1Brijlal Biyani Science College, Amravati-444605, India
2Bharatiya Mahavidyalaya, Amravati-444605, India
3S.G.B. Amravati University, Amravati-444602, India
Corresponding author email: deeplaxmikulkarni26@gmail.com
Article Publishing History
Received: 15/01/2025
Accepted After Revision: 29/03/2025
The diversity of Araneae (spiders) in agricultural ecosystems plays a crucial role in pest regulation and ecosystem balance. This study analyses the diversity of spiders in Amravati’s agricultural fields, concentrating on two important Kharif crops that are cultivated using the mixed crop method: soyabeans and pigeon peas. The research highlights the species composition, abundance, and distribution of spiders within these crops, emphasizing the role of spiders as natural predators in controlling insect pests. A total of 1014 individual spiders, representing 41 genera and 14 families, and 28 taxa were identified across the surveyed sites.
Four families—Araneidae, Lycosidae, Thomisidae, and Salticidae were found to be the most abundant, with Salticidae exhibiting the highest species diversity. Peak species diversity was recorded from August to November, with a noticeable decline in orb-weavers (Araneidae) following harvest of the soybean crop. By comparing spider communities across pigeon pea and soybean fields, the study identifies the influence of crop type, habitat, and environmental factors on spider diversity. The findings highlight the significant role of spiders in agroecosystems, particularly in the management of pest populations in Kharif crop cultivation. This work contributes to a better understanding of arachnid biodiversity in agricultural landscapes and its potential benefits for pest control in the region of Amravati, India.
Araneae, Diversity, Agriculture Fields, Pigeon Pea, Soybean.
Laharia R, Kulkarni D, Hadole P.Assessment of the Spider Fauna from Agricultural Agroecosystems Around Amravati, Maharashtra, India. Biosc.Biotech.Res.Comm. 2025;18(1).
Laharia R, Kulkarni D, Hadole P. Assessment of the Spider Fauna from Agricultural Agroecosystems Around Amravati, Maharashtra, India. Biosc.Biotech.Res.Comm. 2024;18(1). Available from: <a href=”https://bit.ly/2U8EBeg”>https://bit.ly/2U8EBeg</a>
INTRODUCTION
Pigeon pea and soybean are amongst the most important and widely cultivated pulse and oilseed crops in Maharashtra, India. Area under cultivation pigeon pea 102.1ha and soybean 317.6 ha with productivity of 753 kg/ha and 764 kg/ha; however, the actual production is 242.8 t and 76.9t for the two crops. One of the factors for lower production is the infestation of insect pests, which attack all the stages of plants. Researchers have identified pest infestations across various regions of the country as a critical issue, with severe consequences, including farmer suicides (Singh and Singh, 1991; Rao et al., 2002; Akhilesh and Parasnath, 2003; Daniel et al., 2018). To mitigate crop losses to economically manageable levels or prevent them entirely, the application of chemical pesticides has become a widely adopted approach. However, due to the adverse effects of these chemicals on soil health, water quality, and farmer well-being, there is an urgent need for eco-friendly pest control methods.
Agricultural experts advocate for the use of natural predators, cultural practices, pest-resistant crop varieties, and transgenic crops to enhance productivity while minimizing environmental harm. Arthropods, representing the largest proportion of animal biomass and biodiversity in agro-ecosystems, play a vital role in delivering essential ecosystem services. They function as pollinators, predators, decomposers, and nutrient recyclers, contributing significantly to ecological balance. Due to their rapid responsiveness to environmental changes, arthropods also serve as valuable bio-indicators. Among these, predatory arthropods are particularly beneficial in agroecosystems, as they help regulate and suppress populations of phytophagous pests, thereby influencing the guild diversity of other invertebrates. Spiders, in particular, are recognized for their exclusive predatory behavior, which has a profound impact on agro-ecosystem dynamics (Samiayyan, 2014; Michalko et al., 2019, Smith et al., 2020; Kumar & Patel, 2022 Wadhwa and Malik 2024, Nik et al 2025).
The extent of their influence on prey populations is closely linked to spider density or biomass (Greenstone, 1999; Riechert, 1999; Sunderland and Samu, 2000; Liu et al., 2015). These findings underscore the ecological importance of spiders and other predatory arthropods in maintaining the stability and sustainability of agricultural systems. Research has demonstrated that ecosystems with greater species diversity exhibit higher stability compared to those with limited species richness. Arthropod predators, in particular, play a crucial role in regulating pest populations (Chang and Kareiva, 1999; Symondson et al., 2002; Wadhwa and Malik 2024 and Nik et al 2025).
Despite this understanding, knowledge of spider diversity in agroecosystems, specifically in pigeon pea and soybean fields in the Amravati region, remains fragmented. To address this gap, the present study was conducted to explore the diversity, species richness, and ecological dynamics of spider communities within these cropping systems.
MATERIAL AND METHODS
Study area: The study area, Amravati, is geographically located at coordinates 20.93°N and 77.75°E, with an average elevation of 343 meters above sea level. The region falls within the rainfed agricultural zone of the Amravati division, where crop production is heavily reliant on seasonal precipitation.The research focused on spider fauna within crop fields practicing intercropping, specifically cultivating pigeon pea and soybean. These two crops were selected due to their contrasting growth patterns, which provided a unique ecological context for studying spider diversity and community dynamics in agroecosystems.Four field sites were selected for the study.
Sampling duration and area under investigation: In the selected fields, spiders were observed and collected twice a month during the two kharif seasons i.e., July 2020 to December 2020 and July 2021 to December, total 12 surveys for each season. Amravati district is situated right in the center of the northern border of Maharashtra. It lies between 20.93°N 77.75°E. It is 343 metres above sea level on average. Major agriculture of the study area is in the rain-fed region of the Amravati division and the crops entirely depend on the amount of precipitation.
Collection Method: Spider specimens were collected using a combination of pitfall traps, aerial and ground hand collection methods, and vegetation beating techniques. Visual inspections were conducted on crop plants, surrounding vegetation, tree trunks, leaf litter, and other microhabitats within and around the selected crop fields. For documentation and identification, a Nikon D3200 camera equipped with a Nikkor 42x Wide Optical Zoom ED VR lens was utilized to capture high-resolution images of the specimens.
Spider Identification and Data analysis: Collected specimens were categorized by relative abundance in descending order and preserved in labeled containers with 70% ethanol for taxonomic identification and quantification under laboratory conditions. A digital photographic database was developed to facilitate morphological analysis using a Magnus MSZ-BI Zoom Stereo Binocular Microscope integrated with a MagCam DC-5 digital camera, enabling high-resolution imaging of diagnostic features. Taxonomic classification at the family level was conducted using Jocque and Dippenaar-Schoeman’s Spider Families of the World (2007). Genus- and species-level identifications were cross-referenced with published Indian literature (monographs, books) and supplemented by international research publications. To ensure accuracy, supplementary validation was performed using the World Spider Catalog (2025) and additional peer-reviewed resources from global repositories.
The study presented here has assessed spider species diversity across the target agroecosystems. To evaluate sampling completeness, species richness and community diversity were quantified using the Shannon-Wiener diversity index (H’) (Ravi et al., 2015), which integrates both species abundance and evenness to characterize ecological patterns in the sampled locations.
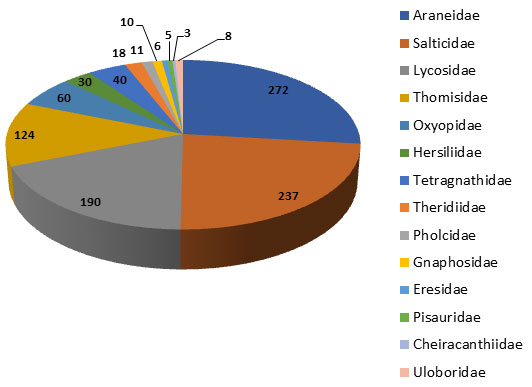
Results and Discussion: An inventory of spiders that were collected from the soybean and pigeon pea agro-ecosystem is listed in Table 1. In total, 1014 individuals were collected, comprising 41 genera from 14 familiesand 28 taxa have been identified at the species level. Of the 14 families, four dominant families were reported common for both cropping seasons. From Table 1, the result shows that Araneidae and Salticidae recorded the highest abundance of spiders with a total number of 272 and 237 spiders, followed by Lycosidae 190 and Thomisidae 124, while Cheiracanthiidae recorded the lowest abundance.
Peak species diversity was observed between August and November, with species richness during the 2020 Kharif season exceeding that of the 2021 season. This interannual variability in spider diversity metrics may reflect subtle shifts in climatic parameters (e.g., temperature, precipitation) between the two study periods, highlighting the sensitivity of arthropod communities to environmental fluctuations in rainfed agroecosystems (Smith et al., 2020; Kumar & Patel, 2022 Wadhwa and Malik 2024, Nik et al 2025).
While the family Araneidae exhibited the highest relative abundance in both cropping seasons, followed by Lycosidae and Thomisidae, Salticidae emerged as the most species-rich family across both study periods. This observation aligns with prior findings documenting that spider families such as Araneidae, Lycosidae, Thomisidae, and Salticidae—alongside Linyphiidae, Oxyopidae, and Tetragnathidae—are ecologically significant in agroecosystems due to their roles in regulating pest populations through natural predation (Memahet al., 2014; Deshmukh, 2017). The dominance of these families in the current study corroborates their established prevalence and functional importance in agricultural habitats, reinforcing their potential as bioindicators and biocontrol agents in integrated pest management strategies.
Spatial distribution of spiders within agroecosystems is strongly mediated by vegetation structure rather than random dispersion, reflecting their reliance on habitat complexity for foraging and web-building. A marked decline in orb-weaver (Araneidae) populations post-soybean harvest was observed, directly correlating with reduced vegetation density, underscoring the critical role of plant architecture in sustaining spider guilds.
Such spider–plant associations may exhibit mutualistic dynamics, wherein spiders enhance plant fitness via predation on herbivorous pests, while plants provide structural refugia and optimal microhabitats for spider survival and reproduction (Vasconcellos-Neto et al., 2020). This interplay is particularly relevant in soybean and pigeon pea systems, which are vulnerable to early-stage infestations by pests such as semiloopers, Helicoverpa, girdle beetles, pod borers, aphids, jassids, and whiteflies (Dwarka et al., 2021; Kennedy & Lekshmi, 2022 Nik et al 2025).
Peak species diversity, observed between August and November across both Kharif seasons, aligns with periods of maximal vegetative growth and structural complexity, further emphasizing the temporal dependency of spider communities on crop phenology and agroecological conditions. These findings highlight the importance of vegetation management in conserving spider biodiversity and enhancing their ecosystem services in pest regulation.Spiders are strongly influenced by plant architecture, rather than being randomly distributed in the vegetation.
The decline in orb-weavers after the soybean crop was harvested was noted, correlating with the loss of vegetation. Spider–plant associations can be considered mutualistic when spiders confer protective or nutritional benefits that enhance plant fitness, while plants, in turn, provide spiders with shelter and optimal foraging habitats (Vasconcellos-Neto et al., 2020). Soybean and pigeon pea are likely to be attacked by the Semilooper, Helicoverpa, girdle beetle, Pod borer aphids, jassids, and white fly, etc. in early stages of growth (Dwarka, et al., 2021; Kennedy and Lekshmi, 2022 Wadhwa and Malik 2024).
The maximum species diversity was noted from August to November for both the kharif seasons studied. According to Suana et al. (2004), the diversity of spider species is also influenced by factors such as habitat type, plant growth period, and landscape structure. Spiders are very sensitive to the variations in abiotic conditions, and Pitilinet al. (2019) observed that spiders influence the pest populations in the field and these are also influenced. Decline was noted in orb-weavers after the soybean crop was harvested, correlating with the loss of habitat (Fig. 1(d)).
This study documents novel baseline data on salticid (jumping spider) assemblages within agroecosystems, expanding known arachnofaunal records for Maharashtra, India. The findings align with established ecological principles demonstrating that spider-mediated pest suppression exhibits functional density-dependence, wherein higher predator abundance enhances trophic regulation of phytophagous arthropods (Greenstone, 1999; Riechert, 1999; Sunderland &Samu, 2000; Prashanthakumara&Venkateshwarlu, 2017).
These results corroborate the hypothesis that maintaining threshold abundance levels of spiders—particularly generalist predators like Salticidae—is critical for optimizing biocontrol efficacy in cropping systems. Such density-dependent interactions underscore the necessity of habitat management strategies that conserve spider populations to bolster ecosystem resilience and reduce reliance on synthetic pesticides.
Figure 1: Selected crop fields with mix crops of Soyabean and Pigeon Pea (Field a,b,c,d)

Table 1. List of spider species recorded from agroecosystems
| S.No. | Family | Genera | Taxa | Total |
| 1. | Araneidae
(Clerck, 1757) |
Argiope aemula (Walckenaer, 1841) | 1 | 17 |
| Argiope sp. | 2 | 29 | ||
| Bijoaraneus mitificus(Simon, 1886) * | 3 | 10 | ||
| Cyclosa bifida (Doleschall, 1859) | 4 | 7 | ||
| Cyclosa confraga(Thorell, 1892) | 5 | 4 | ||
| Cyclosa Sp. | 6 | 13 | ||
| Cyrtophora citricola* (Forsskål, 1775) | 7 | 5 | ||
| Eriovixia excelsa (Simon, 1889) | 8 | 26 | ||
| Guizygiella indica (Tikader& Bal, 1980) | 9 | 22 | ||
| Larinia sp. | 10 | 14 | ||
| Neoscona mukerjei (Tikader, 1980) | 11 | 28 | ||
| Neoscona theisi (Walckenaer, 1841) | 12 | 39 | ||
| Neoscona vigilans (Blackwall, 1865) | 13 | 21 | ||
| Neoscona sp. | 14 | 37 | ||
| 2. | CheiracanthiidaeWagner, 1887 | Cheiracanthium inornatumO. Pickard-Cambridge, 1874 | 15 | 3 |
| 3. | Eresidae
(C. L. Koch, 1845) |
Stegodyphus sarasinorum (Karsch,1892) | 16 | 6 |
| 4. | Gnaphosidae (Banks, 1892) | Drassodes sp. | 17 | 7 |
| Zelotes sp. | 18 | 3 | ||
| 5. | Hersiliidae
(Thorell, 1869) |
Hersilia savignyi (Lucas 1836) | 19 | 9 |
Hersilia sp. |
20 | 21 | ||
| 6. | Lycosidae
(Sundevall, 1833) |
Arctosa lesserti(Reimoser, 1934) | 21 | 11 |
| Hippasa sp | 22 | 14 | ||
| Lycosa poonaensis (Tikader& Malhotra, 1980 | 23 | 23 | ||
| Lycosa sp. | 24 | 39 | ||
Pardosa pseudoannulata (Bösenberg& Strand, 1906) |
25 | 25 | ||
| Pardosa sutherlandi (Gravely, 1924) | 26 | 19 | ||
| Pardosa sp. | 27 | 27 | ||
| Wadicosa fidelis (O. Pickard-Cambridge, 1872) | 28 | 32 | ||
| 7. | Oxyopidae
(Thorell, 1869) |
Oxyopes hindostanicus(Pocock, 1901) | 29 | 8 |
| Oxyopes sp. | 30 | 26 | ||
| Peucetia viridana(Stoliczka, 1869) | 31 | 9 | ||
| Peucetia sp. | 32 | 17 | ||
| 8. | Pholcidae (C. L. Koch, 1850) | Pholcus sp. | 33 | 11 |
| 9 | Pisauridae (Simon, 1890) | Nilus phipsoni (F. O. Pickard-Cambridge, 1898) | 34 | 5 |
| 10. | Salticidae (Blackwall, 1841) | Chrysilla sp. | 35 | 10 |
| Hasarius adansoni (Audouin, 1826) * | 36 | 24 | ||
| Hyllus semicupreus (Simon, 1885) | | 37 | 38 | ||
| Menemerus bivittatus(Dufour, 1831) | 38 | 20 | ||
| Myrmarachne sp. | 39 | 19 | ||
| Phintella sp. | 40 | 38 | ||
| Plexippus paykulli * (Audouin, 1826) | 41 | 45 | ||
| Pseudicius sp. | 42 | 20 | ||
| Telamonia dimidiata(Simon, 1899) | 43 | 23 | ||
| Thyene imperialis(Rossi, 1846) * | 44 | 10 | ||
| 11. | Tetragnathidae (Menge, 1866) | Leucauge decorata (Blackwall, 1864) | 45 | 26 |
| Tetragnatha sp. | 46 | 14 | ||
| 12. | Theridiidae (Sundevall, 1833) | Theridion sp. | 47 | 18 |
| 13. | Thomisidae (Sundevall, 1833) | Indoxysticus minutus * (Tikader, 1960) | 48 | 17 |
| Massuria sp. | 49 | 21 | ||
| Misumena sp. | 50 | 12 | ||
| Runcinia sp. | 51 | 25 | ||
| Thomisus sp. | 52 | 49 | ||
| 14. | Uloboridae
(Thorell, 1869) |
Uloborussp. | 53 | 8 |
| Total | 14 | 41 | 53 | 1014 |
Table 2. Total number of species from individual families
| Sr. No. | Family | Total Species Noted |
| 1 | Araneidae (Clerck, 1757) | 272 |
| 2 | Salticidae (Blackwall, 1841) | 237 |
| 3 | Lycosidae(Sundevall, 1833) | 190 |
| 4 | Thomisidae (Sundevall, 1833) | 124 |
| 5 | Oxyopidae(Thorell, 1869) | 60 |
| 6 | Hersiliidae(Thorell, 1869) | 30 |
| 7 | Tetragnathidae (Menge, 1866) | 40 |
| 8 | Theridiidae (Sundevall, 1833) | 18 |
| 9 | Pholcidae (C. L. Koch, 1850) | 11 |
| 10 | Gnaphosidae (Banks, 1892) | 10 |
| 11 | Eresidae(C. L. Koch, 1845) | 06 |
| 12 | Pisauridae (Simon, 1890) | 05 |
| 13 | CheiracanthiidaeWagner, 1887 | 03 |
| 14 | Uloboridae(Thorell, 1869) | 08 |
| Total | 1014 |
Figure 2: Total percentage of spider species recorded during the study
The biodiversity analysis indices revealed that Shannon-Weiner Diversity Index (H’) was 2.089 in 2020 and 2.766 in 2021 kharif, Species Richness Index (a) was (4.135 in 2020 and 4.22 in 2021 kharif ) and Evenness Index (E1) was (0.104 in 2020 and 0.095 in 2021 kharif). These indicate that the species diversity and evenness indices during 2020 kharif were more abundant compared to those of 2021 kharif; and species richness was more or less equal and exhibited a similar diversification in both seasons.
CONCLUSION
The present study documented lower overall spider diversity and abundance compared to previous studies in the region. This decline may be attributed to the frequent use of chemical pest control methods and fluctuating weather conditions observed during the study period in the area. The Shannon-Weiner index highlights the habitat’s potential for conserving spider diversity. Consequently, understanding spider diversity and species richness is essential for advancing their role as biocontrol agents in agroecosystems. Additionally, the data gathered will serve as a valuable resource for researchers interested in studying the spider fauna of Amravati, Maharashtra (India).
ACKNOWLEDGEMENTS
The authors extend their sincere gratitude to the late Dr. G. N. Vankhede, former Professor and Head of the Department of Zoology at Sant Gadge Baba Amravati University, Amravati, Maharashtra, for his enduring mentorship, scholarly inspiration, and foundational guidance that influenced the conceptualization and pursuit of this research initiative. We would like to express our sincere gratitude to the farmers who generously helped us during this study.
Availability of data and material: All the data generated and analyzed during the study are included in the main manuscript and will be available with the corresponding author on a reasonable request..
Competing interests: The author declares that they have no competing interests.
Funding: NA
REFERENCES
Akhilesh, K and Parasnath (2003) Pest complex and their population dynamics on an early variety of pigeon UPAS – 120 at Varanasi. Indian Journal of Entomology Vol. 65 No.4 Pages 453-460.
Feledyn-Szewczyk B, Kus J, Stalenga J, et al. (2016) The Role of Biological Diversity in Agroecosystems and Organic Farming. Organic Farming – A Promising Way of Food Production. InTech. Available at: http://dx.doi.org/10.5772/61353.
Chang, G C and Kareiva P (1999) The case for indigenous generalists in biological control. In: Theoretical approaches to biological control, (Eds.) Hawkins, B.A. and H.V. Cornell. Cambridge University Press, Cambridge, United Kingdom. Pages 103-115.
Daniel J, Alfred & Narayanasami, Chitra & Mariyappan, Mathialagan. (2018). Diversity of Harmful and Beneficial Insect Fauna in Pigeonpea (Cajanus cajan (L.)) Ecosystem in Tamil Nadu, India. International Journal of Current Microbiology and Applied Sciences. 7. 396-402. 10.20546/ijcmas.2018.708.045.
Dolly, K and Mishra A (2008) Ant Community Variation in Urban and agricultural Ecosystems inVadodara District (Gujarat State), Western India, Asian Myrmecology Vol 2 Pages 85- 93.
Greenstone, M H (1999) Spider predation: How and why we study it. Journal of Arachnology Vol. 27 Pages 333-342.
https://agricoop.nic.in/sites/default/files/MH12%20-%20Amravati.pdf.
Vasconcellos-Neto J., Y. F. Messas, H. da Silva Souza, German A. (2017) Ecological Approach, Behaviour and Ecology of Spiders, Springer International Publishing AG , C. Viera, M.O. Gonzaga (eds.) pages 165-213.
John, S K and Jeeva K. L (2022) Holistic Pest Management Strategies in Tropical Plant Species. 10.5772/intechopen.105104.
Keswani S, Hadole, P and Rajoria, A (2012) Checklist of Spiders (Arachnida: Araneae) from India. Indian Journal of Arachnology. Vol. 1 No.1 Pages 1-129.
Memah, V. V., Max Tulung, Jootje Warouw, Redsway R.T.D. Maramis (2014) Diversity of Spider Species in Some Agricultural Crops in North Sulawesi, Indonesia, International Journal of Scientific & Engineering Research, Vol 5, Issue 6 Pages 70-75.
Nik JMJ J Cunniffe and Stephen Parnell (2025) Assessing delimiting strategies to identify the infested zones of quarantine plant pests and diseases Scientific Reports volume 15, Article number: 5610
Pitilin R B, Prado J, Brescovit A D and Buschini M L T (2019) Climatic conditions drive the abundance and diversity of spider community in an Atlantic forest fragment Oecologia Australis Vol.23 No.1 Pages 39-55.
Prashanthakumara S. M and M. Venkateshwarlu (2017) Diversity and Distribution Of Spider Fauna In Different Ecosystems Of Chikmagalur Parts Of Western Ghats, Karnataka International Journal of Innovative Research and Advanced Studies (IJIRAS), Volume 4 No.7 Pages 20-24.
Rao, R. G. V, K. B. Saxena, P. Yang Shiying and T. Weiguang (2002) Insect pest problems of pigeon pea in Gangni and Hainan province of China. International. Chickpea and Pigeon pea, New Letters, Pages 48.
Ravi N, Sravanthy Ch, Sammaiah Ch. and Thirupathaiah M (2015) Biodiversity of Ants (Insecta-Hymenoptera) in Agro- Ecosystem and Grassland in Jammikunta, Karimnagar District, Telangana, India, Journal of Environment, Vol. 4 No.1 Pages 11-16.
Riechert S E and Lockley T (1984) “Spiders as Biological Control Agents,” Annual Review of Entomology, Vol. 29, Pages 299-320.
Singh, H K and Singh, H N (1991) Some major pest incidence on certain late cultivars of pigeon pea Cajanuscajan L. during half pod formation stage. Ind. J. Ent. Vol.53 No. 2 Pages 298-303.
Suana I W, Solihin D D, Buchori D, Manuwoto S, and Triwidodo H (2004) Komunitaslaba-laba pada lansekappersawahan di Cianjur Hayati, Vol 11 Pages 145-152.
Sunderland K and Samu F (2000) Effects of agricultural diversification on the abundance, distribution and pest control potential of spiders: a review. Entomologia Experimentalis et Applicata. Vol 95 Pages 1-13.
Synmondson, W O C, Sunderland, KD and Greenstone, M H (2002) Can generalist predators be effective biocontrol agents, Annual Review of Entomology Vol 47 Pages 561-594.
Wadhwa D and Kamal Malik (2024) A generalizable and interpretable model for early warning of pest-induced crop diseases using environmental data Computers and Electronics in Agriculture Volume 227, Part 1,109472
World Spider Catalog (2025). World Spider Catalog. Version 26. Natural History Museum Bern, online at http://wsc.nmbe.ch, accessed on {10-02-2025}. doi: 10.24436/2


