1Laboratory of the State Collection of Entomoacariphages and Primary Assessment of Biological Plant
Protection Products, Federal Research Center of Biological Plant Protection, Krasnodar, Russia
2Biotechnology Sector, Federal Research Center of Biological Plant Protection, Krasnodar, Russia
Corresponding author email: irina-agasyeva@mail.ru
Article Publishing History
Received: 25/10/2021
Accepted After Revision: 22/12/2021
One of the promising entomophages capable of controlling the abundance of the codling moth is Habrobracon hebetor Say. Natural populations of the gabrobragon can reduce the number of caterpillars of the corn moth to 22%, the garden moth to 35%, the cotton moth to 45%, and the gamma moth to 30%. This work aims to assess the parasitic activity of the gabrobragon as a regulator of the codling moth abundance in various geographic populations, to select a host insect for its mass breeding in laboratory conditions, and to assess the molecular genetic variability of the structure of H. hebetor populations. The capture of natural populations of the gabrobragon H. hebetor was carried out in apple orchards in Krasnodar Krai and Stavropol Krai of Russia using cassettes in which caterpillars of the codling moth were placed. As a result of the research, the natural starting population of the gabrobragon H. hebetor was captured, and a method for their maintenance and breeding was developed.
The most effective host insect is the wax moth (Galleria mellonela L.), which resulted in 195 adults, compared to 98 of the mill moth (Ephestia kuhniella Zell.). The gabrobragon population introduced into the apple tree cenosis continued its reproduction in natural conditions and largely suppressed the number and harmfulness of the codling moth. The RAPD analysis of the Krasnodar and Stavropol populations of Habrobracon hebetor Say revealed a high level of DNA polymorphism and genetic diversity in the studied geographic populations of the gabrobragon. At the same time, intrapopulation variability was 87.1%, while interpopulation variability accounted for 12.9% of the total indicator. The limited gene flow (Nm = 3.298) results in relatively low identity (GI = 0.906) between populations and significant interpopulation variability. This indicates that the analyzed insect samples probably represent different geographic populations of the H. hebetor ectoparasite.
Apple Tree, Codling Moth, DNA Polymorphism, Gabrobragon Habrobracon hebetor Say, RAPD Analysis
Agasyeva I, Besedina E, Ismailov V, Nefedova M, Nastasiy A. Biological Features and Molecular Genetic Structure of Habrobracon hebetor Populations. Biosc.Biotech.Res.Comm. 2021;14(4).
Agasyeva I, Besedina E, Ismailov V, Nefedova M, Nastasiy A. Biological Features and Molecular Genetic Structure of
Habrobracon hebetor Populations. Biosc.Biotech.Res.Comm. 2021;14(4). Available from: <a href=”https://bit.ly/3DYngpS“>https://bit.ly/3DYngpS</a>
Copyright © Agasyeva et al., This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) https://creativecommns.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Habrobracon hebetor Say is a hymenoptera parasite of many types of Lepidoptera pests in India, Pakistan, South Africa, Egypt, Canada, Western Europe, Central Asia, southern regions of Russia, Crimea, Transcaucasia, the Caucasus, Ukraine and Moldova (Dulgerova 1994; Agasieva et al. 2019; Piekarska-Boniecka et al. 2019) It affects caterpillars of cotton moth, corn stalk moth, apple, plum, oriental moth, mill, acacia, boxwood moth and other (over 60 species) lepidoptera pests that actively colonize vegetable, fodder, fruit crops, cotton, soybeans and corn (Amarasekarea et al. 2016; Chouinard et al. 2019).
H. hebetor is a small insect from the order Hymenoptera of the Braconidae family. A female finds a caterpillar, paralyzes it, and then lays her eggs on the caterpillar’s body. The number of eggs laid per caterpillar can reach 45, while the gabrobragon larva, while developing, feeds on the hemolymph of the caterpillar, of which only the outer covers remain (Kovalenkov et al. 1995; Chouinard et al. 2019).
The duration of development of one generation of the gabrobragon is 9–14 days. Natural bragon is present in all stations; during the growing season it migrates from one to another (Jumaev et al. 2017; Chouinard et al. 2019). Natural populations of the gabrobragon are able to reduce the number of caterpillars of the corn moth to 22%, garden moth – up to 35%, cotton moth – up to 45%, and gamma moths – up to 30% (Kovalenkov et al. 1995). For many years, the entomophage has been the object of mass breeding and use against a number of harmful lepidoptera species. The biological efficacy of a gabrobragon propagated in artificial conditions at low release rates (1-3 thousand individuals / ha) against a corn moth, cotton moth, acacia moth reaches 70-90% (Kovalenkov et al. 1995; Agasieva et al. 2019).
It is used in the open field for biological protection of tomato, corn, soybeans, cotton, sunflower, apple against cotton moth (Helicoverpa armigera Hb.), corn stalk moth (Ostrinia nubilalis Hbn.), acacia moth (Cyella zinckenella Fr.), codling moth (Cydia pomonella L.) Average daily temperature in the range of 25-30°C, the lifespan of the imago of at least 15 days, and the relative humidity of 70-80% are optimal for the parasitic activity of the gabrobragon. However, a number of researchers noted that the trophic relationships of H. hebetor vary significantly, both in laboratory and in the field (Frolov 2014; Amarasekarea et al. 2016; Piekarska-Boniecka et al. 2019).
Thus, it is artificially divided into pyralid, moth, leaf-roller and other races. In all likelihood, as a result of microevolutionary processes, many biological parameters of this species have changed, such as food specialization, stationary distribution, migration abilities, and morphogenetic structure of populations, which may complicate its application in biological plant protection programs. In this regard, the study of the biological characteristics of H. hebetor will make it possible to identify the reasons for the variability of the structure of gabrobragon populations and the prospects for its further use for biological control of a number of harmful Lepidoptera (Chouinard et al. 2019).
The aim of the present work is to assess the parasitic activity of the gabrobragon as a regulator of the codling moth abundance in various geographic populations, to select a host insect for its mass breeding in laboratory conditions, and to assess the molecular genetic variability of the structure of H. hebetor Say populations.
MATERIAL AND METHODS
The capture of natural populations of the gabrobragon H hebetor was carried out in apple orchards in Krasnodar Krai and Stavropol Krai of Russia using cassettes in which caterpillars of the codling moth were placed. Mass breeding of gabrobragon for biological control of the codling moth was carried out using the wax moth (Galleria mellonela L.) as a host insect of middle-aged caterpillars. In clean glass jars with a capacity of 1 liter, we put 100 g of artificial nutrient medium (modification A) and 7-10 cocoons of galleria (before the butterflies leave them). The jars are covered with glass lids and placed in a dark thermostat with a temperature of 28-30 oC and an air humidity of 70-75%.
The emerged butterflies laid eggs directly on the medium, from which in 12-15 days caterpillars hatched, which immediately penetrated into the medium. In jars, caterpillars developed up to the 3rd age. Then the contents of the jars were divided into two portions, which were placed in new 1 liter jars, previously filled with a medium with a layer of 6-7 cm. This amount of medium was sufficient until the caterpillars were fully grown. Then galleria caterpillars of older ages, 100-400 ind. were placed in glass jars with a capacity of 1-3 liters with corrugated paper and infected with gabrobragon.
In each jar, 30-50 females of the parasite were introduced, which paralyzed the caterpillars and laiid eggs on them. After 10-14 days adults flown out, which were packaged in plastic containers or glass jars and released into apple orchards during the appearance of middle-aged codling moth caterpillars at the rate of 1-2 thousand ind. per 1 hectare. Efficacy assessment of the bioagent was carried out using cassettes with caterpillars of the codling moth and trapping belts placed in the experimental garden and control plots of the garden.
Figure 1: Cassettes with codling moth caterpillars for catching gabrobragon in an apple orchard.

We determined the parasitic activity of natural gabrobragon populations and the dynamics of its numbers according to the damage degree of the caterpillars. When assessing the genetic structure of various geographic populations of H. hebetor, the object of the study was a sample of insects (n = 20) from the Krasnodar and Stavropol populations. Laboratory experiments were performed using the following equipment: iCycler amplifier (Bio Rad, USA), Sub Cell-GT electrophoresis devices (Bio Rad, USA), Power Pac-Basic electrophoresis power supply (Bio Rad, USA), transilluminator ECX-20-M (Vilber Lourmat, France), microcentrifuge “MiniSpin” (Eppendorf, Germany), thermostat for microtubes “Thermo 24” (Biokom, Russia).
DNA isolation was performed from adult insects (imago), amplification (RAPD-PCR) and electrophoresis in 1.8% agarose – as we described earlier (Kil et al., 2016b). In the PCR reaction, four primers highly specific for H. hebetor DNA were used: OPA05, OPA10, OPB04, UBC519 (Kil et al. 2016a; Kil et al. 2016b; Kil et al. 2018). The primers were synthesized by LCC Evrogen (Moscow); DNA polymerase, buffer, and other necessary components for PCR were supplied by Sibenzyme (Moscow). Genetic diversity, DNA polymorphism, and genetic similarity were assessed using Nei and Shennon, from the POPGENE version 1.31 software package (Yeh et al. 1997; Kil 2019).
Statistical data processing was performed using the Statistica 13.0 software package with the Duncan’s test. The studies were carried out on the basis of the laboratory of the State Collection of Entomoacariphages and the primary assessment of biological plant protection products of the Federal Research Center of Biological Plant Protection (FRCBPP), Russia, Krasnodar.
RESULTS AND DISCUSSION
One of the promising entomophages capable of controlling the abundance of the codling moth is the gabrobragon H. hebetor. In (2019) and (2020), in the apple orchards of the FRCBPP (Krasnodar Krai) and in the SSPK Sady Stavropolya (Stavropol Krai), natural starting populations of the gabrobragon H. hebetor were caught on bait cassettes with older caterpillars of the codling moth. Local ecotypes were of practical value, which were captured in order to clarify their bioecological features and the possibility of practical application to protect the apple tree from the codling moth.
Biological features and morphological signs of gabrobragon: ectoparasite wintering occured in a state of diapause at the imago stage. The flown out of the gabrobragon occured not earlier than April when the temperature was about + 15°C. The entomophage feeds on nectar and pollen from flowering plants, for example, weeds (wild radish, dandelion, shepherd’s purse, etc.), were accumulated in fruit gardens and vineyards (Agasieva et al. 2019; Piekarska-Boniecka et al. 2019).
After feeding, mating and searching for target host species that may appeared on crops took place. On sunny, windless days at a temperature of + 25 … + 30°C, the maximum searching and parasitic activity of the ectoparasite was noted. The males of the gabrobragon were polygamous, the females were monogamous. The search for an insect host for laying eggs was based on the orientation of females to odors produced by the host’s fodder plant, as well as by the caterpillars themselves and their metabolic products.
While attracting the parasite, the combination of various plants and phytophagous insects – objects of infection by the gabrobragon – played a huge role. Under the laboratory conditions, a host insect and optimal conditions for its mass reproduction, storage and maintenance were selected. Caterpillars of the wax moth (G. mellonella) and caterpillars of the mill moth (E. kuhniella) were tested as host insects. The effectiveness of each species was determined by the number of paralyzed caterpillars and the emergence of parasites of the filial generation (Table 1) (Chouinard et al. 2019).
Table 1. The number of gabrobragon emerged depending on the host insect
| Host insect | Number of caterpillars, ind. | Number of gabrobragon used for infestation, ind. | Number of cocoons formed, pcs. | Number of the emerged gabrobragon, ind. |
| Ephestia kuhniella Zell. | 100 | 25 | 106 | 98 |
| Galleria mellonela L. | 100 | 25 | 123 | 195 |
| As a result of statistical processing by Student’s t-test, the following values were obtained: temp. = -3.77003, t0.01 = 4.6041, t0.05 = 2.7764, which rejects the null hypothesis, therefore, the options are statistically significantly different. | ||||
As the data presented in (Table 6) show, the most effective host insect was the wax moth (G. mellonela), in the variant with which the yield of adults was 195 individuals, in comparison with 98 on the mill moth (E. kuhniella).
The timing of the release of entomophages and the developmental phases of the target insect were of paramount importance, especially for obtaining the maximum effect from the use of parasitic Hymenoptera. In this connection, in laboratory conditions, an assessment of the parasitic activity of the gabrobragon was carried out, depending on the age structure of the caterpillars of the codling moth (Table 2) (Chouinard et al. 2019).
Table 2. Parasitic activity of the ectoparasite Habrobracon hebetor Say against the codling moth (Cydia pomonella L.)
| Codling moth, caterpillars | Number of caterpillars, ind. | Among them: | Number of cocoons formed, pcs. | Number of emerged parasites, % | |
| paralyzed, % | parasitized, % | ||||
| Krasnodar Krai | |||||
| Older age | 42 | 100 d | 76,19 d | 32 d | 78,13 c |
| Middle age | 34 | 100 c | 64,71 c | 22 c | 54,55 b |
| Young age | 37 | 0 a | 0 a | 0 a | 0 a |
| Stavropol Krai | |||||
| Older age | 45 | 22,22 b | 4,44 b | 0 b | 0 a |
| Middle age | 39 | 5,13 a | 0 a | 0 a | 0 a |
| Young age | 35 | 0 a | 0 a | 0 a | 0 a |
| *Note: there are no statistically significant differences according to Duncan’s test at the 95% probability level between the options marked with the same letter indices, when compared within the columns. | |||||
As the data presented in (Table 2) show, gabrobragon effectively paralyzed the caterpillars of the codling moth of middle and older ages (caught in Krasnodar Krai), the emergence of parasites of which was 54.8 and 78.6%, respectively. The Stavropol population was not very effective against caterpillars of the codling moth of middle and young ages. Thus, it was found that the new moth population of the gabrobragon, propagated under laboratory conditions, met all the requirements for assessment as a bioagent for regulating the abundance of the codling moth (Chouinard et al. 2019).
In an organic apple orchard at the Shcherbakov farm, gabrobragons were released on the autumn ripening varieties Liberty and Florin. Studies on the use of H. hebetor were carried out over two years (2019) and (2020). Reproducing under natural conditions, the ectoparasite, upon reaching a sufficient population density, significantly regulated the number of the pest. As a result of the experiment, we observed the uniform dispersal of the gabrobragon over the entire garden area. The evidence was the infestation of caterpillars in cassettes installed in the apple orchard. The damage degree by gabrobracons of pest caterpillars was 50-65%, fruit damage was 3.5% (Chouinard et al. 2019; Agasieva et al. 2019).
During the growing season, cardboard trapping belts were installed on apple tree boles to record the dynamics of the pest abundance and the damage of caterpillars by the hymenopteran parasites. As a result of regular (weekly) observations, caterpillars of the codling moth infected with gabrobragon and empty cocoons of the parasite that flew out were revealed. The general parasitism of the pest caterpillars in the trapping belts reached 60-70% (Figure 2).Thus, the gabrobragon population introduced into the apple tree cenosis continued its reproduction in natural conditions and largely suppressed the number and harmfulness of the codling moth (Chouinard et al. 2019).
Figure 2. 1: A trapping belt installed on an apple tree; 2 – gabrobragon cocoons, after the parasite leaves them.

RAPD analysis of two geographic populations of the entomophage H. hebetor was carried out using four primers: OPA05, OPA10, OPB04, UBC519. The results of the RAPD analysis of the two studied insect populations using these primers are shown on electrophoregrams (Figure 3) (Agasieva et al. 2019).
Figure 3: Electropherograms of the products of DNA amplification of gabrobragon populations in 1.8% agarose with various RAPD primers. Tracks: 1-10 – Krasnodar; 11-20 – Stavropol; M – molecular weight markers (base pairs)
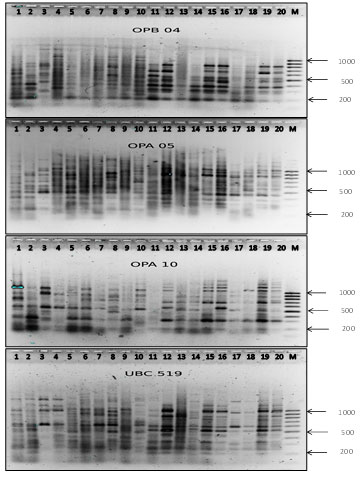
As a result of the PCR analysis, 62 RAPD loci were obtained, ranging in size from 100 to 1500 base pairs (Table 3).
Table 3. Number of loci per primer and sizes of DNA fragments in RAPD analysis of gabrobragon populations.
| RAPD-primer | Base pairs 5’-3’ subsequence | Number of loci | Sizes of DNA fragments (b.p.) |
| OPA 05 | AGGGGTCTTG | 13 | ≈ 1100-100 |
| ОРА 10 | GTGATCGCAG | 16 | ≈ 1500-100 |
| ОРВ 04 | GGACTGGAGT | 13 | ≈ 1100-100 |
| UBC 519 | ACCGGACACT | 20 | ≈ 1300-100 |
Molecular genetic analysis of H. hebetor revealed a high level of DNA polymorphism (P = 80.7-82.3%) and genetic diversity (H = 0.256-0.283) in the studied geographic samples and the absence of significant differences between them in these indicators (table 4)
Table 4. DNA polymorphism and genetic diversity of gabrobragon populations by RAPD primers (62 loci
| Sample from the population | P (%) | H ± SD* | I ± SD* |
| Krasnodar
|
82,3 | 0,256±0,171 | 0,394±0,236 |
| Stavropol
|
80,7 | 0,283±0,189 | 0,422±0,259 |
| *tfact ≤ t05 – differences are not significant;
P -% of polymorphic loci in the population; H – genetic diversity according to Nei (1973); I – Shannon informational index; ± SD is the standard deviation. |
|||
Genetic diversity (according to Nei) within geographic samples (Hs = 0.270) accounted for 87.1% of the total genetic diversity (Hs = 0.310) (Table 5). The revealed ratio of intra- and inter-population variability, estimated by the Shannon Index (I), showed similar data. The level of genetic flow between populations was Nm = 3.298, and the coefficient of genetic differentiation was Gst = 0.132. The Gst value confirmed the fact that 12.9% of the total genetic variability falls on the share of variability between populations, which determines the differentiation between samples (Agasieva et al. 2019).
Table 5. Total genetic variability of gabrobragon populations for all RAPD loci (n = 62)
| Indicator | Pt (%) | Ht | Hs | Gst | Nm |
| Value
(± SD) |
100.0 | 0.310±0.018 | 0.270±0.015 | 0.132 | 3,298 |
| Pt -% of polymorphic loci for all samples;
Нt – the general genetic variability in the population; Нs – genetic variability within populations; Gst – the coefficient of genetic differentiation; Nm – an indicator of gene flow between populations. |
|||||
Analysis of genetic differences between the studied insect samples showed that their genetic similarity was relatively low (genetic identity (GI) = 0.906) (table 6).
Table 6. Genetic distances (GD) (under the diagonal) and genetic identity (GI) (over the diagonal)
between gabrobragon populations (according to Nei, 1978)
| Sample from the population | Krasnodar | Stavropol |
| Krasnodar | – | 0.906 |
| Stavropol | 0.099 | – |
CONCLUSION
The fidings of the present study indicates that the analyzed insect samples were likely to represent different geographic populations of the H. hebetor ectoparasite, which was confirmed by the data of biological studies. Thus, the RAPD analysis of the Krasnodar and Stavropol populations of H. hebetor revealed a high level of DNA polymorphism and genetic diversity in the studied geographic populations of the gabrobragon. At the same time, intrapopulation variability was 87.1%, while interpopulation variability accounted for 12.9% of the total indicator. The limited gene flow (Nm = 3.298) resulted in relatively low identity (GI = 0.906) between populations and significant interpopulation variability.
ACKNOWLEDGEMENTS
This study was financially supported by the Kuban Science Foundation within the framework of the scientific project No. MFI-20.1 / 65.
REFERENCES
Agasieva, IS, Ismailov, VYa, Fedorenco, EV, et al. (2019). Elaboration of biological control methods of the apple moth for organic fruit farming technologies. Pomiculture and small fruits culture in Russia Vol 56 Pages 96-105. DOI: 10.31676/2073-4948-2019-56-96-105.
Amarasekarea, KG, Shearer, PW and Mills, NJ (2016). Testing the selectivity of pesticide effects on natural enemies in laboratory bioassays Biological Control Vol 102 Pages 7-16. DOI: 10.1016/j.biocontrol.2015.10.015.
Chouinard, G, Morin, Y and Cormier, D (2019). Seasonal Biology and Behaviour of the Predatory Mirid Hyaliodes Vitripennis, a Beneficial Insect of Apple Orchards in Quebec, Canada. Acta Horticulturae Vol 1261 Pages 235–242. DOI: 10.17660/actahortic.2019.1261.34.
Dulgerova, VA (1994). Development of technology for breeding gabrobragon. Environmentally friendly and pesticide-free technologies for obtaining crop products In: collection of the materials of the All-Russian scientific and production meeting Pushchino Krasnodar (1).
Frolov, AN (2014). Biotic factors of depression of the corn moth. Bulletin of plant protection Vol 2 Pages 37-47.
Jumaev, RA, Kimsanboev, XX, Adilov, MM, et al. (2017). The technology of rearing Braconidae in vitro in biolaboratory. European science review No 3-4 Pages 3-5 DOI: 10.20534/ESR-17-3.4-3-5.
Kil, VI (2009). Methods for assessing DNA polymorphism of insect populations using PCR (RAPD- and ISSR-PCR): Methodological recommendations LLC Education-Yug Krasnodar.
Kil, VI, Besedina, EN, Agasyeva, IS, et al. (2016a). DNA polymorphism and genetic diversity of the Krasnodar population of Habrobracon hebetor East European Scientific Journal No 12 Pages 49-51.
Kil, VI, Besedina, EN, Agasyeva, IS, et al. (2016b). RAPD analysis of laboratory and natural populations of Habrobracon hebetor. In: Scientific symposium: Advanced Biotechnologies – Achievements and Prospects, Chisinau, Republica Moldova: CZU (4). https://ibn.idsi.md/sites/default/files/imag_file/teze_Simpozion_2016_2.pdf
Kil, VI, Besedina, EN, Balaban, AT, et al. (2018). Identification of Habrobracon hebetor populations by RAPD markers. Russian agricultural science No 4 Pages 36-43 DOI: 10.31857/S250026270000546-5.
Kovalenkov, VG, Ismailov, VYa and Tyurina, NM (1995). Ecological protection against pests of tomato, bell pepper and corn (under the conditions of the North Caucasus region) Production of ecologically safe crop products Pushchino Russia Pages 186-195.
Kovalenkov, VG, Tyurin, NM and Ismailov, VYa (1995). Breeding technology and application of gabrobragon ectoparasite Methodical instructions RAAS VNIIBZR Russia.
Piekarska-Boniecka, H, Rzanska-Wieczorek, M, Siatkowski, I, et al. (2019). Controlling the abundance of the rose tortrix moth Archips rosana (L.) by parasitoids in apple orchards in Wielkopolska, Poland. Plant Protect. Sci. Vol 55 Pages 266-273 DOI: 10.17221/9/2019-pps.
Yeh, FC, Yang, RC, Boyle, TBJ, et al. (1997). POPGENE, the user-friendly shareware for population genetic analysis. Computer program and documentation. Molecular Biology and Biotechnology Centre. University of Alberta, Edmonton Pages 58-65.


