1Chemistry Department, Xavier University-Ateneo de Cagayan, Corrales Avenue, Cagayan de Oro City 9000, Philippines
Corresponding author Email: aangcajas@xu.edu.ph
Article Publishing History
Received: 25/03/2019
Accepted After Revision: 05/05/2019
A novel adsorbent starch-graft-N-methyl-N-vinylacetamide (starch-g-NMVA) was synthesized using microwave assisted graft copolymerization. Monomers of N-methyl-N-vinylacteamide (NMVA) was grafted to potato starch using microwave irradiation and potassium peroxydisulfate (KPS) as free radical initiator. The highest % grafting (%G) obtained was 10.03% under the optimum conditions of 0.55 M NMVA and 90 seconds exposure time, keeping the initiator, starch, and microwave power fixed at 0.0014M, 0.1 g, and 1200W respectively. FTIR and SEM analyses confirmed that the monomer was grafted successfully. The graft copolymer was investigated for its efficiency in removing Cu(II) ions from aqueous solutions at different concentrations. Using the Box-Behnken method the most favorable pH, adsorbent dose, and initial Cu(II) concentration generated for the adsorption was 5.5, 100 mg and 100 ppm, respectively. The resulting adsorbent is able to remove 52.9% of Cu(II) ions in an aqueous solution at these conditions. The Langmuir isotherm best described the adsorption property of the system with a correlation coefficient (R2) of 0.9361. On the basis of this model, the maximum adsorption capacity (Qo) of the starch-g-NMVA was calculated to be 46.9749 mg/g. This study indicated that starch-g-NMVA is a good alternative adsorbent for the removal of Cu (II) from aqueous solution. Further desorption and optimization studies will help us know the applicability of this adsorbent in removing metals from a simulated wastewater setup.
Starch, Graft Copolymerization, Copper (Ii) Ions, Box Behnken Design, N-Methyl-N-Vinylacetamide
Villocillo C. B, Angcajas A. B. Biosorption of Copper (II) Ions in Aqueous Solution by Microwave-Synthesized Starch-Graft-N-Methyl-N-Vinylacetamide. Biosc.Biotech.Res.Comm. 2019;12(2).
Villocillo C. B, Angcajas A. B. Biosorption of Copper (II) Ions in Aqueous Solution by Microwave-Synthesized Starch-Graft-N-Methyl-N-Vinylacetamide. Biosc.Biotech.Res.Comm. 2019;12(2). Available from: https://bit.ly/2IbEF2A
Copyright © Villocillo and Angcajas, This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
Introduction
Technological and industrial advancements have always posed danger to the environment as it is associated to increase in heavy metal contamination in water and other parts of the ecosystem. Mining operations, fertilizer production, batteries, pesticides, and paper industries may directly or indirectly discharge heavy metal wastewaters into the environment (Mrinalini et al, 2015). Many of these heavy metal ions, i.e, ions of zinc, copper, nickel, mercury, lead and chromium, are toxic and non-biodegradable (Zhu et al., 2012). Because of their non-biodegradability, heavy metals tend to accumulate in living organisms, causing various diseases and disorders (Hasret et al., 2012). Copper, although an essential compound to plants and animals, becomes toxic when biological requirements are exceeded. Some toxic effects of copper in the body is potentially damaging protein, lipid, and DNA (Moroncola et al, 2016). Removal of heavy metals like copper from waste waters is important to maintain a suitable quality of water. Filtration, adsorption, and chemical precipitation are some methods used in removing heavy metal ions from wastewater or aqueous solution. Adsorption technology has recently become a real alternative to traditional wastewater treatment due to its relative simplicity and efficiency (Paksamut et al., 2018).
This involves a mass transfer process by which a substance is transferred from the liquid phase to the surface of the solid, and becomes bound by physical and/or chemical interactions (Zhu et al., 2012). Chemical adsorption, also called activated adsorption, results from the chemical interaction between the adsorbent and the adsorbate. Chemisorption occurs only as a monolayer and substances chemisorbed on solid surface are hardly removed because of stronger forces (Mehta et al., 2014). High adsorption capacity can be observed from biosorbent with abundant amino and carboxyl groups (Zhang et al., 2017). The presence of functionalities such as hydroxyl groups (-OH) on the surface of the adsorbent allows introduction of several heavy metal groups.
The amount of adsorbed ions onto the adsorbent could depend on the acidity of the medium (pH), initial heavy metal ion concentration, and adsorbent dose. Other factors include temperature and contact time. The acidity of the solution or pH is one of the most important parameters controlling the uptake of heavy metals from wastewater and aqueous solutions since it determines the surface charge of the adsorbent and the degree of ionization and speciation of the adsorbent (Abdel et al., 2011). In a study done by Tumin et al. (2002), the uptake of copper increased significantly when the pH was at 4 to 5 and adsorption capacity decreased slightly in pH range of 6 to 9. Using a Box-Behnken design, it was observed that % metal adsorption increased as the pH of the metal-containing aqueous solution increased from 2.5 to 5.5 (Ocreto et al., 2019).
Adsorbent dosage is another important parameter which influences the extent of metal uptake from the solution. Studies report that percent metal removal increases as the adsorbent dosage increases due to the introduction of more adsorption or binding sites and the availability of more surface area for metal attachment (Onundi et al., 2010). Manivannan et al. (2015) reported that the amount of copper increased with the increase in adsorbent dose and reached a maximum value after a particular dose. Additionally, adsorption capacities increased as the dosage of natural bioadsorbents and contact time increased during Cu (II) removal (Paksamut et al., 2018).
The use of synthetic polymer as toxic metal ion adsorbent is a possible approach for preventing environmental pollution and recycling metals. Synthetic adsorbents are mostly composed of petroleum-based polymers which are usually non-renewable and non-biodegradable. Generally, synthetic adsorbents are discarded in landfills or treated by incineration after the adsorption process for metal ions. Natural polymer such as starch is a more attractive raw material for industrial applications because it is renewable, abundantly available, and fully biodegradable. It also has numerous hydroxyl groups that can be chemically modified to design adsorbent materials of interest (Singh et al., 2012).
In a study of Cankaya (2016), starch methacrylate, prepared by esterification of primary -OH group of starch, was grafted with N-cyclohexyl acrylamide and methyl methacrylate monomers via free radical polymerization. Chemical modification of natural polymers via grafting can be achieved through the use of microwave radiation to generate free radical sites on the natural polymer backbone (Mostafa et al., 2013). Fosso-Kankeu et al. (2016) reported a 107.1 % conversion to guar gum-graft-poly-ethyl acrylate during copolymerization of ethylacrylate and guar gum by microwave irradiation at 900 MW and 3 minutes exposure. Reaction variables, such as initiators, composition of reaction mixture, microwave power, and exposure time played a key roles during grafting copolymerization using microwave irradiation (Karthika et al, 2014).
This study involves the synthesis of potato starch grafted with N-methyl-N-vinylacetamide and its application in the removal of copper in copper solutions. Box-Behnken design under response surface methodology was used to identify the optimized adsorption condition (Tarangini et al., 2009). The grafted starch was characterized and the amount of copper removed was quantitatively analyzed using Atomic Absorption Spectroscopy.
Materials and Methods
Potato starch, N-methyl-N-vinylacetamide (NMVA, 98%), reagent grade potassium peroxydisulfate (KPS, ≥ 99% ), ethanol (≥ 99.5%), and copper sulfate pentahydrate (≥ 99.5%) were purchased from Sigma-Aldrich (Singapore) and were used without any pretreatment.
NMVA grafting onto potato starch under microwave irradiation
The grafting procedure employed in this study was adapted from Pandey et al. (2012) with slight modification. Potato starch (0.1g) was dissolved in 25 mL distilled water in a 150 mL open-necked flask. To this solution, calculated amounts of KPS (0.0014M) and NMVA (0.35 – 0.55M) were added together and the total volume of the resulting solution was adjusted to 25 mL. The flask was then exposed at 100% (1200 W) microwave power for 90 and 120 seconds in a Samsung domestic microwave oven (Samsung, MW73B, Malaysia) with a microwave frequency of 2450 MHz. Potato starch-graft-N-methyl-N-vinylacetamide (starch-g-NMVA) was precipitated by pouring the reaction mixture in ethanol to dissolve homopolymers of NMVA. The mixture was centrifuged and the separated copolymer was oven-dried at 50°C up to a constant mass. Grafting parameters such as % grafting, % grafting efficiency, % conversion, and % homopolymer were then calculated using the following equations:
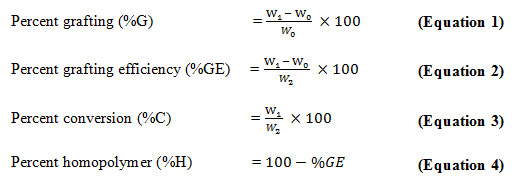
where W0 is the weight of starch, W1 is the weight of the grafted starch, and W2 is the weight of NMVA.
Partial characterization of starch-g-NMVA
The synthesized starch graft copolymer (starch-g-NMVA) was subjected to partial characterization using FTIR and SEM to evaluate its physical and chemical properties. The IR spectra of potato starch and starch-g-NMVA samples were obtained with a Fourier Transform Infrared Spectrometer (Perkin-Elmer, Spectrum 100, USA) and using the range 500-4000 cm-1 to provide the proof of grafting. Surface morphology of starch-g-NMVA was analyzed using a Scanning Electron Microscope (JEOL, JSM 5300, USA).
Optimization of Cu(II) removal
Adsorption conditions such as initial pH (1.5 to 5.5), initial concentration of Cu(II) (5-100 ppm) and adsorbent dose (10-100 mg) were optimized simultaneously. The optimization experiments were carried out at a working volume of 25 mL, and agitated in the shaker apparatus with a speed of 120 rpm at room temperature for 240 minutes. After agitation, the mixtures were filtered through Whatman 0.45 mm filter paper and the amount of adsorbed metal ions was determined using the Atomic Absorption Spectrophotometer (Perkin-Elmer, AAnalyst 400, USA). Control experiments were carried out to show that no sorption occurs on either glassware or filtration systems. The pH of the reaction mixture was adjusted to the desired value using either 0.5 M hydrochloric acid or 0.5 M sodium hydroxide.
Box-Behnken design
Box-Behnken statistical experiment design and the response surface methodology by Minitab Version 17.1.0 was employed to investigate the combined effect of pH, adsorbent dose, and initial copper concentration. Each independent variable was studied at three different levels: low, medium and high, coded as -1, 0, +1, respectively. The center point of the design was replicated three times for the estimation of error.
The experimental data were analyzed by fitting to a second order polynomial model, which was statistically validated by performing Analysis of Variance (ANOVA) and lack-of-fit test to evaluate the significance of the model.The performance of the process was evaluated by analyzing the response (y), which is the percent removal of copper, that (E depends on the input factors X1, X2,…, Xk, and the relationship between the response parameters is described by
![]()
Where f is the real response function the format of which is unknown and is the residual factor associated with the experiment. The surface represented by f(Xi, Xj) is called a response surface. The response can be represented graphically, either in the three-dimensional space or as contour plots that help visualize the shape of the response surface.
For RSM, the most commonly used second order polynomial equation developed to fit the experimental data and determine the relevant model terms can be written as:
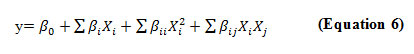
Where β0 is the constant coefficient, βi is the slope or linear effect of the input factor. βixj is the linear by linear interaction effect between the input factor xj and βii is the quadratic effect of input factor xj.
Adsorption Isotherm Study
Adsorption data was fitted to the Langmuir and Freundlich isotherms. The Langmuir isotherm was expressed in the linear form as with the equation:

where Ce is the equilibrium concentration (mg/L) and qe the amount adsorbed at equilibrium (mg/g). The Langmuir constants Qo (mg/g) represent the monolayer adsorption capacity and b (L/mg) relates the heat of adsorption.The essential feature of the Langmuir adsorption can be expressed by means of RL, a dimensionless constant referred to as separation factor or equilibrium parameter for predicting whether an adsorption system is favorable or unfavorable. RL is calculated using the following equation:

where C0 is the initial Cu(II) concentration (mg/L). If RL values lies between 0 and 1, the adsorption is favorable.
The Freundlich isotherm describes the heterogeneous surface energies by multilayer adsorption and is expressed in linear form as given:
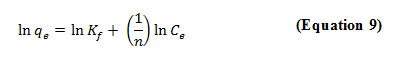
where Kf (mg/g),is roughly an indicator of the adsorption capacity and 1/n is the adsorption intensity.
Results and Discussion
Starch was grafted with N-methyl-N-vinylacetamide (NMVA) by microwave assisted method using potassium peroxydisulfate (KPS) as a free radical initiator. Grafting was carried out in aqueous medium. The microwave energy absorbed by the water molecules resulted to dielectric heating of the reaction medium. Moreover, microwaves have lowering effect on Gibbs energy of activation of reactions. With these effects of microwave in the reaction medium, peroxydisulfate decomposed into sulfate ion radicals quickly. The resulting sulfate ion radicals interact with water to give free hydroxyl radicals. The primary free radicals (sulfate ion radicals and/or the hydroxyl radicals) combine with vinylacetamide to give monomer free radicals. Since homopolymerization is reported to be faster than graft copolymerization, the growing homopolymer free radical abstracts hydrogen from the starch molecule to result in a macro radical to which more NMVA moieties become attached to form a chain. This chain will grow until it combines with other chain to give the graft copolymer (Singh et al., 2007). A proposed reaction mechanism of graft copolymerization involving starch (StOH) and free radical species (R• or SO4– and OH–) introduced by Kalia et al., 2013.
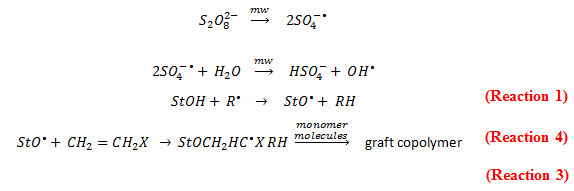
The resulting graft, starch-g-NMVA, was gel-like upon adding ethanol. After drying, the graft copolymer was hard and plastic-like. The powdered form of the graft copolymer is shown in Figure 1.
 |
Figure 1: Dried starch-g-NMVA copolymer |
Optimum grafting conditions
The effect of varying the concentration of the NMVA monomer along with the effect of exposure time on grafting parameters were studied.
Effect of monomer concentration
The effect of NMVA on grafting percentage was investigated by varying its’ concentration from 0.35 to 0.55 M. Results showed that % grafting increased with increasing NMVA concentration (Table 1).
Table 1: %Grafting and %Grafting Efficiency as a function of monomer concentration of 0.0014M potassium peroxydisulfate (KPS), starch (0.1g/25mL), at 2450 W and 90 seconds exposure time.
| N-methyl-N-vinylacetamide (NMVA) | % Grafting | %Grafting Efficiency | %Conversion | %Homopolymer |
| 0.35 M | 7.78 0.064 | 1.85 0.064 | 25.64 0.015 | 98.15 0.015 |
| 0.45 M | 9.44 0.085 | 2.30 0.085 | 26.58 0.100 | 97.71 0.010 |
| 0.55 M | 10.0 0.036 | 2.41 0.014 | 26.38 0.241 | 97.60 0.014 |
Values are average of five individual samples (n=5), expressed as mean ± standard deviation
The grafting percentage (%G) indicates the increase in weight of original starch subjected to grafting with a monomer while grafting efficiency percentage (%GE) is the fraction of monomer converted to graft polymer. In general, the grafting efficiency depends on the monomer concentration. This is true with the result obtained in this study as presented in Table 1. The increase in monomer concentration resulted to increase in %GE. However, the increase in %GE obtained in this study is lower compared to other studies (Nadiah et al., 2016). Low %GE means that less monomer was used in grafting and most was wasted in side reactions and homopolymer formation (Nadiah et al., 2016). The increase in %GE observed is an indication that important functional groups is present on the starch backbone and these groups will play an important role in the adsorption process.
Percentage conversion (%C) is taken as the ratio of the weight of the grafted copolymer to the weight of the monomer. It is observed that % C decreased from 0.45 to 0.55 M concentration of NMVA. This may be due to the large ratio of NMVA to initiator KPS. In this case, KPS is relatively deficient to the superabundant NMVA monomers. Hence, though the absolute amount of grafted copolymer increases, as evidenced by the increase in %G, %C is observed to decrease. The same observation was made by Liu et al. (2005).
This direct relation between the percent graft yield and monomer concentration within the experimental range studied can be attributed to the molecular collisions triggered by the increased NMVA population in the vicinity of starch molecules. Hence, grafting increased. On the other hand, the percentage of homopolymer (%H) showed a reverse trend with respect to %GE. This behavior can be attributed to accumulation of monomer at close proximity of the starch backbone. (Castañeda et al., 2012)
Effect of exposure time
To investigate the effect of time on graft copolymerization, the reaction was carried out using the exposure times 90 and 120 seconds. As shown in Figure 2, an increasing trend of grafting percentage was observed after reaction of starch with different concentrations of NMVA (0.35 M, 0.45 M, 0.55 M) at fixed microwave radiation exposure time of 90 seconds.
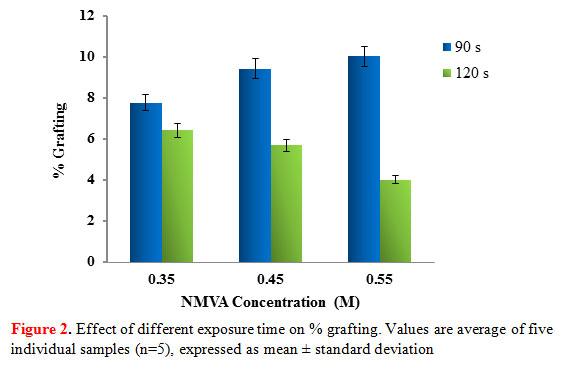 |
Figure 2: Effect of different exposure time on % grafting. Values are average of five individual samples (n=5), expressed as mean ± standard deviation |
However, at longer exposure of 120 seconds, there was a decrease in the grafting percentage. This can be attributed to the depletion of initiator concentrations as the reaction proceeds. Also, more homopolymers are formed compared to the graft copolymer at higher exposure time. When homopolymerization increases, the viscosity of the reaction medium also increases which creates hindrance in the movement of the free radicals toward active sites, resulting in less grafting percentage (Lakshmi et al., 2011).
The highest %G which is 10.03% was obtained by exposing a reaction mixture containing 0.55 M of NMVA, 0.0014 M of KPS initiator and starch (0.1 g) to 1200W microwave power for 90 seconds. The starch-g-NMVA representative sample containing the maximum %G was characterized using FTIR and SEM analysis and was used for the adsorption experiments.
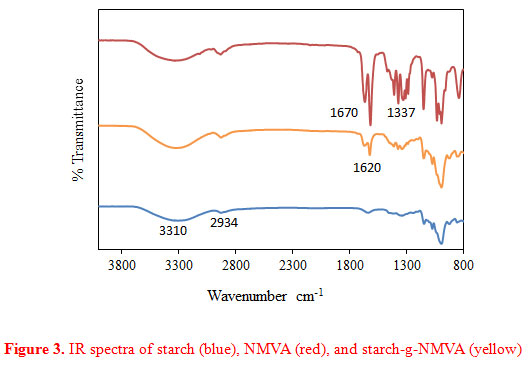 |
Figure 3: IR spectra of starch (blue), NMVA (red), and starch-g-NMVA (yellow) |
Characterization of the starch-g-NMVA
Potato starch, NMVA, and starch-g-NMVA were subjected to FT-IR to examine the functional groups present. As shown in Figure 3, the IR spectrum of starch (blue) showed a wide band at around 3300 cm-1. This can be attributed to the O-H stretching of starch. This result verifies the polyhydroxy nature of starch molecule. Furthermore, the appearance of adsorption band at around 2934 cm-1 can be attributed to the asymmetric stretching of CH groups.
In the spectrum of NMVA (red), the peak at 1670 cm-1 confirms the carbonyl functional group in the structure of NMVA. This carbonyl group is reported to enhance metal adsorption (Ozturk et al., 2015). The peak at 1620 cm-1 confirms the vinylic carbon present in the monomer while the 1337 cm-1 is for the C-N stretching of the amide group. In the case of starch-g-NMVA spectrum, it is observed that there is variation in the intensity of C-H stretching vibration and shifting of peak from 2940 cm-1 to 2980 cm-1. Additional peaks at 1337cm-1 and 1620 cm-1 indicate the added functionality of the graft copolymer. However, there is a peak appearing at 1620 cm-1 indicating C=C groups present. This can be due to the impurity of the grafted copolymer. The % homopolymer is large (97.6%) thus there are still unreacted monomer molecules which were not removed thus accounting for the C=C peak appearing in the spectrum of starch-g-NMVA.
In the study of Kalia et al. (2013), the surface of the pure starch appeared to have oval granules that have smooth surfaces but are irregular in size and shape. The SEM image (Figure 4) obtained after grafting of our starch and exposure to microwaves showed aggravation in the surface of the starch granules. Irregular rough surface can be seen where the monomers of NMVA were grafted. The microwave irradiation employed caused the heating energy to penetrate deep inside the granules causing them to bursts outside and formed indents in the surface. The same result was observed from the study conducted by Nadiah et al.(2016) on the effect of microwave heating on potato and tapioca starches in water suspension.
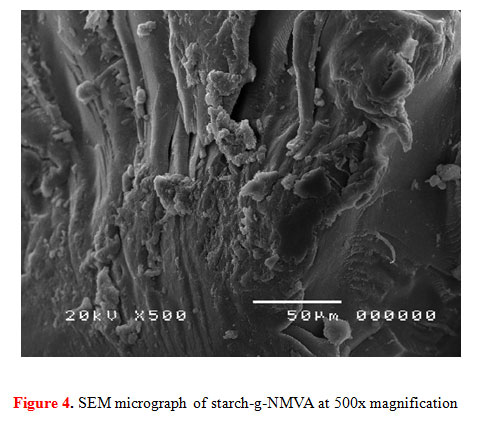 |
Figure 4: SEM micrograph of starch-g-NMVA at 500x magnification |
Optimization of adsorption parameters
In this study, only the parameters adsorbent dose, pH, and initial Cu(II) concentration and its effect on the efficiency of Cu(II) removal by starch-g-NMVA were studied. Optimization of the removal of Cu(II) ions from aqueous solutions for three parameters were carried out with Box-Behnken statistical design. Using the Minitab 17.1.0 software, a total of 15 experiments were generated and the theoretical percentage removal of Cu(II) are compared with the experimental values, as shown in Table 2. The theoretical % removal was also generated by the Minitab software based on the experimental results.
Table 2: Box-Behnken results for Cu(II) adsorption onto starch-g-NMVA.
| Run Order | Biosorption Parameters | % Cu (II) Adsorbed | |||
| P | A | C | experimental | theoretical | |
| 1 | -1 | 0 | +1 | 7.1416 | 5.1876 |
| 2 | 0 | 0 | 0 | 8.9305 | 10.6168 |
| 3 | +1 | -1 | 0 | 24.0418 | 23.5302 |
| 4 | +1 | 0 | +1 | 32.1254 | 34.8879 |
| 5 | 0 | 0 | 0 | 12.2139 | 10.6168 |
| 6 | 0 | +1 | -1 | 15.2749 | 17.5257 |
| 7 | 0 | -1 | +1 | 16.8189 | 14.5681 |
| 8 | 0 | -1 | -1 | 7.5415 | 6.0991 |
| 9 | -1 | 0 | -1 | 2.9289 | 0.1664 |
| 10 | 0 | 0 | 0 | 10.7059 | 10.6168 |
| 11 | -1 | -1 | 0 | 6.4854 | 10.6902 |
| 12 | 0 | +1 | +1 | 23.5426 | 24.9849 |
| 13 | -1 | +1 | 0 | 7.1829 | 7.6945 |
| 14 | +1 | +1 | 0 | 52.5741 | 48.3693 |
| 15 | +1 | 0 | -1 | 22.0267 | 23.9808 |
Legend: P = pH (-1=1.5, 0=3.5, +1=5.5)
A = Adsorbent dose (-1=10mg, 0=55mg, +1=100mg)
C = initial Cu(II) concentration (-1=5mg/L, 0=52.5mg/L, +1=100mg/L)
By applying multiple regression analysis on the design matrix and the responses given, the following second-order polynomial equation was established to explain the Cu(II) removal efficiency which can then be used to obtain the theoretical percentage removal by substituting the values of the parameters from the specific runs.
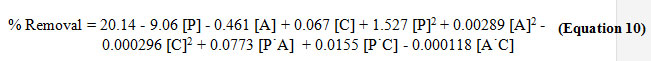
Regression analysis suggests strong correlation between theoretical and experimental percentage Cu(II) removal. The experimental results fit to the Box-Behnken model; hence this model can explain the relationship of the different parameters considered in this experiment. With this, the Box-Behnken model was reliable enough to be used in estimating the best parameter conditions. Analysis of variance (ANOVA) was conducted to test the significance of the second-order polynomial equation for the experimental data. The F-test and p-value determines the significance of each process parameter on the Cu(II) adsorption onto starch-g-NMVA. A p-value of less than 0.05 and a calculated F-value higher than the tabulated F-value would indicate statistical result.
The quadratic model was highly significant, as was evident from the low p-value of 0.004. Furthermore, the calculated F-value (Fcal = 15.95) was found to be greater than the tabulated F-value (Fα,df,(n-df-1) = F0.05,9,7 = Ftab = 3.68) at 5% level, indicating that the computed Fisher’s variance ratio at this level was large enough to justify a high degree of the quadratic model and also to indicate that the treatment combinations or runs are highly significant, as similarly reported by others (54). Since Fcal > Ftab (15.95 > 3.68), The Fisher’s F-test concluded with 95% certainty that the regression model explained a significant amount of the variation in the dependent variable.
All the three parameters pH, adsorbent dose, and initial Cu(II) concentration are significant. However in the 2-way interactions, only the interaction between pH and adsorbent dose was significant. Thus signifies that pH and adsorbent dose have considerable contribution in the model. The high value of the coefficient of determination (R2 = 0.9663) as determined by the Minitab software indicates that 96% of the variability in the response is explained by the model.Metal uptake depends on pH and is related to both the functional groups on the adsorbent surface and the metal chemistry in solution, which affects the surface charge of the adsorbent and the degree of ionization of the adsorbate (Tumin et al., 2008). In Figure 5A, it can be observed that % removal increased with pH. At pH 1.5, the adsorption capacity is low due to the increase in positive charge density on the surface sites, and thus electrostatic repulsion occurs between the metal ions and the edge groups with positive charge on the surface. At pH 5.5, the surface charge of the graft copolymer becomes negatively charged, which enhances Cu(II) adsorption through electrostatic attraction (Tumin et al., 2008).
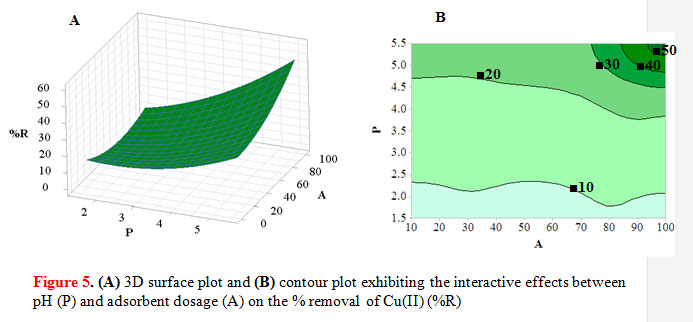 |
Figure 5: (A) 3D surface plot and (B) contour plot exhibiting the interactive effects between pH (P) and adsorbent dosage (A) on the % removal of Cu(II) (%R) |
It was observed that an increase in the removal of Cu(II) ions is caused by an increase in initial Cu(II) concentration. At lower Cu(II) concentration, the ratio of number of moles of metal ion to the available adsorption sites is low, and therefore the amount adsorbed per unit adsorbent increases slowly. With increasing metal ion concentration, there is an increase in the amount of metal ions adsorbed due to increased driving force of the metal ions toward the active sites on the adsorbent (Abdel Salam et al., 2011).
The effect of adsorbent dose on the % removal of Cu(II) ions is shown simultaneously with pH (Figure 5) . The % removal was observed to increase as the adsorbent dose increased from 10 to 100 mg. At a low dose of 10 mg starch-g-NMVA, there is tight competition between the Cu(II) ions due to the limited number of available binding sites; hence a low % removal was attained. An increase in adsorbent dose to 100 mg would cause a corresponding increase in % removal due to more adsorption sites that are available for Cu(II) uptake (Tumin et al., 2008). Table 3 summarizes the best parameters obtained from the statistical software.
Table 3: Best adsorption parameter values for maximum Cu(II) removal
| Adsorption parameters | Best values |
| % Removal | 52.90 |
| pH | 5.5 |
| Adsorbent dose (mg) | 100 |
| Initial Cu(II) concentration (mg/L) | 100 |
Adsorption isotherm studies
Adsorption isotherms are critical for design purposes since it describes how the adsorbate and the adsorbents interact with each other. It can be made into a model equation where it expresses the relation between the amount of solute adsorbed and the concentration of the solute in the fluid phase. For any adsorption operation the correlation of equilibrium data using an equation is essential. In this study, two isotherm equations were adopted: the Freundlich isotherm equation and the Langmuir isotherm equation.The adsorption of Cu(II) onto the starch-g-NMVA adsorbent fits the Langmuir model. The plot of 1/qe against 1/Ce , as shown in Figure 6, gives a straight line with r2 value of 0.9381. The best fit line obtained from plotting log qe against log Ce for a Freundlich model (Figure 7) gives an r2 value of only 0.8914.
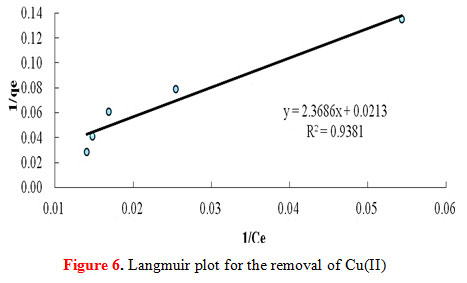 |
Figure 6: Langmuir plot for the removal of Cu(II) |
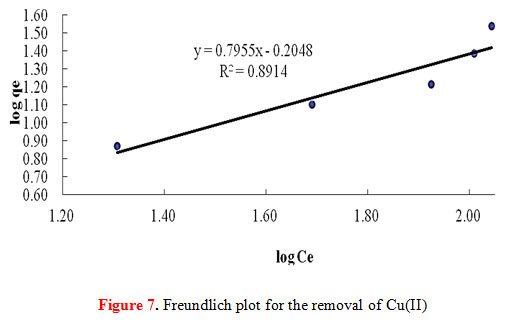 |
Figure 7: Freundlich plot for the removal of Cu(II) |
Table 4: Langmuir and Freundlich isotherm parameters for the adsorption of Cu(II) onto starch-g-NMVA.
| Isotherm | Parameter | Value | R2 |
| Langmuir | KL | 0.07350 | 0.9361 |
| Qo | 46.9749 | ||
| b | 0.0504 | ||
| Freundlich | Kf | 0.6240 | 0.8914 |
| n | 1.2571 | ||
| 1/n | 0.7955 |
The Langmuir equation assumes that during maximum adsorption a saturated mono-layer of adsorbate molecules formed on the adsorbent surface, the energy of adsorption is constant, and there is no transmigration of adsorbate in the plane of the surface (Zenasni et al., 2012). A large value of the Langmuir adsorption equilibrium constant, b implies strong bonding of Cu(II) to the graft copolymer. This is due to the chemical modification by grafting N-methyl-N-vinylacetamide on starch, thus, increasing its metal binding abilities. Additionally, the characteristic equilibrium parameter, KL, which is found to be 0.0735, indicates favorable adsorption for all initial concentration (Co) studied as it lies between the values of 0 and 1. The Langmuir monolayer adsorption capacity (Qo) has a value of 46.97 mg/g which is the amount of the metal required to occupy all the available sites per unit mass of the sample (Zenasni et al., 2012).
The ability of Freundlich model to fit the experiment data was also examined. For this case, the plot of log qe vs log Ce (Figure 7) was employed to generate the intensity of adsorption (1/n) and adsorption capacity Kf which were calculated from the slope and intercept of the plot respectively. The Freundlich constants Kf and n obtained from this study were 0.6240 and 1.2571 respectively. The intercept Kf value is an indicator of the adsorption capacity of the adsorbent while the slope 1/n indicates the effect of concentration on the adsorption capacity and represents adsorption intensity. The magnitudes of Kf and n values obtained show easy separation of Cu(II) ions from the aqueous solution and indicate favorable adsorption. As seen from Table 5, n value was found to be good enough for separation as it is greater than 1.
Consequently, the sorption process of metal ions on starch-g-NMVA follows the Langmuir isotherm model, where the metal ions are taken up independently on a single type of binding site in such a way that the uptake of the first metal ion does not affect the sorption of the next ion.
Conclusion
In this study, starch was grafted with monomers of N-methyl-N-vinylacetamide (NMVA) using microwave irradiation and potassium peroxydisulfate (KPS) as radical initiator. Variation of the monomer concentration and exposure time yielded the highest % grafting which is 10.03%. It was obtained at 0.55 M of NMVA and 90 seconds exposure time keeping the starch, KPS, and microwave power at 0.1g, 0.0014M, and 1200W respectively. The graft copolymer was also investigated for its efficiency in removing Cu(II) ions from aqueous solutions at different concentrations. Using the Box-Behnken method, the best parameter conditions of 100 mg/L initial Cu(II) concentration, 100 mg adsorbent dose and a pH of 5.5 were predicted to yield percent Cu(II) removal of 52.90%. The adsorption data were also fitted to both Langmuir and Freundlich isotherms and it was found out that Langmuir isotherm best described the equilibrium data with R2 = 0.9361, which signifies that a homogeneous adsorption takes place between Cu(II) ions and starch-g-NMVA. Starch-graft-N-methyl-N-vinylacetamide was proven to be an effective adsorbent for the removal of Cu(II) ions from aqueous solution with an adsorption capacity of 46.9749 mg/g.
Conflicts of Interests
The authors have no conflict of interest to declare.
Acknowledgement
The authors wish to acknowledge the Kinaadman Research Center (KRC) of Xavier University-Ateneo de Cagayan for the research assistance provided through the Kinaadman Support for Student Research (KSSR).
References
- Abdel Salam, O.E., Reiad, N.A. and ElShafei, M.M. (2011). A Study of the Removal Characteristics of Heavy Metals from Wastewater by Low-Cost Adsorbents. J. Adv. Res. 2, 297-303.
- Çankaya, N. (2016). Synthesis of graft copolymers onto starch and its semiconducting properties. Results in Physics 6 (2016) 538–542.
- Castañeda, M. Mirasol, M. S., Raymundo, L. A. and Solidum J. (2012). Biosorption and Desorption of Lead (Pb+2) from Simulated Waste Water Using Freshwater Snail Shells, Melanoides tuberculata Muller (Family Thiaridae). 2nd International Conference on Environment and BioScience. 44, 54-59.
- Fosso-Kankeu, E., Waanders, F., Maloy, E. (2016). Copolymerization of ethyl acrylate onto guar gum for the adsorption of Mg(II) and Ca(II) ions. Desalination and Water Treatment. 57. 1-10.
- Hasret, E., Ipekoglu, M., Altintas, S. and Ipekoglu, N. A. (2012). Microwave-assisted synthesis of hydroxyapatite for the removal of lead(II) from aqueous solutions. Environ. Sci. Pollut. Res. 19, 2766-2775.
- Kalia, S. and Sabaa, M. W. (2013). Polymer Grafting: A Versatile Means to Modify Polysaccharides. Polysaccharide Based Graft Copolymers. Springer: Heidelberg. p 1-14, 59-110.
- Karthika, JS. and Vishalakshi, B. (2014). Microwave-Assisted Synthesis and Characterization of Poly(2-(dimethylamino)ethyl methacrylate) Grafted Gellan Gum. International Journal of Polymer Anal. Charact., 19: 709–720.
- Lakshmi, P., Vijayalakshmi, K. and Sudha, P. N. (2011). Synthesis and characterization of graft copolymers of nylon 6 with maleic anhydride and methylmethacrylate. Arch. Appl. Sci. Res. 3 (6), 351-363.
- Liu, Y. H., Li, Y., Yang, L., Liu, Y. and Bai, L. (2005). Graft copolymerization of methyl acrylate onto sodium alginate initiated by potassium diperiodatocuprate(III). Polimery. 50, 37-42.
- Manivannan, P., Arivoli, S. and Mohammed, S. R. (2015). Mechanistic studies of copper (II) ion adsorption on activated Hibiscus sabdariffa stem nano carbon. J. Chem. Pharm. Res. 7(12), 654-664.
- Mrinalini, K., Anuja, A.B. (2015). Removal of heavy metals from water (Cu and Pb) Using household waste as an adsorbent, J. Biorem. Biodegrad. 6, 1–6.
- Mehta, M. and Chorawala, K. (2014). Adsorptive Removal of Dye From Industrial Dye Effluents Using Low-Cost Adsorbents: A Review. Int. Journal of Engineering Research and Applications. 12. 40-44.
- Moroncola BA., Alegbe MJ, Giwa-Ajeniya AO, Shotonwa IO, Ashilokun AO, Sobola AO. (2016). Removal of copper, cobalt and zinc from aqueous solution using Musa sapientum (Plantain Peel) as bioadsorbent. Int. J. Multidiscip. Res. Dev. 3 22-8.
- Mostafa, Kh. M. and EL-Sanabary, AA. (2013). Synthesis and Characterization of Novel Smart Flocculant Based on Poly(MAam)‐Pregelled Starch Graft Copolymers and Their Degraded Products. Adv. Polym. Technol. 32, 21339.
- Nadiah, N. I.; Uthumporn, U.; Syahariza, Z. A. (2016). Effect of Microwave Heating on Potato and Tapioca Starches in Water Suspension. IJASEIT. 6(1), 61-68.
- Ocreto, J., Go, C., Chua, J., Apacible, C., Vilando, A. (2019). Competitive effects for the adsorption of copper, cadmium and lead ions using modified activated carbon from bamboo. MATEC Web of Conferences 268, 06021.
- Onundi, Y. B., Mamun, A. A., Al Khatib, M. F. and Ahmed, Y.M. (2010). Adsorption of copper, nickel and lead ions from synthetic semiconductor industrial wastewater by palm shell activated carbon. Int. J. Environ. Sci. Tech. 7 (4), 751-758.
- Ozturk, D.; Sahan, T. (2015). Design and Optimization of Cu(II) Adsorption Conditions from Aqueous Solutions by Low-Cost Adsorbent Pumice with Response Surface Methodology. Pol. J. Environ. Stud. 24 (4), 1749-1756.
- Paksamut, J. and Boonsong, P. (2018). Removal of Copper (II) Ions in Aqueous Solutions Using Tannin-Rich Plants as Natural Bio-Adsorbents. IOP Conf. Ser.: Mater. Sci. Eng. 317.
- Pandey, S.; Mishra, S. B. (2012). Microwave synthesized xanthan gum-g-poly(ethylacrylate): An efficient Pb2+ ion binder. Carbohydrate Polymers.90, 370-379.
- Singh, V., Tiwari, A., Pandey, S. and Singh, K. (2007). Synthesis and characterization of electrical conducting chitosan-graft-polyaniline. eXPRESS Polymer Letters. 1 (1), 51-58.
- Tarangini, K., Kumar, A., Satpathy, G.R. and Sangal, V.K. (2009). Statistical Optimization of Process Parameters for Cr (VI) Biosorption onto Mixed Cultures of Pseudomonas aeruginosa and Bacillus subtilis. Clean. 37 (4-5), 319-327.
- Tumin, N. J., Chuah, A. L., Zawani, Z. and Abdul Rashid, S. (2008). Adsorption of copper from aqueous solution by Elais guineensis kernel activated carbon. JESTEC. 3(2), 180-189.
- Zhang, C., Su, J., Zhu, H., Xiong, J., Liu, X., Li, D., Chen, Y., and Li, Y. (2017). The removal of heavy metal ions from aqueous solutions by amine functionalized cellulose pretreated with microwave-H2O2. RSC Adv., 2017, 7, 34182
- Zenasni, M. A., Benfarhi, S., Merlin, A., Molina, S., George, B. and Meroufel, B. (2012). Adsorption of Cu(II) on maghnite from aqueous solution: Effects of pH, initial concentration, interaction time and temperature. Natural Science. 4 (11), 856-868.
- Zhu, L., Zhang, L. and Tang, Y. (2012). Synthesis of Montmorillonite/Poly(acrylic acid-co-2-acrylamido-2-methyl-1-propane sulfonic acid) Superabsorbent Composite and the Study of its Adsorption. Bull. Korean Chem. Soc.33 (5), 1669-1674


