blue) biosorption by giant Duck weed (Spirodela polyrrhiza). Environmental Pollution. 125; 385–392.
Zazouli MA, Mahvi AH, Mahdavi Y, Balarak D. (2015). Isothermic and kinetic modeling of fluoride removal from water by means of the natural biosorbents Sorghum and Canola. Fluoride. 48;15-22.
Balarak D, Mahdavi Y, Bazrafshan E, Mahvi AH, Esfandyari Y. (2016). Adsorption of fluoride from aqueous solutions by Carbon nanotubes: determination of equilibrium, kinetic, and thermodynamic parameters. Fluoride. 49;71-83.
Kumar PS, Ramalingam S, Senthamarai C, Niranjanaa M. (2010). Adsorption of dye from aqueous solution by Cashew nut shell: Studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Desalination. 261;52–60.


