1Aryabhatta Centre for Nanoscience and Nanotechnology, Aryabhatta Knowledge University, Patna-800001, India
2University Department of Chemistry, T.M. Bhagalpur University, Bhagalpur-812007, India
3University Department of Physics, T.M. Bhagalpur University, Bhagalpur-812007, India
Corresponding author email: prasad_k@tmbuniv.ac.in
Article Publishing History
Received: 05/10/2020
Accepted After Revision: 05/12/2020
This rationale of the present work is to amalgamate the benefits of ethnomedicine and nanotechnology by fabricating biogenic platinum nanoparticles to look for possible alternative treatment to cure fatal diseases like cancer. Biogenic Platinum nanoparticles were fabricated from the leaves of medicinal plant of Piper betle. The fabrication was ascertained in the umbrella of tools that help in its characterization and include spectroscopic techniques like UV-vis and FTIR along with X-Ray diffraction and Scanning electron microscopy. The cytotoxicity assay against Lung cancer cell line (A549) was carried out by the MTT assay technique to check the cytotoxic efficacy of Platinum nanoparticles in comparison to that of the ethnomedicinal Piper betle plant extract. It was affirmed that both PtNPs and plant extract reduces the cell viability of cancer cells but at all the doses PtNPs proved to be more efficient in their cytotoxic effect in comparison to the extract. This work is a small step towards foreshadowing possible alternative therapeutics for cancer and revolutioninzing our ancient ethnomedicine.
Ethnomedicine, Nanotechnology, Platinum Nanoparticles, Piper betle, Lung Cancer Cell Line (A549).
Jha B, Zamani S, Jha A. K, Prasad K. Biogenic Platinum as Nanomedicine: A Synergism of Ethnomedicine and Nanotechnology. Biosc.Biotech.Res.Comm. 2020;13(4).
Jha B, Zamani S, Jha A. K, Prasad K. Biogenic Platinum as Nanomedicine: A Synergism of Ethnomedicine and Nanotechnology. Biosc.Biotech.Res.Comm. 2020;13(4). Available from: https://bit.ly/3elcX37
Copyright © Jha et al., This is an Open Access Article distributed under the Terms of the Creative Commons Attribution License (CC-BY) https://creativecommons.org/licenses/by/4.0/, which permits unrestricted use distribution and reproduction in any medium, provide the original author and source are credited.
INTRODUCTION
Ethnomedicine or traditional herbal medicine is an ancient science that is known for curing disease using indigenous plants. Plants as therapeutic agents can be used in various ways like isolating bioactive compounds for direct use as drugs or directly using the whole plant/part of it as an herbal remedy. Toxicity concerns with use of plants are less likely as compared to chemicals. Each medicinal plant is blessed with an array of phytochemicals like alkaloids, flavonoids, terpenes, tannins, organic acids etc. that play important roles in several biological activities, including antibacterial, antifungal, anti-inflammatory, antitumour etc. (Madhumita et al., 2020).
This treasure of diverse organic compounds in these plants is also known to play an important role in nanotransformation through molecular level modifications. Thus, making plants a suitable candidate for nanoparticle synthesis. On the other hand, the path-breaking, scientifically fascinating branch of nanoscience and nanotechnology has witnessed a revolution since its genesis in the exploration of matter at reduced dimensions. The special functionality flaunted by nanosized matter has been recognized leading to their wide applications in various sectors; biomedical being one of them. Platinum nanoparticles are one of the eminent nanoparticles with immense usage and practical applications in the biomedical sector that allured scientists to search out various fabrication routes for their synthesis (Jha et al., 2018a).
This work is an attempt to synergize the ethnomedicine and nanotechnology streams by fabricating biogenic platinum nanoparticles from the time-honored plant, Piper betel well known for its medicinal values. The cytotoxic potential of the rich phytoconstituents of the leaves against the cancer cell line is a proven fact (Abdul Rahman et al., 2014, Gundala et al., 2014, Ng et al., 2014). Although the effect of bare platinum as a cytotoxic agent is reported in chunks platinum as conjugates have been experimentally proven to be effective against a wide range of cancer cells, (Porcel et al 2010, Oberai et al 2014, Cheng et al., 2016, Johnstone et al., 2016, Jha et al 2018b, Madhumita, et al. (2020).
This work aims to amalgamate the benefits of ethnomedicine and nanotechnology by fabricating platinum from the medicinal leaves extract. The active metabolites besides helping in fabricating the platinum also bind with the nanoparticles and stabilize thereby reducing their toxicity. This adds on therapeutic advantage to the synthesized nanoparticles and also drastically reduces the side effect. This effort/approach escorts the development of alternative therapeutic treatment for fatal diseases like cancer.
MATERIAL AND METHODS
Fresh and healthy leaves of Piper betle plant were procured and thoroughly washed initially with tap water and then after with distilled water. In order to disinfect it was further sterilized with ethanol and left for some time later washed with sterile water and finally air dried. 10 gm of this air dried leaves was measured and finally chopped maintaining sterile environment. The chopped leaves were put in a beaker with 100 ml of 50% ethanol and placed on a boiling steam bath for 15 minutes till the solution turned green. When cooled the solution was double filtered to be used as source extract for aiding nanotransformation. 0.025 M Platinum salt solution in distilled water using analytical grade Platinum (IV) chloride salt from Hi-media Lab Pvt. Ltd., Mumbai, India was prepared by dissolving appropriate weight. 20ml of plant extract salt solution was diluted by 80 ml of distilled water and was placed on boiling water bath. 10ml Pt salt solution was added maintaining the basic pH conditions through the salt solution of analytical grade sodium hydrogen carbonate (Hi-media Lab Pvt. Ltd., Mumbai).
The plant aided nanotransformation to obtain PtNPs was allowed to happen at the temperature of boiling water bath. As the reaction proceeded the nanoparticles formed in the duration of 40-45 min as a colloidal solution which on cooling was centrifuged at 10,000 rpm for 20 minutes. The pellet obtained after discarding the clear supernatant was washed two times with distilled water. After removal of moisture content, it was characterized before carrying out further investigation. The ethanolic extract of the medicinal plant was also retained to check its medicinal values in comparison to PtNPs.
The fabrication of PtNPs was ascertained by characterization techniques. These techniques help to congregate information needed for the preferment of fabrication techniques and further property based applicability of nanoparticles. The biosynthesized PtNPs were characterized to know about their properties, dimensions, morphology etc. through various sophisticated instruments like UV-Vis spectroscopy, FTIR spectroscopy, X-Ray diffraction and Scanning electron microscopy. Perkin Elmer spectrophotometer, UK was used to record the UV-vis. Absorbance spectrum of the nanoparticle solution. The degree of precursor Pt metal ions conversion to their respective PtNPs was assessed by UV-Vis spectra (Behzadi et al., 2015, Hartland, 2006).
The FTIR spectrum of the dried PtNPs was collected in transmission mode in between the wavelength range of 4000-400 cm-1 by Perkin Elmer, UK, FTIR spectrophotometer. Bruker D8-Advance diffractometer with Cu-Kα radiation source aided in recording the X-Ray diffraction pattern of fabricated PtNPs (Bunaciu et al., 2015). It was used to determine the crystal structure and average particulate size. The SEM micrograph recorded by the EVO 18, Carl Zeiss Microscopy Ltd., helped in exploring the microstructural studies of biogenic PtNPs. The imaging provides topographical information about the nanoparticles.
It is of pivotal interest to assess the cytotoxicity of promising compounds intended for pharmaceutical use. The cytotoxicity of PtNPs was monitored by metabolic and imaging assays. MTT assay or metabolic assay provides information about the alterations in metabolism of cells in response to toxicities by promising compounds (Berridge et al., 2005) while imaging assays are employed to actuate the cytotoxic changes due to treatment. Lung cancer cell line (A549) were undertaken for the comparison of cytotoxic assay of medicinal plant extract and PtNPs (Gazdar et al., 2010). The cell line was cultured in RPMI 1640 media with 10% FBS and antibiotic pen-strep, all obtained from Hi Media Mumbai, India. The cells were placed in two 96 well plate with each well containing 5×103cells in 200µl per well. For the growth of mammalian cells physiological pH and temperature is maintained for 24 hr at 37ºC in a 5% CO2.
The other day, the cells were treated with various concentrations of 5, 10, 25, 50, 75, 100, 150 and 200µg/ml of plant extract and PtNPs respectively. After a treatment period of 24hrs the media was aspirated and 100µL of MTT (Hi Media Mumbai, India) of concentration 5mg/ml in 1X PBS was appended to each well. Negative control for the experiment was a well with media containing no drug. After 4hrs of incubation in an incubator the MTT was removed from the 96 well plates and 100µL of DMSO was added per well. The readings were taken at 570nm and cell viability was represented as percent cell viability. The experiment was executed in duplicates for statistical analysis and reliability of results.
RESULTS AND DISCUSSION
Biogenic PtNPs fabrication: The medicinal plant extract of Piper betle served as a cocktail of multifunctional reactants acting both as reducing and stabilizing agents (Alam et al., 2013). In the present context the ethanolic extract of Piper betle with ethnomedicinal properties is store house of rich phytochemicals with abundant alkaloids, terpenoids, flavonoids and steroids that aid up in nanotransformation (Punuri et al., 2012). The metabolites act as reducing agents providing the electron to metal ion for nanotransformation. The reduction potential of the metal ion and the reducing metabolites both determines the effectiveness of the concerned bio-generators. With the development of nucleation centre by formation of elemental atoms, the nucleation process begins and the particle growth stages follows (Thanh et al., 2014). The diminishing size results in high surface energy leading to aggregation of nanoparticles.
The plant extract serving as a capping agent comes into action by surrounding the nanoparticles with biocompatible metabolites (Alam et al., 2013). The preliminary confirmation of this transformation was the visual inspection. On proper maintenance of processing parameters, the plant metabolites acting both as reducing and capping agents acts on salt solution to produce initial Pt nuclei that later undergo subsequent growth to give rise to stable, homogenous and capped PtNPs. The mixing of the precursor salt to the extract resulted in gradual colour change that after a duration of 40-45 minutes turned black confirming the completion of the reaction, (Attard et al., 2012, Venu et al., 2011 Jha et al 2018b).
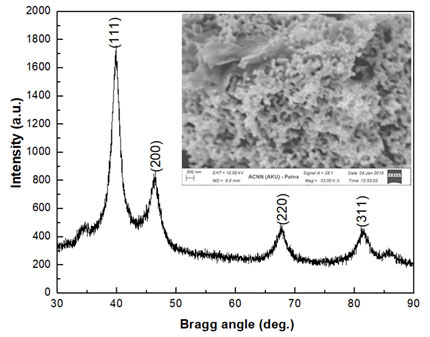
Structural and Microstructural Analysis of PtNPs: The further appliance of biogenic platinum nano entities is dependent on monitoring of its fabrication and properties which was achieved through characterization tools. Elucidation of PtNPs has been resoluted by numerous characterization techniques. The crystalline nature of biogenic PtNPs was established by the X-ray diffraction analysis. The Bragg reflections were observed as peaks in the graph plotted by Origin 8.5 data analysis and graphing software with the intensity on Y-axis and two times of Bragg angle (2θ) on the X-axis. As depicted in Figure 1, four peaks or reflection in the pattern was obtained each due to diffracted X-rays from a specific set of planes in the crystalline structure (Bunaciu et al., 2015). Miller indices were assigned to the peak as (111), (200), (220), (311) and (222) which depicted face-centered cubic (fcc) structure.
Figure 1: X-ray diffraction pattern and scanning electron microscope image, 3300 KX (inset) of PtNPs synthesized from the ethanolic extract of Piper betle leaves.
Applying Debye-Scherrer formula: D = 0.94λ/βcosθ (where β = full width at half maxima of the peak) the apparent crystallite size was estimated to be ~7nm which was in accord with standard literature (ICDD no. #88-2343). The lattice parameter and volume of the cubic crystal structured PtNPs was estimated to be 3.9120Å and 59.868Å3 respectively and were compared to the standard crystallographic bulk data to assess the squeezing in dimensions. The lowering in unit cell volume as observed could be attributed to nanosizing effect or quantum sizing effect (Jha and Prasad, 2010). The lattice strain value of PtNPs as observed was ~0.511 which owes to advantage of biosynthetic protocol. Upon interaction of the beam of electrons with the PtNPs the scanned surface of the nanoparticles was observed in the form of SEM micrograph as depicted by Figure 1, inset. The surface topography of PtNPs turned out to be nearly spherical structures.
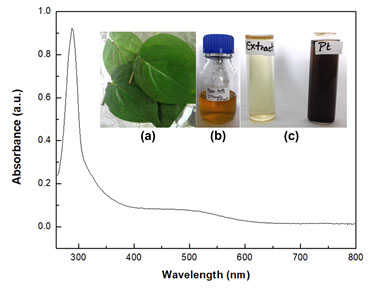
UV-Vis. and FTIR spectra of PtNPs: The UV-Vis spectrum of the PtNPs fabricated by Piper betle leaves is shown in Figure 2. Besides, insets of Figure 2 depict (a) the Piper betle leaves, (b) ethanolic extract of Piper betle leaves and (c) diluted ethanolic extract of Piper betle leaves and PtNPs colloid. This spectrum studies comprehend the information for the final formation of nanoparticles. The colour generation of PtNPs (inset, Figure 2c) owe to the excitation of surface plasmon resonance caused on interaction with candidate metabolites. The λmax or the wavelength of maximum absorbance was centralized at 288nm for PtNPs which ascertained the characteristic absorbance of platinum nanoparticles.
Figure 2: UV-Visible spectrum of PtNPs synthesized from the ethanolic extract of Piper betle leaves. Inset shows the (a) Piper betle leaves, (b) ethanolic extract of Piper betle leaves and (c) diluted ethanolic extract of Piper betle leaves and PtNPs in colloid form.
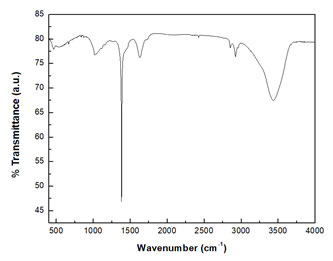
The spectrum showed long tail towards the longer wavelength that could be due to greater size distribution in these particles. FTIR spectroscopy is a label-free technique that enables extraction of biochemical information of the material under consideration. In the present work the fabricated PtNPs when subjected to infrared radiation results in a spectrum as presented in Figure 3. The metabolites aiding in nanotransformation tend to adhere to the fabricated PtNPs and the varied bonds of the metabolite absorbs varied intensities resulting in spectral domains. Each spectral peak is directly correlated to the bonds leading to deciphering and chemical identification.
Figure 3: FTIR spectrum of PtNPs synthesized from the ethanolic extract of Piper betle leaves.
The PtNPs surrounded by ethnomedicinal plant residues show intense and broad peak in the range of 3400 cm-1 with two peaks at 3693 cm-1 and 3448 cm-1 which owes to alcohol –OH stretching vibration and intramolecular H-bond. Polyphenol is the main metabolite present in the medicinal Piper betle leaves which is bestowed with phenolic hydroxyl groups (Tsao, 2010).The peak around 1780 cm-1 may be due to C=O bond of anhydride, aldehyde, ketone, ester, acid etc. of flavones, quinines. The band at about 1633 cm-1 is due to C=C stretching and 1513 cm-1 is owing to C=N stretching. The set of bands associated with some splitting; centered at 1600 cm-1 and 1500 cm-1 is due to aromatic ring vibrations (Coates, 2006). The fingerprint region 1500 cm-1 – 400 cm-1 has diverse vibrational peaks. The FTIR data communicates of the potential interaction of medicinal plant metabolites and the PtNPs that aid both in fabrication and functionalization.
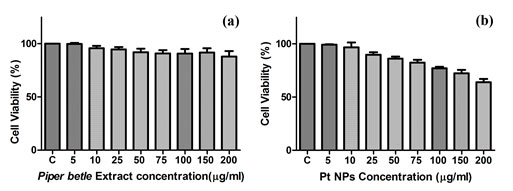
Comparative analysis of cytotoxicity potential of plant extract and PtNPs: The MTT assay using lung cancer cell line (A549) of PtNPs was done along with that of plant extract with a range of dose concentrations for 24hrs duration in triplicates to check the cytotoxic efficacy of nanoparticles in comparison to that of the ethnomedicinal Piper betle plant extract. The cell viability data are expressed in Figure 4(a) and 4(b). The viability of cells for each concentration was compared to that of plant extract treated viability at the same dose. Both the data was presented as mean±SE of experiments done in triplicates. It was observed that PtNPs cutoff the growth of cancer cells significantly (***p<0.001) with inhibition of 36.01%of cells as compared to 12.06% (*p<0.05) of cells killed by plant extract at the maximal dose of 200µg/ml. It was seen that the reduction in cell viability by both the plant extract and the PtNPs is directly proportional to the dose increment.
Figure 4: Cytotoxicity of (a) Piper betle leaves extract and (b) PtNPs on lung cancer cell line (A549).
The cell viability after PtNPs treatment at the dose of 5µg/ml was 99.35% (difference not significant p>0.05), at 10µg/ml was 96.75% (difference not significant p>0.05), at 25µg/ml was 89.77% (**p<0.01), at 50µg/ml was 86.11%(**p<0.01), at 75µg/ml was 82.34% (**p<0.01), at 100µg/ml was 77.05% (***p<0.001), at 150µg/ml was 72.31% (***p<0.001) and at 200µg/ml was 63.99% (***p<0.001) as compared to 99.81% (difference not significant p>0.05) at the treatment dose of 5µg/ml, 95.82%(difference not significant p>0.05) at the dose of 10µg/ml, 94.68% (difference not significant p>0.05) at the dose of 25µg/ml, 92.06% (*p<0.05) at the dose of 50µg/ml, 90.89%(*p<0.05) at the dose of 75µg/ml, 90.74% (*p<0.05) at the dose of 100µg/ml, 91.66% (*p<0.05) at the dose of 150µg/ml, 87.94% (*p<0.05) at the dose of 200µg/ml of the Piper betle plant leaves extract. The results showed that different concentrations of PtNPs and plant extract have varied cytotoxic effects on A549 cell line.
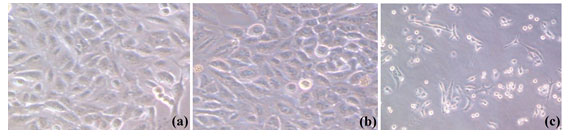
The cytotoxicity increased with the increase in the dose concentration (Mishra et al., 2012) and it was affirmed that both PtNPs and plant extract reduces the cell viability of cancer cells but at all the doses PtNPs proved to be more efficient in their cytotoxic effect in comparison to the extract. The images captured by the phase contrast microscope at the highest experimental dose of 200µg/ml of plant extract and PtNPs (Figure 5) showed a noticeable effect on the cultured cells with alterations in the morphology of the cells as compared to control (untreated) cells owing to cytotoxicity of both plant extract and PtNPs. In comparison to the untreated cells the treated cells contracted, loss in adherence was observed leading to floating of cells in the media. Both plant extract and the PtNPs treated cells exhibited morphological alterations. The effect as observed was more significant for the PtNPs.
Figure 5: Morphological alterations of lung cancer cell line (A549) (a) untreated cells, (b) treated with Piper betle leaves extract, and (c) treated with PtNPs synthesized form Piper betle leaves extract.
CONCLUSION
This study counts on the fact that exploration of medicinal plants in nanofabrication avenues has many persisting scopes. The therapeutic potentiality of nanoparticles has been a matter of contention since long. The nanometer dimension owned by the nano-entities and the biological components forecasts the synergism among them and is the basis for probable applications of these entities as nanomedicine. Cellular level experiments with A549 epithelial lung cancer cell line elucidated the therapeutic efficacy of biogenic PtNPs fabricated from the ethnomedicinal plant Piper betle. In the reference frame of plant extract PtNPs were more efficient.
This inference opens up avenues to revolutionize the ethnomedicine in conjunction with nanotechnology as it is presumed that plant extract moieties provides some coating material to the bare nanoparticles which besides reducing toxicity add on therapeutic advantage to the synthesized PtNPs thereby reducing the side effects. The in vitro cytotoxicity effect of biologically fabricated and functionalized PtNPs against A549 as witnessed proves to be a leading-edge venture, foretelling the potentiality of these nanoparticles for cancer therapeutics. This work is a small step towards foreshadowing possible alternative therapeutics for cancer and revolutionizing our ancient ethnomedicine. It attempts to remould the ethnomedicine by conjunction with nanotechnology.
Authors Contributions: All authors have contributed equally in bringing out this research work.
Conflict of Interest: None.
REFERENCES
Abdul Rahman, A., A Jamal, A.R., Harun, R., Mohd Mokhtar, N., Wan Ngah, W.Z., (2014) Gamma-tocotrienol and hydroxy-chavicol synergistically inhibits growth and induces apoptosis of human glioma cells. BMC Complement. Altern. Med. 14, 213. https://doi.org/10.1186/1472-6882-14-213
Alam, M.N., Roy, N., Mandal, D., Begum, N.A., (2013). Green chemistry for nanochemistry: exploring medicinal plants for the biogenic synthesis of metal NPs with fine-tuned properties. RSC Adv. 3, 11935. https://doi.org/10.1039/c3ra23133j
Attard, G., Casadesús, M., Macaskie, L.E., Deplanche, K., (2012). Biosynthesis of Platinum Nanoparticles by Escherichia coli MC4100: Can Such Nanoparticles Exhibit Intrinsic Surface Enantioselectivity? Langmuir 28, 5267–5274. https://doi.org/10.1021/la204495z
Behzadi, S., Ghasemi, F., Ghalkhani, M., Ashkarran, A.A., F., Mahmoudi, M., (2015). Determination of nanoparticles using UV-Vis spectra. Nanoscale 7, 5134–5139. https://doi.org/10.1039/C4NR00580E
Berridge, M.V., Herst, P.M., Tan, A.S., (2005). Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction, in: Biotechnology Annual Review. Elsevier, pp. 127–152. https://doi.org/10.1016/S1387-2656(05)11004-7
Bunaciu, A.A., Udriştioiu, E. Gabriela, Aboul-Enein, H.Y., (2015). X-Ray Diffraction: Instrumentation and Applications. Crit. Rev. Anal. Chem. 45, 289–299. https://doi.org/10.1080/10408347.2014.949616
Cheng, H.-J., Wu, T.-H., Chien, C.-T., Tu, H.-W., Cha, T.-S., Lin, S.-Y., (2016). Corrosion-Activated Chemotherapeutic Function of Nanoparticulate Platinum as a Cisplatin Resistance-Overcoming Prodrug with Limited Autophagy Induction. Small 12, 6124–6133. https://doi.org/10.1002/smll.201602374
Coates, J., (2006). Interpretation of Infrared Spectra, A Practical Approach, in: Meyers, R.A. (Ed.), Encyclopedia of Analytical Chemistry. John Wiley & Sons, Ltd, Chichester, UK, p. a5606. https://doi.org/10.1002/9780470027318.a5606
Gazdar, A.F., Girard, L., Lockwood, W.W., Lam, W.L., Minna, J.D., (2010). Lung Cancer Cell Lines as Tools for Biomedical Discovery and Research. JNCI J. Natl. Cancer Inst. 102, 1310–1321. https://doi.org/10.1093/jnci/djq279
Gundala, S.R., Yang, C., Mukkavilli, R., Paranjpe, R., Brahmbhatt, M., Pannu, V., Cheng, A., Reid, M.D., Aneja, R., (2014). Hydroxychavicol, a betel leaf component, inhibits prostate cancer through ROS-driven DNA damage and apoptosis. Toxicol. Appl. Pharmacol. 280, 86–96. https://doi.org/10.1016/j.taap.2014.07.012
Hartland, G.V., (2006). Coherent Excitation Of Vibrational Modes In Metallic Nanoparticles. Annu. Rev. Phys. Chem. 57, 403–430. https://doi.org/10.1146/annurev.physchem.57.032905.104533
Jha, A.K., Prasad, K., (2010). Green Synthesis of Silver Nanoparticles Using Cycas Leaf. Int. J. Green Nanotechnol. Phys. Chem. 1, P110–P117. https://doi.org/10.1080/19430871003684572
Jha, B., Jha, A.K., Prasad, K., (2018a). Plants as Fabricators of Biogenic Platinum Nanoparticles: A Gambit Endeavour, in: Prasad, R., Jha, A.K., Prasad, K. (Eds.), Exploring the Realms of Nature for Nanosynthesis, Nanotechnology in the Life Sciences. Springer International Publishing, Cham, pp. 147–170. https://doi.org/10.1007/978-3-319-99570-0_7
Jha, B., Rao, M., Chattopadhyay, A., Bandyopadhyay, A., Prasad, K., Jha, A.K.,( 2018b) Punica granatum fabricated platinum nanoparticles: A therapeutic pill for breast cancer. Presented at the 2ND International Conference On Condensed Matter and Applied Physics (ICC 2017), Bikaner, India, p. 030087. https://doi.org/10.1063/1.5032422
Johnstone, T.C., Suntharalingam, K., Lippard, S.J., (2016) The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 116, 3436–3486. https://doi.org/10.1021/acs.chemrev.5b00597
Madhumita, M., Guha, P., Nag, A., (2020). Bio‐actives of betel leaf (Piper betle L.): A comprehensive review on extraction, isolation, characterization, and biological activity. Phytother. Res. 34, 2609–2627. https://doi.org/10.1002/ptr.6715
Mishra, A., Mehdi, S.J., Irshad, Md., Ali, A., Sardar, M., Moshahid, M., Rizvi, A., (2012). Effect of Biologically Synthesized Silver Nanoparticles on Human Cancer Cells. Sci. Adv. Mater. 4, 1200–1206. https://doi.org/10.1166/sam.2012.1414
Ng, P.L., Rajab, N.F., Then, S.M., Mohd Yusof, Y.A., Wan Ngah, W.Z., Pin, K.Y., Looi, M.L., (2014) Piper betle leaf extract enhances the cytotoxicity effect of 5-fluorouracil in inhibiting the growth of HT29 and HCT116 colon cancer cells. J. Zhejiang Univ.-Sci. B 15, 692–700. https://doi.org/10.1631/jzus.B1300303
Oberoi, H.S., Nukolova, N.V., Kabanov, A.V., (2014). Nanocarriers for delivery of platinum anticancer drugs☆ 50.
Porcel, E., Liehn, S., Remita, H., Usami, N., Kobayashi, K., Furusawa, Y., Sech, C.L., Lacombe, S., (2010). Platinum nanoparticles: a promising material for future cancer therapy? Nanotechnology 21, 085103. https://doi.org/10.1088/0957-4484/21/8/085103
Punuri, J.B., Sharma, P., Sibyala, S., Tamuli, R., Bora, U., (2012). Piper betle-mediated green synthesis of biocompatible gold nanoparticles. Int. Nano Lett. 2, 18. https://doi.org/10.1186/2228-5326-2-18
Thanh, N.T.K., Maclean, N., Mahiddine, S., (2014). Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 114, 7610–7630. https://doi.org/10.1021/cr400544s
Tsao, R., (2010). Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2, 1231–1246. https://doi.org/10.3390/nu2121231
Venu, R., Ramulu, T.S., Anandakumar, S., Rani, V.S., Kim, C.G., (2011). Bio-directed synthesis of platinum nanoparticles using aqueous honey solutions and their catalytic applications. Colloids Surf. Physicochem. Eng. Asp. 384, 733–738. https://doi.org/10.1016/j.colsurfa.2011.05.045