1Biology Department, Faculty of Science, King Abdulaziz University Jeddah, Saudi Arabia.
2Botany and Microbiology Department, Faculty of Science, Kafrelsheikh University, Egypt.
Corresponding author email: magdammali@hotmail.com
Article Publishing History
Received: 28/04/2021
Accepted After Revision: 25/06/2021
The removal of heavy metals by actinomycetes has been the subject of many investigations as they used as potential heavy metal sorbents. This study aimed to evaluate chromium biosorption activity of some actinomycetes isolated from wastewater sample collected Industrial Wastewater Treatment Plant (IWWTP), Jeddah, Saudi Arabia. About 35 different isolates of actinomycetes were obtained on starch nitrate medium with 100 ppm of chromium ions. The removal of chromium from growth medium was maximum by isolate FM2 which was selected and identified as a species belonging to the genus Streptomyces. The 16S rRNA sequence of this isolate showed the highest similarity (98%) with Streptomyces mutabilis and identified as S. mutabilis FM2. The growth of the previous isolates was determined after 5 days at 30◦C in the presence of different chromium oxide concentrations, 50- 300 mg/l. The minimum inhibitory concentration (MIC) of this isolate for Cr (VI) was 135 mg/l. By dead biomass of the previous isolate FM2 (1g/l), the biosorption capacity measured by Plasma Atomic Emission Spectrometer ICPE-9000 of the bacterial strain for Cr (VI) ion was 800 mg/l which was 38% of the initial metal ion concentration.
The maximum biosorption process for the tested isolate was recorded at pH 7, optimum temperature at 45◦C. Biosorbent mass of 0 – 1.5 g was tested for removal of chromium. The result showed that the adsorption capacities against the heavy metal Cr (VI) were increased with increasing the weight of the used dry biomass. The genus Streptomyces was the most potent in removing of heavy metals and S. mutabilis biomass has a good potential to be used in removal of chromium from wastewater. Their use in real life situation can alleviate pollution and increase the quality of water for human consumption and sanitary purposes. Initial concentration of metal ion, pH and cell biomass affect chromium biosorption process by the dried cells of Streptomyces mutabilis.
Biosorption. MTC, Biomass, Streptomyces Mutabilis, Biomass, Wastewater, Bioremediation.
Zabermawi N. O, Bakran F. M, Jastaniah S. D, Noor S. O, Aly M. M. Biosorption of Chromium Ions by Streptomyces Mutabilis Isolated from Industrial Wastewater Treatment Plant. Biosc.Biotech.Res.Comm. 2021;14(2).
Zabermawi N. O, Bakran F. M, Jastaniah S. D, Noor S. O, Aly M. M.Biosorption of Chromium Ions by Streptomyces mutabilis Isolated from Industrial Wastewater Treatment Plant. Biosc.Biotech.Res.Comm. 2021;14(2). Available from: <a href=”https://bit.ly/3pb1nx1“>https://bit.ly/3pb1nx1</a>
Copyright © Zabermawi et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
One of the growing problem over the world is water pollution by chemical specially with heavy metals which showed serious side effects on human health and its environment. Several metals were highly toxic at low concentrations and through food chains, they accumulated in liver, kidney and other humans and animal tissues (Singh et al., 2011, Sahmoune, 2016). Physical or chemical methods like activated carbon adsorption, ion exchange, membrane filtration, and chemical precipitation can be applied to remove heavy metals from wastewater (Wang and Chen, 2009, Lakherwal, 2014). Nanocomposites adsorbents were applied to the removal of heavy metals from aqueous solution (Lu et al., 2017). Ion imprinted polymers has been used for elimination of heavy metal ions at low concentrations in complicated matrices (Cai et al., 2013, Fu et al., 2016). Heavy metals bioremediation methods received increasing attention because they are more safe and easily to be applied (Saiano et al., 2005, Yin et al., 2016).
Through the two decades, biosorption method is efficient technique, cost effective and alternative for water and wastewater treatments (Ahmad et al., 2018). Biosorption methods were briefly studied by many authors (Al-Homaidan et al., 2014, Vendruscolo et al., 2017, Abed et al., 2020). Biosorption is a physicochemical process which involves the removal of various pollutants, such as heavy metals from solution by biological materials (Fadel et al., 2017, Yang and Wang, 2009) . Different types of microorganisms, such as bacteria, algae, fungi and yeast cells can remove heavy metals from aqueous solutions (Vijayaraghavan and Balasubramanian, 2015, Podder and Majumder, 2016). The removal rate of Cr6+ has been explored by the optimized removal conditions by Bacillus subtilis strain SZMC 6179J and the initial pH, initial Cr6+ concentration (mg/l), time (hrs) and inoculation percentage (%) affect removal process. The optimal conditions for removal by the previous isolate were at pH 5.0, incubation time 24.0 hrs, inoculation percentage 4.6% (v/v) and initial concentration of Cr6+ 55.0 mg/l (Liu et al., 2020).
Actinomycetes, particularly Streptomyces species have been tested for uptake metals with very encouraging results (Gupta et al., 2015). Streptomyces species are stable and are not subject to the drastic treatments, and possess advantages such as low investment cost, and high treatment efficiencies (Mosbah and Sahmoune, 2013). Among the actinomycetes, the genus Streptomyces have produced useful secondary metabolites and the major source of a new bioactive molecule and good antibiotic producing organisms (Takahashi and Omura, 2003). Streptomyces are gram-positive filamentous bacteria with high guanine/cytosine content. Streptomyces strains characterized by a complex life cycle and the production of an amount of secondary metabolites which often find a use in medicinal, in applications (Hopwood, 2006). Streptomyces cells have excellent role in heavy metal removals and degradation of different pollutions. The aim of this study is to investigate the use of Streptomyces mutabilis biomass as an adsorbent for removing heavy metals from industrial wastewater solutions such as chromium which could reach to the underground water, causing harmful effects on human, animals, and plants.
MATERIAL AND METHODS
Collection of soil samples: For the present investigation, soil samples were collected from different contaminated soil area and were used for Actinomycete isolation. All samples were collected from Industrial Wastewater Treatment Plant located in Jeddah. Each sample was collected in sterile test tubes and stored at 4°C until used.
Isolation and purification of chromium resistance bacterial isolate: About one gram of each soil samples was suspended in 9 ml of sterilized distilled water and serial dilution was done. One ml of each dilution was separately added to the surface of a plate containing starch nitrate agar medium and incubated at 30°C for 5 days. The obtained bacteria colonies were transferred to new plates until obtained of pure colonies which purified by streaking on soiled agar medium. The selected bacterial colonies were purified and transferred to slants on Starch nitrate agar for preservation at 4◦C. Long preservation of strains was in starch broth plus 50% sterile glycerol and stored at -20◦C until used (Ramesh and Mathivanan, 2009, Kannabiran, 2010).
Morphological, physiological and biochemical characterization of the bacterial isolates: Contaminated soil samples from Industrial Wastewater Treatment Plant, located in Jeddah, were used for actinomycetes isolation on Starch nitrate agar medium as described by Bakan et al., (2019). All actinomycete isolates were screened for Chromium resistant activity on starch nitrate agar medium containing 100 mg/l . After 5 days at 30◦C, the most resistance isolated were screened on medium contained different concentrations of Cr(VI) and minimum inhibitory concentration (MIC) was determined (Bakan et al., 2019). Strain FM2, the most resistant isolate, was preliminarily identified according to traditional morphological criteria, including morphology and growth pattern on starch nitrate agar for example.
Characteristics of the bacterial colonies on the agar plate, morphology of substrate and aerial hyphae, the morphology of spores, color of the produced pigment were carried out (Shirling and Gottileb, 1966). Also, the selected bacterial isolate FM2 was cultivated on different media plates for example; Yeast extract -malt extract agar (ISP-2), In-organic salts-starch iron agar (ISP-4), and Tyrosine agar (ISP-7) plates and identified according to morphology, physiology and biochemical characters. The cellular morphology of the bacterial isolate FM2 was examined under light microscope. Moreover, biochemical characterization of the isolate was carried out as described in International actinomycetes isolates Project including biochemical identification tests such as catalase, citrate oxidase, indole production (Pridham and Lyons, 1961).
Molecular identification: Genomic DNA from each isolate was obtained using the QIAamp DNA Mini Kit (Weisburg et al., 1991). Then, the concentration and purity of DNA were determined. Princess Al-Jawhara Center of Excellence in Research of Hereditary Disorders, the 16S rDNA gene was amplified by PCR using the forward primer 5’-AGTTTGATCATGGTCAG-3’ and the reverse primer 5’-GGTTACCTTGTTACGACT-3’. The DNA sequence was compared to the GenBank database at the National Center for Biotechnology Information (NCBI) using the BLAST program (Saitou and Nei, 1987).
Study of the minimal inhibitory concentrations: The actinobacteria isolate FM2 was screened for heavy metal- resistant activity in starch nitrate agar medium, after preparing of the medium it was sterilized by autoclaving at 121°C for 15 min, then different concentration of each sterile metal salt solution was added a to molten cooled starch nitrate agar medium immediately before pouring to the plates and solidification. Microbial growth was used as the qualitative parameter of mean resistance after seven days of incubation at 30°C. Testing for tolerance of the microorganisms ended when complete inhibition of the growth was observed on the nutrient agar with metal supplementation (Koushalshahi et al., 2012, Daboor et al., 2014). This method was used to determine the minimum inhibitory concentration (MIC) for each heavy metal at which there is no colony growth in the three copies. This method was used to give a rapid screening, but it is a qualitative estimation (Abbas and Edwards, 1989).
Biomass preparation of actinobacteria for biosorbent process: The biomass of actinobacteria (biosorbent) was prepared by Saurav and Kannabiran (2011b), (Latha et al., 2015). the tested isolate FM2 was cultivated in 500 ml flasks containing 100 ml of starch nitrite broth medium and was kept shaking in an orbital rotary shaker at 130 rpm for 10 days at 30◦C. Then, the cultures were harvested by centrifugation at 4500 rpm for 15 min and were washed three times with distilled water. The pellet was kept in glass petri dishes and dried at 70◦C for 24 h. After the biomass was dried, it was crushed in a blender to powder. Then, it was used for further studies.
Preparation of metal solutions: The heavy metals used for the screening of metal resistance in actinobacterial isolate were chromium (Cr). The salt solutions were prepared from analytical-grade chemicals such as Chromium (VI) oxide, sterilized separately for 15 min at 110°C (Saurav and Kannabiran, 2009) and preserved at 4℃ until used. The concentrations of metal ion were prepared from stock solutions of 100,000 mg/l. Fresh dilutions were used for each study. The pH of each test solution was adjusted to the required value by using 1 M NaOH and 1N HCl (Latha et al., 2015).
Biosorption experiments: The chromium removal ability of the actinomycete isolate FM2 was determined by measuring the level of chromium uptake following the method of Saurav and Kannabiran (2011b) with slight modifications. The isolated strain was tested to biosorption of Cr (VI) metal salt solution at different concentrations (50–300 mg/l) then in different concentrations (0.5, 1.0, 2.0, 2.5, 3.0 g/l of biomass obtained from tested strain. The dried biomass of the actinomycete isolate was suspended and the pH was adjusted to 7.0. Then the flasks were kept shaking in an orbital rotary shaker at 130 rpm for one week after that the filtrates were analyzed for chromium concentration by Plasma Atomic Emission Spectrometer ICPE-9000. The metal removal efficiency (MRE) was calculated by using the following equation.
![]()
Where MRE: metal removal efficiency, Ci represents the initial chromium metal ion and Cf represents the final chromium metal ion concentration.
Effect of pH and temperature on Heavy Metal Removal : The dry biomass (0.25 g) of the Actinomycete isolate FM2 was incubated into a series of 100 ml conical flasks containing either 25 ml of distilled water with 320 mg/l of chromium (VI) oxide. The pH was varied from 4 to 10 (4, 6, 7, 8 and 10). On the other hand, the effect of different temperatures (24, 28, 30, 35 and 45◦C) on biosorption capacity was detected. The pH of the medium was adjusted using dilute HCl or NaOH. Then, all flaks were incubated at 30◦C and 130 rpm for 5 days. The percentage biosorption of metal ions was calculated at the all tested pH values.
Concentration of Heavy Metals Residuals in the Bacterial Biomass : To detect the residual of chromium found in the biomass of the isolate which they involved in bioremediation technique, the biomass fragments were collected after centrifugation at 4500 rpm in 15 minutes. then the samples pallets were collected and oven-dried at 70∘C about 24 h. After that, each sample (0.5 g) was then digested through addition of 5 ml of hydrochloric (HCl) acid and 5 ml sulphuric acid (H2SO4). After heating gently until the samples were digested (formation of a clear solution above the residue), the volume was adjusted to 10 ml with distilled water, pH was adjusted to 7 with 5 M NaOH and the solutions were analyzed for metals concentrations Cr (VI) using Plasma Atomic Emission Spectrometer (ICPE-9000).
Applied Biosorption Experiments: This experiment was detected the ability of dead bacterial biomass FM2 to removal heavy metals (Cr(VI)) from industrial wastewater. At first, the content of different heavy metals of waste water was analysis of by Plasma Atomic Emission Spectrometer ICPE-9000. Then, two conical flask (250 ml) containing 100 ml of waste water from Bani Malek Station, with 0.5 g of dry dead bacterial biomass, obtained from tested, was kept shaking at 130 rpm for one week at 30C. After that the content of each flask was filtered through filter paper and the filtrate was analyzed for metal concentration by Plasma Atomic Emission Spectrometer ICPE-9000. The percentage biosorption of metal ions was calculated as follows:
![]()
RESULTS AND DISCUSSION
The actinobacteria with potential heavy metal biosorption ability were selected for identification. The morphological, culture and biochemical characteristics of the strain were investigated and recorded in the Table 1. The isolate was Gram positive, not acid fast, with non-motile cells and substrate and aerial mycelia were well developed. The color of the isolate, on starch nitrate was gray. The growth on solid medium and examined under light microscope were recorded (Table 2, Figure 1). The growth and shapes on different agar media were appeared in Table 3 and Figures 2.
Table 1. The selected actinomycetes, color, growth, mycelia and production of melanin pigment
| Isolates | Color | Growth (100 ppm Cr) | Substrate and aerial mycelia | Melanin pigment | |
| On agar medium | Dry weight/l | ||||
| FM1 | Pink | + | 0.17±0.01 | Well developed | +ve |
| FM2 | Gray | ++++ | 0.37±0.02 | Well developed | -ve |
| FM4 | Black | ++ | 0.30±0.02 | Well developed | +ve |
| FM9 | Pink | ++ | 0.23±0.02 | Well developed | -ve |
| FM11 | Gray | ++ | 0.30±0.09 | Well developed | -ve |
| FM15 | White | ++ | 0.21±0.01 | Well developed | -ve |
+: Poor growth, ++: Moderate growth, +++: Good growth, -ve: No pigment, +ve: Detected pigment
Table 2. Morphological characteristics of the selected isolate FM2
| Characteristics | Results | Characteristics | Results |
| Gram stain | Gram-positive | Aerial mycelium | Present |
| Colony size | Discrete | Sporangia | Absent |
| Acid fast stain | Negative | Optimum temperature | 30 °C |
| Motility | Absent | Optimum pH range | 6.5 – 8.0 |
| Respiration | Aerobic | Melanin pigment | Positive |
| Substrate Mycelium | Branched | Catalase | Positive |
| Spore chain | Positive | Penicillin | Sensitive |
| Motile spores | Absent | Cephalosporin | Resistance |
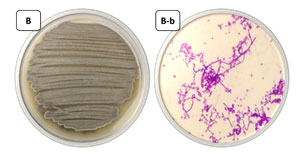
Figure 1: The selected actinomycete isolate FM2, B: grown on starch nitrate agar medium, B-b: under light microscope x1000.
The isolate FM2 grow well on starch nitrate agar, In-organic salt starch iron agar ISP4, and tyrosine agar was recorded on ISP-7, while moderate growth on yeast extract malt extract agar (ISP-2), E-Medium (ISP-9), Glycerol asparagine agar (ISP-5), and dextrose agar. On the other hand, the isolate FM2 use different carbon and nitrogen sources, it was found that it used strongly the carbon sources, glucose, sucrose, lactose, and maltose while moderate utilization was recorded by isolate FM2 for starch, dextrose and fructose. Moreover, different nitrogen sources, yeast extract and peptone were well utilized by isolate FM2.
Table 3. Growth of FM2 isolate on different media growth for 5 days at 30◦C.
| Media | Growth | Color of aerial mycelium | Color of substrate mycelium | Soluble pigment |
| Starch Nitrate | Heavy | Grey | Grey | Light grey |
| Yeast extract-malt extract (ISP-2) | Moderate | Creamy | Creamy | Light brown |
| In-organic salt-starch iron (ISP4) | Heavy | Dark grey | Grey | Creamy |
| Glycerol asparagine (ISP-5) | Moderate | White | White | No- pigment |
| Tyrosine medium (ISP-7) | Heavy | Light grey | Light grey | No- pigment |
| E-Medium (ISP-9) | Moderate | Page | Page | No- pigment |
| Dextrose medium | Moderate | Creamy | Creamy | Yellow pigment |
Figure 2: Growth of bacterial isolate FM2 on different agar media
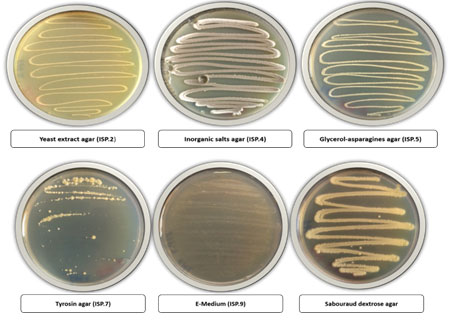
Table 4: Growth of bacterial isolate FM2 on different carbon and nitrogen sources
| Carbon sources | Glucose | Sucrose | Starch | Lactose | Dextrose | Maltose | Fructose |
| Results | + + + | + + + | + + | + + + | + + | + + + | + + |
| Nitrogen source | Ammonium sulfate | Ammonium chloride | Sodium Nitrate | Potassium nitrate | Glycine | Vanillin | Peptone |
| Results | ± | + | + | + | + + | + | +++ |
+++: high utilization, ++: moderate utilization, +: weak utilization, ±: very weak utilization -: no utilization
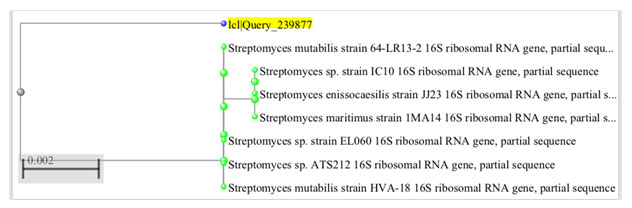
Molecular Identification: The selected strain shown the partial sequencing of 16S rRNA gene of the strain on both directions yielded 16S rDNA nucleotide sequence length with 1300 base pairs. The BLAST search of 16S rDNA sequence of the strain showed the highest (98%) similarity with Streptomyces mutabilis strain 64-LR13-2, Streptomyces mutabilis strain HBUM83834, and Streptomyces sp. Strain ELO60 (Figure 3).
Figure 3: Phylogenetic tree based on 16s rRNA for Streptomyces mutabilis FM2.
Minimum Inhibitory Concentration (MIC) of Chromium: In this study, the actinomycetes isolate from soil were explored for their bioremediation capabilities to prove that they have potential in bringing down the intensity of heavy metals in media. The inhibitory effects of Cr (VI) on bacterial growth were investigated on starch nitrate agar medium. The MIC was 135 mg/l for Cr (VI) as shown in Figure 4 and Table 4.
Figure 4: Effect of different concentration of chromium (50-135 mg/l) on growth to the isolate FM2.

Table 5. Actinomycetes isolates tolerance to different chromium concentrations.
| Cr (VI) (mg/l) | 50 | 70 | 100 | 115 | 125 | 120 | 125 | 130 | 135 | 150 |
| FM 2 | ++ + | ++ + | + ++ | + ++ | + + | + + | ++ | + | + | – |
+++: high growth, ++: moderate growth, +: Low growth,-: no growth
Percentages of chromium adsorption by FM2 cells (0.1 g/l) added to different concentrations of chromium were determined. At 50 mg/l Cr, the removal percentage was 100% and decreased by increasing Cr concentrations (Figure 5). The percentage of adsorption was a function of the initial metal concentration. The amounts of metal uptake q (mg/g) by the dead biomass of FM2 at a different metal concentration Cr (VI) are calculated. Table 5 showed the amounts of chromium uptake q (mg/g) by the different concentrations of the dead biomass of isolate FM2. The amounts of metal uptake q (mg/g) by the dead biomass of isolate FM2 was ranged from 23.23% to 5.00% with a maximum adsorption of 34.86 % using 0.12 mg/l of the dry mass. As the biosorbent weight of the tested isolate was increased from 0.12 to 1.50 g, the removal percentage increased from 34.86% to 93.91% for Cr (VI) but the chromium uptake q (mg/g) was decreased.
The results showed the effect of pH on the adsorption of chromium oxide by dead biomass of the tested isolate. The highest biosorption capacity for chromium was at pH7 (Figure 7). Figure 8. showed the effect of different temperatures on the adsorption of chromium by the dead biomass of the tested. Biosorption capacity was analyzed over a temperature range from 24℃ to 45℃. The highest biosorption capacity of chromium by the tested isolate was 100% at 37-45℃. Wastewater was collected from wastewater treatment plant and the selected bacterial isolate was used for chromium removal from wastewater. At the beginning the Chromium concentrations was 11 mg/l. After 7 days of incubation with the tested bacteria, the concentration of chromium decreased to 0.0.
Figure 5: Capacity of chromium adsorption by FM2 isolate (0.1 g/l) on different concentrations of chromium.
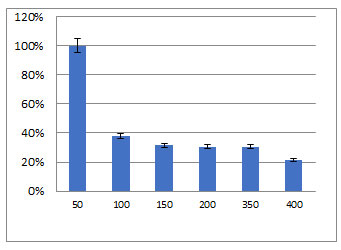
Table 6. Effect of different concentrations of biomass on Chromium (VI) removal from a solution at 320 mg/l.
| Biomass weight
(g/l) |
Final chromium
concentration (mg/l) |
% Biosorption | Specific metal
uptake (mg/g) |
| 0.00 | 320 | 0.00 | 0.00 |
| 0.12 | 208.41 | 34.86 | 23.24 |
| 0.25 | 172.91 | 45.96 | 14.70 |
| 0.50 | 77.90 | 75.65 | 12.10 |
| 0.75 | 64.65 | 79.79 | 8.51 |
| 1.00 | 51.25 | 83.98 | 6.71 |
| 1.50 | 19.48 | 93.91 | 5.00 |
* Specific metal uptake (Q)
Figure 6: Effect of different pH rage on biosorption of Cr(VI) at 50 mg/l by dried cell of bacterial isolate FM2 (0.12 mg/l).
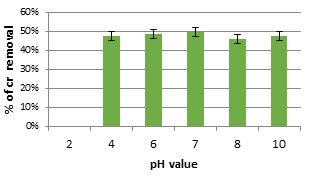
Figure 7: Effect of different temperatures on the biosorption of chromium at 50 mg/l by dried cells of bacterial isolates FM2 (0.12 mg/l).
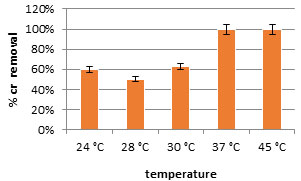
Table 7. Biosorption of some heavy metals from waste water using dry biomass of the isolate FM2.
|
Isolate |
Chromium (VI) Concentration | ||
| At the start (mg/l) | After 7 days (mg/l) | % of Biosorption | |
| FM2 | 11±0.7 | 0.00 | 100 % |
In Saudi Arabia, Jeddah is one of the biggest cities with about three million inhabitants. Large quantities of wastewater returned to the environment and accumulated in underground pools. Only 30 percent went wastewater treatment plants for purification before being dumped in the Red Sea. Most of the wastewater that is accumulated through pipes must be treated for heavy metal removal before dumping into the sea. Without purification, wastewater caused massive damage to the aquatic animals, beaches, air, human health and soil. Sewage treatment plants facilitate and improved wastewater management. The heavy metals such as Fe++, Cu++, Zn++, Ni++, Mn++, Cd++, Pb++ and Cr+++ are toxic pollutants of the environment, wastewater and aquatic live (Amundsen et al., 1997; Wong et al., 2001). Accumulation of these metals in soil, animal tissue is documented (Li et al. 2006, Okoye et al. 2011, Abduljaleel et al. 2012).
Thus, in recent decades removal of heavy metals using conventional physical and chemical methods are urgent but these methods are high expensive, low effectiveness and non eco-friendly. Biosorption methods by biological materials like live or dead microbes of non-pathogenic bacteria, fungi, gut microflora and actinomycetes can be used to remove detoxify and eliminate some heavy metals from soil and solutions. These methods are effective, little price, eco-friendly. easy to applied and had beneficial health effects (Wang and Chen, 2009).
In different ways, actinomycetes played an important role in removal of harmful metals. Thus, in this study we tried to isolate and identify some actinomycete genera from contaminated area that can remove Chromium ions which showed high toxicity to living cells and pollute human environments (water, food, air and soils). Two forms of Chromium are recorded, trivalent (Cr III) which is less toxic to plants and animals and hexavalent (CrVI) which cause human cancer, genotoxicity, acute and chronic toxicity to skin and nervous and immune systems in addition to general environmental toxicity (Bagchi et al. 2002).
As was reported before, actinomycetes showed exceptional resistance properties to some heavy metals and they adaptive themselves to adsorb these metals during continuous exposure (Abbas and Edwards 1989; Amoroso et al. 2000). Koushalshahi et al. (2012) reported that actinomycetes from contaminated samples are resistant to heavy metals compared to those from non-contaminated area. On minimal agar medium, out of 15 isolates, isolate FM2 was the most tolerant isolate (MIC value 135 mg/l). Latha et al. (2015) reported that the MIC was in the ranged from of <50–250 mg/l.
It was identified as species belong to genus Streptomyces according to morphological, physiological, and biochemical characteristics. Identification was confirmed using molecular method. 16S rRNA sequence analysis is a tool used for confirmation of the identification of bacteria (Wallhausser et al. 1964, Muharram et al. 2013). Using 16Sr RNA, he isolate FM2 was identified as Streptomyces mutabilis FM2. The previous method was used by many authors (Dhanasekaran et al. 2012; Saha et al. 2013, Bahamdain et al., 2020, Aly et al., 2020).
The biosorption capacity of Streptomyces mutabilis for Cr(VI) was found to be maximum (100%) at 50 mg/l metal ion concentration using 0.1 g/l of the cell biomass. At pH 7.0 at 37- 45◦C, the higher biosorption ability (100%) was obtained using 0.12 mg/l of the dry material. Increasing the concentration of Cr(VI) decreased biosorption capacity which may due to metal saturation on biosorption material while increasing the weight of the biosorption material, increased the biosorption capacity due to the highest contact between bacterial cell wall and metal ions (Saurav and Kannabiran, 2011a,b). They added that maximum Cr(VI) biosorption by Streptomyces sp. dry cells (3 g/l) was obtained at 100 mg/l at pH 7. biosorption or removal capacity of heavy metal was affected by biosorbent weight, pH and initial metal concentration (Donmez et al. 1999, Yao et al. 2009, Saurav and Kannabiran 2011b) Cr(VI) ions react with several functional groups like the hydroxyl (OH), amine (NH2) and carboxyl (CO) groups, found on the bacterial biomass which play an important role in the metal ions biosorption process. Microbial cell walls contained, peptidoglycan polysaccharide, glycoprotein, glucan, chitin, mannan, phosphomannan and teichoic and teichuronic acids which may adsorb metal ions (Volesky 1990).
The potent Cr(VI) biosorbent Streptomyces mutabilis was used to purify wastewater from heavy metals and to control the problem of bioaccumulation in living cells. Bakran et al. (2019) used two Streptomyces to remove lead from wastewater. In conclusion, Streptomyces mutabilis belong to actinomycetes and showed excellent activity to remove and resist hazardous heavy metals like Cr(VI) with maximum biosorption (100 %) at 0.12 g/l at pH7 and 37-45◦C after 7 days.
REFERENCES
Abbas A and Edwards C (1989) Effects of metals on a range of Streptomyces species. Appl Environ Microbiol 55(8):2030–2035.
Abduljaleel SA, Shuhaimi-othman M, Babji A (2012) Assessment of trace metals contents in chicken (Gallus gallus domesticus) and quail (Coturnix coturnix japonica) tissues from Selangor (Malaysia). Environ Sci Technol 5(6):441–451.
Abed RMM, Shanti M, Muthukrishnan T, Al-Riyami Z, Pracejus B and Moraetis D (2020) The Role of microbial mats in the removal of hexavalent chromium and associated shifts in their bacterial community composition. Front. Microbiol. 11:12.
Ahmad, A., Bhat, A. & Buang, A. (2018). Biosorption of transition metals by freely suspended and Ca-alginate immobilised with Chlorella vulgaris: Kinetic and equilibrium modeling. Journal of cleaner production, 171, 1361-1375.
Al-Homaidan, A. A., Al-Houri, H. J., Al-Hazzani, A. A., Elgaaly, G. & Moubayed, N. M. (2014). Biosorption of copper ions from aqueous solutions by Spirulina platensis biomass. Arabian Journal of Chemistry, 7, 57-62.
Aly M M, Alsiny M N, AbuRas MM. and Jastaniah SD. (2020). Antitumor L-glutaminase production by rare actinomycetes obtained from marine water using submerged fermentation. Bioscience Biotech, Research Combination, 13 (3).
Amoroso MJ, Schubert D, Mitscherlich P, Schumann P, Kothe E (2000) Evidence for high affinity nickel transporter genes in heavy metal resistant Streptomyces. J Basic Microbiol 40(5–6):295–301.
Bagchi D, Stohs SJ, Downs BW, Bagchi M, Preuss HG (2002) Cytotoxicity and oxidative mechanisms of different forms of chromium. Toxicology 180(1):5–22
Bahamdain L A, Abo Aba S E, Sabry A, Amasha R H., Noor S O and Aly M M (2020) Molecular Identification and Phylogenetic Analysis of Some Rare Actinomycetes Obtained from Al-Lith Hot Spring Area of Saudi Arabia. Biosc. Biotech. Res. Comm. Vol 13 (3): 1037-1049.
Bakran F M, Aly M M, Zabermawi N M O (2019) Removal of Some Heavy Metals from Industrial Wastewater by Actinomycetes Isolated From Contaminated Soil. IOSR Journal of Pharmacy and Biological Sciences. Volume 14( 5 ):58-69.
Cai X., Li J., Zhang Z., Yang F., Dong R. and Chen, L. (2013) Novel Pb2+ ion imprinted polymers based on ionic interaction via synergy of dual functional monomers for selective solid-phase extraction of Pb2+ in water samples. ACS Applied Materials & Interfaces, 6, 305-313.
Daboor SM., Haroon AM., Esmael NAE and Hanona S I (2014) Heavy metal adsorption of Streptomyces chromofuscus K101. Journal of Coastal Life Medicine, 2, 431-437.
Dhanasekaran D, Ambika K, Thajuddin N, Panneerselvam A (2012) Allelopathic effect of actinobacterial isolates against selected weeds. Arch Phytopathol Plant Protect 45(5):505–521.
Donmez GC, Aksu Z, Ozturk A, Kutsal T (1999) A comparative studyon heavy metal biosorption characteristics of some algae. Process Biochem 34(9):885–892
Fadel M., Hassanein N. M., Elshafei M. M., Mostafa AH Ahmed MA and Khater H M (2017). Biosorption of manganese from groundwater by biomass of Saccharomyces cerevisiae. Hbrc Journal, 13, 106-113.
Fu J, Wang X, Li J, Ding Y and Chen L(2016) Synthesis of multi-ion imprinted polymers based on dithizone chelation for simultaneous removal of Hg2+, Cd 2+, Ni 2+ and Cu 2+ from aqueous solutions. RSC Advances, 6, 44087-44095.
Gupta V K, Nayak A and Agarwal S (2015). Bioadsorbents for remediation of heavy metals: current status and their future prospects. Environmental Engineering Research, 20, 1-18.
Hopwood DA (2006). Soil to genomics: the Streptomyces chromosome. Annu. Rev. Genet., 40, 1-23.
Koushalshahi MB, Issazadeh K, Tehranifard A, Majid MR, Pahlaviani K, Massiha A (2012) Isolation of Hg and Cu resistant Streptomyces from marine sediments in different regions of the Caspian Sea. Afr J Microbiol Res 6(18):4048–4052.
Kumar, S. & Kannabiran, K. 2010. Antifungal activity of Streptomyces VITSVK5 spp. against drug resistant Aspergillus clinical isolates from pulmonary tuberculosis patients. Journal de Mycologie Médicale/Journal of Medical Mycology, 20, 101-107.
Lakherwal, D. 2014. Adsorption Of Heavy Metals: A Review. International Journal of environmental research and development, 4, 41-48.
Latha s., vinothini G and dhanasekaran D (2015) Chromium [Cr (VI)] biosorption property of the newly isolated actinobacterial probiont Streptomyces werraensis LD22. 3 Biotech, 5, 423-432.
Liu J., Xue, J., Wei, X. Huimin Su and Ruidan Xu (2020) Optimization of Cr6+ Removal by Bacillus subtilis Strain SZMC 6179J from Chromium-Containing Soil. Indian J Microbiol 60, 430–435.
Lu W, Li, J., Sheng, Y., Zhang, X., You J and Chen, L (2017) One-pot synthesis of magnetic iron oxide nanoparticle-multiwalled carbon nanotube composites for enhanced removal of Cr (VI) from aqueous solution. Journal of colloid and interface science, 505, 1134-1146.
Mosbah R and Sahmoune MN (2013) Biosorption of heavy metals by Streptomyces species—an overview. Central European Journal of Chemistry, 11, 1412-1422.
Muharram MM, Abdelkader MS, Alqasoumi SI (2013) Antimicrobial activity of soil actinomycetes isolated from Alkharj KSA. Int J Micr Res 4(1):12–20.
Okoye COB, Ibeto CN, Ihedioha JN (2011) Assessment of heavy metals in chicken feeds sold in south eastern Nigeria. Adv Appl Sci Res 2(3):63–68.
Podder M and Majumder C (2016) Predictive approach for simultaneous biosorption and bioaccumulation of arsenic by Corynebacterium glutamicum MTCC 2745 biofilm supported on NL/MnFe2O4 composite. Journal of Water Process Engineering, 11, 8-31.
Pridham TG and Lyons AJ (1961). Streptomyces albus (Rossi Doria) Waksman et Henrici: taxonomic study of strains labeled Streptomyces albus. J. Bacteriol. 81:431-441.
Ramesh S and Mathivanan N (2009) Screening of marine actinomycetes isolated from the Bay of Bengal, India for antimicrobial activity and industrial enzymes. World Journal of Microbiology and Biotechnology, 25, 2103-2111.
Saha S, Dhanasekaran D, Shanmugapriya S, Latha S (2013) Nocardiopsis sp. SD5: a potent feather degrading rare actinobacterium isolated from feather waste in Tamil Nadu, India. J Basic Microbiol 53:608–616.
Sahmoune MN (2016) The role of biosorbents in the removal of arsenic from water. Chemical Engineering & Technology, 39, 1617-1628.
Saiano F, Ciofalo M, Cacciola SO and Ramirez S (2005). Metal ion adsorption by Phomopsis sp. biomaterial in laboratory experiments and real wastewater treatments. Water Research, 39, 2273-2280.
Saitou N and Nei M (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular biology and evolution, 4, 406-425.
Saurav K, Kannabiran K (2011a) Biosorption of Cd(II) and Pb(II) ions by aqueous solutions of novel alkalophillic Streptomyces spp. biomass. J Ocean Univ China (Ocean Coastal Sea Res) 10(1):61–66.
Saurav K, Kannabiran K (2011b) Biosorption of Cr(III) and Cr(VI) by Streptomyces VITSVK9 spp. Ann Microbiol 61(4):833–841.
Saurav K and Kannabiran K (2009) Chromium heavy metal resistance activity of marine Streptomyces VITSVK5 spp.(GQ848482). Pharmacologyonline, 3, 603-613.
Saurav, K. & Kannabiran, K. 2011. Biosorption of Cd (II) and Pb (II) ions by aqueous solutions of novel alkalophillic Streptomyces VITSVK5 spp. biomass. Journal of Ocean University of China, 10, 61-66.
Singh R, Gautam N, Mishra A and Gupta R (2011) Heavy metals and living systems: An overview. Indian journal of pharmacology, 43, 246.
Takahashi Y and Omura S (2003) Isolation of new actinomycete strains for the screening of new bioactive compounds. The Journal of general and applied microbiology, 49, 141-154.
Vendruscolo F, Da Rocha FGL and Antoniosi Filho NR (2017) Biosorption of hexavalent chromium by microorganisms. International Biodeterioration & Biodegradation, 119, 87-95.
Vijayaraghavan K and Balasubramanian R (2015) Is biosorption suitable for decontamination of metal-bearing wastewaters? A critical review on the state-of-the-art of biosorption processes and future directions. Journal of environmental management, 160, 283-296.
Volesky B (1990) Biosorption and biosorbents. Biosorption of heavy metals. CRC Press, Florida, pp 3–6
Wallhausser KH, Huber G, Nesemann G, Praeve P, Zepf K (1964) Die Antibiotica FF 3582A und B und ihre Identitat mit Nonactin und seinen Honologen. Arzneim Forsch 14:356–360.
Wang J and Chen C (2009) Biosorbent of heavy metals removal and their future. Biotechnol Adv 27(2):195-226
Weisburg WG, Barns SM, Pelletier DA and Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. Journal of bacteriology, 173, 697-703.
Yang J, He M, Wang G (2009) Removal of toxic chromate using free and immobilized Cr(VI)-reducing bacterial cells of Intrasporangium sp. Q5–1. World J Microb Biot 25:1579–1587.
Yao L, Ye ZF, Wang ZY, Ni JR (2009) Characteristics of Pb biosorption with aerobic granular biomass. Chin Sci Bull 53(6):948–953.
Yin K, Lv M, Wang Q, Wu Y, Liao C, Zhang W and Chen L (2016). Simultaneous bioremediation and biodetection of mercury ion through surface display of carboxylesterase E2 from Pseudomonas aeruginosa PA1. Water Research, 103, 383-390.


