Department of Botany and Microbiology, Gurukul Kangri Vishwavidyalaya, Haridwar 249404, Uttarakhand, India
Corresponding author Email: maheshwaridk@gmail.com
Article Publishing History
Received: 22/07/2019
Accepted After Revision: 27/09/2019
The food-borne antibacterial activity of Murraya koenigii, acetone leaves extract (ALE) was investigated against standard strains of Escherichia coli, Listeria monocytogenes and Staphylococcus aureus causing various food-borne diseases in human. The ALE showed a significant inhibition of the L.monocytogenes, S. auresus but the low inhibition of E.coli. The bioactive metabolites analysis of ALE by TLC, HPLC, UV-Vis spectroscopy, FTIR and NMR exhibited the presence of diverse types of bioactive phyto-constituents such as flavonoids, saponins, phenolic bioactive compounds etc. which might be responsible for bacterial inhibition in-vitro. FTIR studies of ALE, revealed the presence of functional groups peaks such as phenol, alkanes, alkenes, aromatic, aliphatic and amine plant bioactive compounds. NMR showed the presence of aliphatic groups and –OH groups compounds which were structurally and functionally similar due to the chemical arrangement of functional groups. Thus, M. koenigii provided a natural remedy for the control of food-borne pathogens.
Antimicrobial activity; FTIR; HPLC;NMR; Phyto-chemical analysis.
Kumar D, Dubey R. C, Maheshwari D. K. Bio-Efficacy of Acetonic Leaf Extract of Murraya koenigii With Reference To Its Antibacterial Spectrum Against Food-Borne Bacteria. Biosc.Biotech.Res.Comm. 2019;12(3).
Kumar D, Dubey R. C, Maheshwari D. K. Bio-Efficacy of Acetonic Leaf Extract of Murraya koenigii With Reference To Its Antibacterial Spectrum Against Food-Borne Bacteria. Biosc.Biotech.Res.Comm. 2019;12(3). Available from: https://bit.ly/2mguZwI
Introduction
Food-borne bacterial diseases in worldwide are proliferate day-by-day and becomes a serious concerns for both consumers and the food fabrication. Majority of food-borne diseases are caused by food spoilage bacteria and other microorganism (Sousa, 2008). Hence, the problem of food contamination arising through bacterial infection is a matter of major concern for the public health in both developed and developing nations (Shi and Zhu, 2009).Food spoilage is a sundry process involving food-borne microorganisms causing loss of 25% world’s food supply and a vast degree of infection illness. Fresh products such as fruits, vegetables, dairy products etc. are most likely to be contaminated by food-borne pathogens such as Campylobacter, Escherichia coli, Listeria monocytogenes, Staphylococcus aureus, etc. (Dhama et al., 2015).The majorly pathogenic bacteria (66%) have played main part in food spoilage or food borne illness (Khare and Rawat, 2018).
A good number of such toxicogenic bacteria have been discovered and few of them such as L. monocytogenes among, causing their survival at even extremely low temperatures where the other bacteria do not grow. The economic burden and food-borne diseases by contamination of food is continuing in the contemporary technical advancement (Ray and Bhunia, 2013). It has been estimated that in developing countries like India, 30% of people suffer from outbreaks of food-borne pathogens (Scallan et al. 2011). Traditional remedy, especially the medicinal plants and their extracts, still play a major role in the developing countries to combat bacterial infections (Pirzada et al., 2009). According to the WHO, the majority of worldwide population depends on traditional remedial system as a resource of natural drugs for human healthcare (Agyare et al. 2018).
The folk medicine makes valuable remedial for several diseases due to very low side effects (Zhang et al. 2016). The active phytochemical constituents of medicinal plants have bio-efficacy such as antioxidant, insecticidal and antibacterial activities. The folk medicine and its different parts extract exhibit significant anti-bacterial activity without any adverse serious side effects to host. The leaves of M. koenigii contain good sources of antioxidant activity, revamp the food protection and diminish food-borne infections, and can be used as preservatives (Genena et al. 2008). M. koenigii is a medicinal plant belongs to the family Rutaceae, is native to India and Sri Lanka. Its leaves are used in recipes in India and neighboring countries as spices due to aromatic nature. The leaves of M. koenigii work in relief from the food-borne pathogenic infection, vomiting and dysentery. The present work is therefore, aimed to study the bio-efficacy of ALE against both Gram-positive and Gram-negative food-borne bacteria because of their significance its combat human food-borne pathogens.
Materials and Methods
Plant material: The healthy leaves of M. koenigii were collected from their natural habitat growing at different locations of district Haridwar, Uttarakhand (29.945°N North 78.163° East) during September to October 2015 and 2016. The plant was identified following of authentic literature based on its characteristic features and a herbarium was kept in the Department of Botany and Microbiology, Gurukula Kangri Vishwavidyalaya, Haridwar, India.
Bacterial strains: The standard cultures of Escherichia coli MTCC 25922, Staphylococcus aureus MTCC 25923 and Listeria monocytogenes MTCC 657 were procured from the Microbial Type Culture Collection, (MTCC), Chandigarh, India. The test bacteria were sub-cultured onto nutrient agar medium in order to determine their viability. Stock cultures were maintained on nutrient agar slants at 4°C and inoculated in nutrient broth at 37°C prior to further use.
Extraction of plant material: The M. koenigii leaves were washed with distilled water and the dried leaves were pulverized to get powder in form. 200 g leaf powder was used for bioactive chemical extraction using acetone as solvent. Acetonic leaf extract (ALE) was concentrated by vacuum evaporator under the control temperature and pressure to obtain a gummy/semi-solid mass, which was preserved in a refrigerator at 4°C for further uses (Oniszczuk and Podgórski, 2015).
Antibacterial activity: The selected food-borne bacterial strains were prepared by transferring microbial inocula from stock cultures to test tubes containing Mueller-Hinton Broth (MHB) and incubated at 37˚C for 24 h. Antibacterial activity was tested by agar well- diffusion method (Correa et al., 2017). 100 μL of diluted inoculum of 105cfu mL–1 of 24 h old cultures of E.coli, L. monocytogenes and S. aureus were separately mixed in Mueller Hinton Agar (MHA) medium, with thorough shaking. Medium was poured in sterilized Petri plates and were allowed to solidify. A sterile cork borer of (6 mm diam.) was punch wells in medium. The stock extract of ALE (100 %) was diluted using acetone solvent to get 25, 50 and 75% concentrations for measuring antibacterial activity. DMSO (dimethyl sulphoxide) was used as control. The plates were incubated at 37°C for 24 h for the antibacterial activity (Balouiri et al., 2016). The data was interpreted on the basis of the size of the diameter of zone of inhibition (mm).
Phytochemical screening: The acetonic leaf extract (ALE) was subjected for qualitative analysis of phytoconstituents viz., alkaloids, amino acids, flavonoids, saponins and tannins following Trease and Evans (1983). Dragendorff’s test for alkaloid was carried out in which diluted hydrochloric acid(0.1 mL) and Dragendorff’s reagent(0.1 mL) were added separately in 2 mL of ALE in test tubes. After proper mixing, formation of orange brown colored precipitate indicated the presence of alkaloids. For saponins test ALE (1 mL) was separately diluted with distilled water up to 20 mL and vigorously shaken in a graduated cylinder for 15 min. Development of stable foam indicated the presence of saponins; this test is also known as foam formation test. ALE (5 mL) was separately treated with 1 mL of 10% aqueous lead acetate solution. Formation of yellow colored precipitate indicates the presence of flavonoids. For tannins analysis, ALE (5 mL) were separately allowed to react with 1 mL of 5% ferric chloride solution. Appearance of greenish black color indicated the presence of tannins. ALE (2 mL) and 0.25% ninhydrin reagent were added in fresh test tube and boiled for few minutes. Formation of blue color indicated the presence of total amino acids.
Thin layer chromatography (TLC):For quantitative analysis of different bioactive marker, ALE was applied on prepared TLC role and developed in a TLC chamber saturated with different suitable mobile phase such as ethyl acetate: methanol (3:1), hexane : chloroform : methanol (5:1:1) and chloroform : methanol (5:1). Based on clear bands and proper Rf values, the chloroform: methanol (5:1) was found the most suitable solvent system for the separation of bioactive metabolites. (Ramallo et al., 2006).
Fourier transform infrared spectroscopy (FTIR): FTIR was conducted following Kumar et al. (2014). The Infra-red spectra of acetone extractwas analysis for the determination of functional groups responsible for biological activities. ALE was mixed with KBr (spectroscopic grade) and pressed to form 1-mm pellet. Perfectly dried powder of the ALE was placed on the sample chamber of Nicolet Avatar 330 FTIR spectrometer (Thermo Electron Co., Madison, WI, USA) for the record of spectra and in the range of 600–3600 cm-1. The absorption frequencies appeared in functional group region as well as fingerprint region of the spectra was observed to record FT-IR spectral (Liu et al., 2006).
UV-Vis spectrophotometry: For the UV-VIS spectrophotometer (Perkin Elmer, USA Model: Lambda 950) analysis, the sample was prepared by diluting to 1:10 within the same solvent used in extraction of material (Do et al., 2014). The extract was examined under visible and UV light in the wavelength range 200-800 nm. The UV-visible spectra were performed to identify the compounds containing σ- bonds, π-bonds, lone pair of electrons and aromatic rings. The UV-VIS spectrum was observed both in visible and UV-VIS light lambda 200-800 nm as given by Maji et al (2016).
HPLC analysis: HPLC analysis was performed by Perkin Elmer Series 200 system in isocratic conditions using a C-18 (250mm x 4.6 mm, 5μm) at 25°C. Running conditions included injection volume, 20µl; mobile phase, methanol: acetic acid (0.4%) (800: 200 v/v); flow rate (1 ml/min). M. koenigii ALE (2.5 mg) was dissolved with 5 ml acetone. Bioactive compounds present in test sample were identified by chromatographic peaks with the retention time (RT) at 220 nm and 254 nm by UV detector. HPLC analysis was performed according to the method of Altun et al. (2002).
1H and 13C NMR analysis: Sample was dissolved in respective dutrirated solvents (CDCl3), 600 µl was poured in NMR tube and observed on the applied magnetic field (Tachibana et al., 2001), to obtain the Nuclear Magnetic Resonance (DRX-300Mega Hz Bruker, Switzerland).
Results and Discussion
Phytochemical screening: Preliminary phytochemical analysis of ALE of M. koenigii revealed the presence of saponins, alkaloids, amino acids and flavonoids. However, terpenes was not detected in ALE of M. koenigii (Table 1).
Antibacterial activity: ALE caused broad zone of inhibition. Significantly, wider zone of inhibition of actively growing bacteria on surface of Petri plates indicated the antibacterial potentials of ALE. ALE had the maximum activity against L. monocytogenes (12 mm), while the minimum inhibition was recorded against E. coli (7 mm). The zone of inhibition at 100% concentrations of ALE was most prominent followed by 75, 50, and 25 %. Which was corresponding to concentration the order of effectiveness of ALE was L. monocytogenes >S. aureus >E. coli on the basis of sensitivity (Table 1).
Table 1: Antibacterial activity of ALE of M.Koenigii against food-borne bacteria.
| ALE | Zone of inhibition | |||
| % | L.monocytogenes | S. aureus | E. coli | |
| 100 | 11 ± 2.5 | 9 ± 1.02 | 7 ± 1.02 | |
| 75 | 10 ± 1.01 | 8 ± 0.87 | 6 ±0.32 | |
| 50 | 8 ± 0.75 | 5 ± 0.72 | 4± 0.12 | |
| 25 | 4 ± 0.45 | 3 ± 0.25 | NI | |
| Control | NI | NI | NI | |
| Erythromycin | 25 | 23 | 20 | |
Values are mean of three independent observations ± SD; NI= No inhibition
Thin layer chromatography: Different active metabolites such as the alkaloids, flavonoids, glycosides,terpenoids and saponins with many high resolution bands appeared with different Rfvalues (Figure 1).
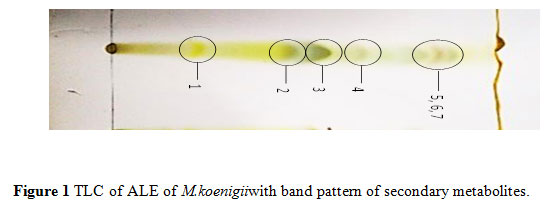 |
Figure 1 TLC of ALE of M.koenigii with band pattern of secondary metabolites. |
Fourier transform infrared spectroscopy (FTIR): The functional groups of bioactive chemicals of ALE both known and unknown bands appeared at 3959.59 – 553.53cm-1. The intense broad absorbance at 3515.99 cm-1 was the characteristic of the hydroxyl functional group in alcohols and phenolic compounds. The absorbance was relatively intense and broad at 3515 cm-1 characterized for hydroxyl functional groups. Two bands at 3444.63, 3259.47 and a weaker band at 1600.81 cm-1 exhibited amine group. Further, intense and weaker absorptions were observed in the C-H aromatic bands at 3130.25, 2960.53 cm-1, respectively. On the other hand, low intensity of the absorption was observed in the two aldehyde bands at 2812.02 and 2704.01 cm-1 similar to another two alkyne bands at2329.85 and 2189.06 cm-1. Single intense absorption peak appeared that determines the hydroxyl band at 3515.99 cm-1, while alkene band at 1361.65 cm-1 has low absorption spectra. The appearance of ester band at 995.20 cm-1 along with others having 4 small alkyl halide bands at 862.12, 754.12, 678.90 and 553.53 cm-1 were significantly diverse but showed majority of alkaloids with distinct secondary metabolites of unknown functional groups referred to the absorption spectra. On the other hand, spectral data of most of the extract confirmed the presence of bioactive groups such as –O-H, -C-H, =C-H, -C≡C, N-H, –C-O, –COOH, C-Cl, C-Br and alkene. Important IR absorption frequencies displayed the presence of C, H, Br and alkane string bioactive compounds (Figure 2).
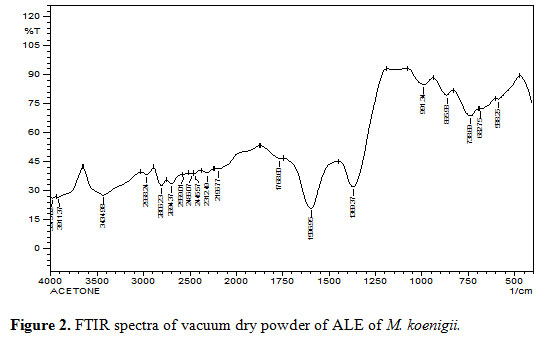 |
Figure 2: FTIR spectra of vacuum dry powder of ALE of M. koenigii. |
UV spectroscopy: The ALE was examined by UV spectroscopy for proximate analysis. The UV-VIS profile in the range of 200 – 800 nm wavelength exhibited the sharpness of the peaks and proper baseline. The flavonoids spectra typically consisted of two absorption maxima in the ranges 200-290 nm and 300-530 nm. The precise position and relative intensities of these maxima gave valuable information in the nature of flavonoids. Occurrence of peaks at 207-557 nm reveals the presents of flavonoids in the M. koenigii (Figure 3).
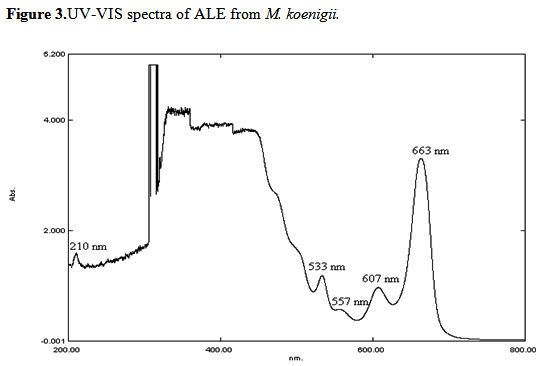 |
Figure 3: UV-VIS spectra of ALE from M. koenigii. |
HPLC analysis: HPLC profiling of ALE showed the presence of bioactive metabolites in different peaks observed at 220 nm and 254 nm in UV. The eight compounds were separated at different retention times at 220 nm and at 254 nm. These spots showed different bioactive constituents (Table 2).
Table 2: Peak list and Rf value of the chromatogram of the ALE of M. koenigii.
| RT | Area | Height | Purity Angle | UV range (nm) |
| 4.364 | 3102730 | 195562 | 6.639 | 220 |
| 9.628 | 266428 | 41190 | 3.636 | 220 |
| 18.936 | 24814 | 178512 | 2.54 | 220 |
| 20.110 | 364394 | 64676 | 0.375 | 220 |
| 21.309 | 762224 | 135192 | 0.222 | 220 |
| 22.782 | 1052445 | 192003 | 0.232 | 220 |
| 4.365 | 47873872 | 2730580 | 6.430 | 254 |
| 7.550 | 126028 | 25924 | 3.784 | 254 |
Nuclear magnetic resonance (NMR) spectrophotometry:1H NMR and 13C NMR spectrum of isolated compound reveals a one strong solvent (CDCl3) peak at 7.264 ppm. NMR spectrum of ALE extract resulted 11 peaks due to the presence of –OH group, C-H and C=O group. In 1H NMR spectrum a less intensive peak appeared at 1.6, 1.7 ppm due to R-CH2-R group and 4.0 ppm for =CH group. The four broad spectrum peaks constitute 2.1, 2.2 ppm for C=O and 2.3, 2.6 ppm spectrum due to the presence of C-N group and one 1.25 ppm for –OH in ALE of M. koenigii (Table 3).
Table 3: 1H-NMR and 13C-NMR spectral data of bioactive constitutents of ALE.
| Type of proton | Chemical shift (δ) ppm | Description of proton |
| ROH | 1.25 | Alcohol |
| C-H | 1.4, 1.5 | Alkane |
| R-CH2-R | 1.6, 1.7 | Alkyl |
| C=O | 2.1, 2.2 | Carbonyl |
| C-N | 2.3, 2.6 | C attached to N and Halogens (Cl, Br, and I) |
| C=O | 3.9 | C attached to O |
| =CH | 4.0 | Alkene |
Foods-borne infectious diseases are serious concern of worldwide. About 250 different food-borne diseases have been reported, and bacteria are the major and common causative agents of two thirds of food borne infectious disease outbreaks (Le et al., 2003). Medicinal plants are widely used against dysentery and food-borne infection throughout the world but only selected plants have been validated by scientific community. M. koenigii leaves are used mainly for flavor and medicinal purpose worldwide shown its potential to serve as food ingredients in India and other Asian countries (Perera and Li., 2012; Shanthala and Jamuna., 2005). We have observed that the leaves of M. koenigii have highly anti-bacterial activity against L. monocytogenes, S. aureus and E. coli.
Recently, Sablania et al. (2019) found that the M. koenigii leaves have antibacterial activity against Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, E. coli and other microorganism. The most significantly inhibitory effect has been observed against L. monocytogenes. ALE resulted in the maximum zone of inhibition against L. monocytogenes followed by S. aureus and quite less against E. coli. Due to the presence of OH (hydroxyl group) at 1.25ppm in ALE, it may be have similar mode of action against bacteria like erythromycin as stated by (Kohanski et al., 2007). Some of the bioactive chemicals may have similar mode of action such as DNA damaging, protein synthesis inhibition and bacteriostatic activity against food-borne bacteria. The anti-bacterial effects against Gram-positive bacteria and Gram-negative may be attributed due to the presence of several bioactive chemicals in ALE (Table 2). The natural bioactive chemicals which contain -OH groups, that were causing of protein damage and membrane lipid inhibition of bacteria (Brogden., 2005).
Similarly, Gupta et al. (2018) reported strong antibacterial properties by M. koenigii leaves extract mainly due to the saponin and active protein. But the action of additional anti-food-borne bioactive compounds of M. koenigii cannot be ruled out. The microbicidal activity of ALE was higher against the Gram-positive bacteria in comparison to the Gram-negative bacteria. Majority of Gram-positive bacteria have been drastic food-borne pathogens in comparison to that of Gram-negative bacteria. The data of UV-VIS spectral absorption showed the presence of flavonoids at absorbance 207-280 nm also reported by Kavitha and Uduman (2017) whereas phenolic acid derivatives at 317-340 nm (Zavoi et al., 2011). In the present study, ALE spectral absorption sharp baseline and peaks were clearly shown in spectral graph. The Preliminary plant bioactive metabolites screening and TLC Rf values ALE of M. koenigii revealed to the presence of diverse type of bioactive metabolites such as alkaloids, amino acid, saponins, flavonoids. On comparison of the leaves shows that the ALE has similar flavonoids and glycosides compounds reported. A similar finding has also been reported as also by Kumar et al., (2013).
Since, the functional groups of bioactive metabolites describe the nature and behavioural characteristics therefore, it is valuable for the preliminary separation and determination of bioactive metabolites constituents, Meepagala et al. (2013) have reported that HPLC chromatograms of ethyl acetate extract of leaves of M. koenegii relative with the peaks of extract retention time 8.2, 9.1 and 10.3 as isomahanine and mahanine according to their chemical name. Shah et al. (2018) found the prominent peak of tannic acid at a retention time of 3.21 min. During our study, the H1 and C-13-NMR spectroscopy analysis of ALE of M. Koenigii showed the presence of the aliphatic group, such as alkane, alkyl and alkene of bioactive chemicals which may be responsible for the anti-bacterial nature of ALE. The NMR spectrum of proton and carbon also predicts the anti-food-borne bioactive metabolites structure on the basis of FTIR and UV-VIS data interpretation as evidenced by previous workers (Pretsch et al., 2013; Sarker et al., 2006)
The results of ALE are useful for the existing information for identification and validation of M. koenigii leaves for the future prospective against food-borne bacteria. The bioactivity of ALE and the therapeutic use of M. koenigii for various ailments and look promising for using the bioactive phyto-chemicals in pure form as an effective natural drug agent in future. Therefore, the leaves of M. koenigii can be used for developing commercial management in food industry; chemical preventative such as benzoic acid and sodium benzoate are also work as antimicrobial which are normally used to expand shelf-life of food products (Nair, 2001).
Conclusion
It may be concluded that the ALE of M. koenigii anti-bacterial efficacy against some food-borne bacterial pathogens due to the presence of several phytochemical compounds such as alkaloid, phenol, flavonoids and tannin were observed. The UV-VIS profile showed the absorbance of flavonoids in the ALE. FTIR analysis confirmed the presence of functional groups of bioactive metabolites such as phenol, alkanes, alcohol and amines, while HPLC outline of M. koenigii showed the presence active metabolites in ALE and the NMR spectrum predicted the structure of bioactive compounds based on data of FTIR and UV-VIS. The results of this study offer a platform for using M. koenigii leaves as herbal alternative for various food-borne disease and infections caused by bacterial pathogens after animal trials. Further work is in progress.
Acknowledgments
The authors are thankful to the Head, of Department of Botany and Microbiology, Gurukula Kangri Vishwa Vidyalaya for providing Laboratory support. The instrumental technical support from CDRI, Lucknow, (India) for providing spectroscopic and analytical data and financial support from the University Grants Commission (UGC) New Delhi provided in the form of RGNF-SC is also acknowledged by one (DK) of us.
References
Agyare, C., Spiegler, V., Asase, A., Scholz, M., Hempel, G., & Hensel, A. (2018). An ethno pharmacological Survey Of Medicinal Plants Traditionally Used For Cancer Treatment In The Ashanti Region, Ghana. Journal Of Ethno pharmacology, 212, 137-152.
Altun, M. L. (2002). HPLC Method For The Analysis Of Paracetamol, Caffeine And Dipyrone. Turkish Journal Of Chemistry, 26(4), 521-528.
Balouiri, M., Sadiki, M., & Ibn Souda, S. K. (2016). Methods For In Vitro Evaluating Antimicrobial Activity: A Review. Journal Of Pharmaceutical Analysis, 6(2), 71-79.
Brogden, K. A. (2005). Antimicrobial Peptides: Pore Formers Or Metabolic Inhibitors In Bacteria?. Nature Reviews Microbiology, 3(3), 238.
Correa, M., Bombardelli, M. C., Fontana, P. D., Bovo, F., Messias-Reason, I. J., Maurer, J. B. B., & Corazza, M. L. (2017). Bioactivity Of Extracts Of Musa paradisiaca L. Obtained With Compressed Propane And Supercritical Co 2. The Journal Of Supercritical Fluids, 122, 63-69.
Dhama, K., Karthik, K., Tiwari, R., Shabbir, M. Z., Barbuddhe, S., Malik, S. V. S., &Singh, R. K. (2015). Listeriosis In Animals, Its Public Health Significance (Food-Borne Zoonosis) And Advances In Diagnosis And Control: A Comprehensive Review. Veterinary Quarterly, 35(4), 211-235.
Do, Q. D., Angkawijaya, A. E., Tran-Nguyen, P. L., Huynh, L. H., Soetaredjo, F. E., Ismadji, S., & Ju, Y. H. (2014). Effect Of Extraction Solvent On Total Phenol Content, Total Flavonoid Content, And Antioxidant Activity Of Limnophila aromatica. Journal Of Food And Drug Analysis, 22(3), 296-302.
Genena, A. K., Hense, H., Smânia Junior, A., &Souza, S. M. D. (2008). Rosemary (Rosmarinus officinalis): A Study Of The Composition, Antioxidant And Antimicrobial Activities Of Extracts Obtained With Supercritical Carbon Dioxide. Food Science And Technology, 28(2), 463-469.
Gupta, D., Kumar, M., & Gupta, V. (2018). An In Vitro Investigation Of Antimicrobial Efficacy Of Euphorbia hirta And Murraya koenigii Against Selected Pathogenic Microorganisms. Asian J Pharm Clin Res, 11(5), 359-363.
Kavitha, R., & Udumanmohideen, A. M. (2017). Exploration Of Phytocompounds In Abelmoschus moschatus Flowers Using Hplc, Uv-Vis And Ftir Techniques. International Journal Of Chemical Studies, 5(6), 702-706.
Khare, S., Tonk, A., & Rawat, A. (2018). Foodborne Diseases Outbreak In India: A Review. Int J Food Sci Nutrition, 3(3), 9-10.
Kohanski, M. A., Dwyer, D. J., Hayete, B., Lawrence, C. A., & Collins, J. J. (2007). A Common Mechanism Of Cellular Death Induced By Bactericidal Antibiotics. Cell, 130(5), 797-810.
Kumar, S. K., Suresh, M., Kumar, S. A., & Kalaiselvi, P. (2014). Bioactive Compounds, Radical Scavenging, Antioxidant Properties And FTIR Spectroscopy Study Of Morinda citrifolia Fruit Extracts. Int J Curr Microbiol Appl Sci, 3, 28-42.
Kumar, S. R., Loveleena, D., & Godwin, S. (2013). Medicinal Property Of Murraya koenigii-A Review. International Research Journal Of Biological Sciences, 2(9), 80-83.
Leloir, Y., Baron, F., &Gautier, M. (2003). Staphylococcus aureus And Food Poisoning. Genet Mol Res, 2(1), 63-76.
Liu, H. X., Sun, S. Q., Lv, G. H., &Chan, K. K. (2006). Study On Angelica And Its Different Extracts By Fourier Transform Infrared Spectroscopy And Two-Dimensional Correlation Ir Spectroscopy. Spectro Chimic Aacta Part A: Molecular And Biomolecular Spectroscopy, 64(2), 321-326.
Maji, J. K., Sharma, S., & Shukla, V. J. (2016). Image Processing And Ultra-Violet And Visible Reflectance Spectroscopy Combined With Chemometrics For Discrimination As Well As Authentication Powder And Extract With Anti-Diabetic Polyherbal Formulation. Int J Pharm Sci Res, 7(8), 325-334.
Meepagala, K. M., Schrader, K. K., &Burandt, C. L. (2013). Antibacterial Compounds From Rutaceae With Activities Against Flavobacterium columnare And Streptococcus iniae. Journal Of Agricultural Chemistry And Environment, 2(04), 90.
Nair, B. (2001). Final Report On The Safety Assessment Of Benzyl Alcohol, Benzoic Acid, And Sodium Benzoate. International Journal Of Toxicology, 20, 23-50.
Oniszczuk, A., & Podgórski, R. (2015). Influence Of Different Extraction Methods On The Quantification Of Selected Flavonoids And Phenolic Acids From Tilia cordata Inflorescence. Industrial Crops And Products, 76, 509-514.
Perera, P. K., & Li, Y. (2012). Functional Herbal Food Ingredients Used In Type 2 Diabetes Mellitus. Pharmacognosy Reviews, 6(11), 37.
Pirzada, A. J., Shaikh, W., Maka, G. A., Shah, S. I. S., And Mughal, S. (2009). Anti-Fungal Activity Of Different Solvent Extracts Of Medicinal Plants Capparis decidua Edgew And Salvadora persicalinn. Against Different Parasitic Fungi. Pak.J. Agric., Agricul.Eng. Vet. Sci. 25(2):26-34
Ramallo, I. A., Zacchino, S. A., &Furlan, R. L. (2006). A Rapid TLC Autographic Method For The Detection Of Xanthine Oxidase Inhibitors And Superoxide Scavengers. Phytochemical Analysis, 17(1), 15-19.
Ray, B., & Bhunia, A. (2013). Fundamental Food Microbiology. CRC Press.
Sablania, V., Bosco, S. J. D., Ahmed, T., & Sarma, V. V. (2019). Antimicrobial And Antioxidant Properties Of Spray Dried Murrayam koenigii Leaf Powder. Journal Of Food Measurement And Characterization, 1-10.
Sarker, S. D., Latif, Z., & Gray, A. I. (2006). Natural Product Isolation. In Natural Products Isolation (Pp. 1-25). Humana Press.
Scallan, E., Hoekstra, R. M., Angulo, F. J., Tauxe, R. V., Widdowson, M. A., Roy, S. L., …& Griffin, P. M. (2011).Foodborne Illness Acquired In The United States-Major Pathogens. Emerging Infectious Diseases, 17(1), 7.
Shah, P., Singh, S. P., Gupta, A. K., &Kumar, A. (2018). Combined hepatoprotective Activity Of Murraya koenigii And Phyllanthus niruri Extracts Against Paracetamol Induced Hepatotoxicity In Alcoholic Rats. Proceedings Of The National Academy Of Sciences, India Section B: Biological Sciences, 1-11.
Shanthala, M., And Jamunaprakash. Acceptability Of Curry Leaf (Murraya koenigii) Incorporated Products And Attitude Toward Consumption. Journal Of Food Processing And Preservation 29, No. 1 (2005): 33-44.
Shi, X., &Zhu, X. (2009). Biofilm Formation And Food Safety In Food Industries. Trends In Food Science & Technology, 20(9), 407-413.
Sieradzki, K., Roberts, R. B., Haber, S. W., &Tomasz, A. (1999). The Development Of Vancomycin Resistance In A Patient With Methicillin-Resistant Staphylococcus aureus Infection. New England Journal Of Medicine, 340(7), 517-523.
Sousa, C. P. D. (2008). The Impact Of Food Manufacturing Practices On Food Borne Diseases. Brazilian Archives Of Biology And Technology, 51(4), 615-623.
Tachibana, Y., Kikuzaki, H., Lajis, N. H., &Nakatani, N. (2001). Antioxidative Activity Of Carbazoles From Murraya koenigii Leaves. Journal Of Agricultural And Food Chemistry, 49(11), 5589-5594.
Trease, G.E. And Evans, M.C. (1983). Textbook Of Pharmacognosy, 12th Ed. Balliere, Tindall, London. 343-383.
Zavoi, S., Fetea, F., Ranga, F., Baciu, A., &Socaciu, C. (2011). Comparative Fingerprint And Extraction Yield Of Medicinal Herb Phenolics With Hepatoprotective Potential, As Determined By Uv-Vis And Ft-Mir Spectroscopy. Notulaebotanicaehortiagrobotanicicluj-Napoca, 39(2), 82-89.
Zhang, C., &Hua, Q. (2016). Applications Of Genome-Scale Metabolic Models In Biotechnology And Systems Medicine. Frontiers In Physiology, 6, 413.


